Abstract
Introduction
The objective of this study was to evaluate the safety and feasibility of the immediate return of patients with ST-elevation myocardial infarction (STEMI) to their originating hospitals after primary percutaneous coronary intervention (PPCI).
Methods
This was a prospective study, conducted between January 2014 and December 2017. All patients with STEMI who were transferred for PPCI and returned back to their referring hospitals (RB group) were included and compared to the onsite STEMI population (OS group). Patient’s demographics, PPCI data, bleeding and adverse cardiovascular events (ACEs) occurring during transfer, hospital stay, and at 1-month follow-up were recorded.
Results
A total of 156 patients in the OS group were compared against 350 patients in the RB group. We found that first medical contact to balloon time and onset of symptoms to balloon time were significantly longer in the RB group than in the OS group [110 ± 67 min vs. 46 ± 35 min (p < 0.0001) and 366 ± 300 min vs. 312 ± 120 min (p = 0.04)], respectively. There were no differences between the RB and OS groups in in-hospital ACEs: 0.3% versus 0% (p = 0.8) for death, 0.3% versus 0.6% (p = 0.79) for reinfarction, 0.6% versus 2% (p = 0.72) for bleeding, and no reported cases of repeat revascularization; and 30-day ACEs: 0.3% versus 0.6% (p = 0.82) for death, 0.3% versus 1.2% (p = 0.68) for reinfarction, 0.6% versus 2% (p = 0.74) for bleeding, and 1.1% versus 1.2% (p = 0.9) for repeat revascularization.
Conclusion
The immediate return of patients with noncomplicated STEMI after PPCI to their referring hospitals is safe and feasible, and can be used as part of an effective reperfusion strategy.
Keywords: Myocardial infarction, Primary percutaneous coronary intervention
1. Introduction
ST-elevation myocardial infarction (STEMI) requires an early reperfusion therapy by either fibrinolysis or primary percutaneous coronary intervention (PPCI). Of note, PPCI is cost-effective and has better reperfusion rates when compared to thrombolytic therapy; hence, it is considered the treatment of choice for patients with STEMI when the delay time from STEMI diagnosis to wire crossing is expected to be less than 120 min [1,2]. Multiple studies have shown that PPCI can significantly reduce mortality, reinfarction, and cerebral complications compared to fibrinolytic treatment [3,4]. Nevertheless, the restricted availability of catheterization laboratories, adequate equipment, and trained personnel still limits its wider adoption in developing countries. To resolve these limitations, patients’ transferring strategies from non PPCI-capable hospitals to tertiary-care centers providing PPCI were developed and proven to improve outcomes in many randomized trials [5–10]. However, this strategy might result in an increased number of PPCIs in tertiary-care centers, which in turn limits the hospital resources, in particular, bed availability in critical care units. Therefore, the concept of immediate return of patients to their parent hospitals was implemented. Few studies investigated the rationale for the same-day return after PPCI. Moreover, most of the prior studies focused on late transfers after PCI and excluded patients with STEMI [11–13]. The aim of the current study was to evaluate the safety and feasibility of the immediate return of patients with STEMI after PPCI to their referring hospitals.
2. Methods
2.1. Study population
We conducted a prospective study between January 2014 and December 2017 in a tertiary-care PPCI-capable center in Al-Qassim region (population of 2 million). Although there are 10 peripheral non-PPCI-capable hospitals in the region, only four centers with a distance of less than 20 km from the tertiary-care center were involved in the study. All study participants were enrolled from Qassim Primary Angioplasty Services (QAPAS) program, which was implemented in 2004 to provide full-time PPCI access to all eligible STEMI patients.
2.2. Patient selection
All patients with STEMI transferred for PPCI and returned back to their referring hospitals between 2014 and 2017 were included in the returned back (RB) group, whereas patients admitted directly from the emergency department for PPCI during 2017 served as an onsite (OS) control group (Fig. 1). Patients with hemodynamic instability (Killip class III or IV, cardiogenic shock, intra-aortic balloon pump insertion, and electrical complications), complicated PPCI (coronary dissection, no-reflow phenomenon, major bleeding, and multivessel PCI), concomitant obstructive left main stenosis, and femoral access without closure device were excluded from the study. The study protocol was approved by the institutional ethics committee (Prince Sultan Cardiac Center Qassim), and all patients signed an informed consent. Demographic data, coronary artery disease risk factors, laboratory results, STEMI location, as well as electrocardiogram and echocardiography variables were reported.
Fig. 1.
Methodology of the study. LM = Left Main coronary aretry; PPCI = primary percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
2.3. Coronary angiography and PCI procedures
All transferred patients were accompanied by a physician and two trained nurses during ambulance transportation. According to the study protocol, a loading dose of aspirin 325 mg, clopidogrel 600 mg, and intravenous heparin 4000 IU were administered during transportation unless given in the referring hospital. Radial access was used for coronary angiography in all patients; however, radial to femoral crossover was applied in cases of vascular access complications, spasm, or technical difficulties. All the standard coronary angiographic views were obtained to determine the number of involved vessels, the extent of coronary artery disease, and the culprit lesion using visual assessment. If required, a thrombus aspiration and/or administration of intravenous glycoprotein IIb/IIIa inhibitors was considered [14,15]. Number and size of the deployed stents and post-stenting dilatation were done according to the culprit lesion morphology. All procedural complications and thrombolysis in myocardial infarction (TIMI) flow grades were recorded. After the procedure, all hemodynamically stable patients without procedural complications were transferred to the referring hospital by the same accompanying medical team.
2.4. Follow-up
Adverse cardiovascular events (ACEs) including reinfarction caused by stent thrombosis or PCI complications, repeat revascularization, electrical complications (ventricular tachycardia or advanced atrioventricular block), rehospitalization because of myocardial ischemia, stroke, and death during hospital stay and at 1 month follow-up were reported for all patients.
2.5. Statistical analysis
The quantitative variables were expressed as mean ± standard deviation and categorical variable were expressed as frequencies. A Student’s t-test was used to compare continuous variables, whereas chi-square test was used for comparison between categorical variables. All statistical analyses were performed using the SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Clinical and angiographic characteristics
Out of 456 patients who were transferred for PPCI from the referring hospitals, 350 (77%) were clinically stable and comprised the RB group (mean age, 51 ± 9 years; 91.3% male). The control group comprised 156 patients who were admitted directly from the emergency department for PPCI (mean age, 54 ± 9 years; 85% male). We found no significant differences between both groups in age, prevalence of coronary artery disease risk factors, and the location of myocardial infarction (Table 1 and Fig. 2). The majority of patients had anterior STEMI (59.5%), followed by inferior (25%), lateral (11.2%), and other location myocardial infarction (4.3%).
Table 1.
Baseline characteristics.
| Onsite PCI (N = 156) |
Returned back PCI (N = 350) |
p | |
|---|---|---|---|
| Age (years) | 54 ± 9 | 51 ± 11 | 0.6 |
| Men | 133 (85) | 319 (91) | 0.86 |
| Diabetes | 46 (29) | 102 (29) | 0.84 |
| Hypertension | 48 (30) | 121 (34) | 0.8 |
| Dyslipidemia | 18 (11) | 5 (2) | 0.08 |
| Smoking | 58 (37) | 135 (38) | 0.9 |
| Family history of CAD | 6 (3.8) | 20 (5.7) | 0.7 |
Data are presented as mean ± SD or n (%).
CAD = coronary artery disease; PCI = percutaneous coronary intervention.
Fig. 2.
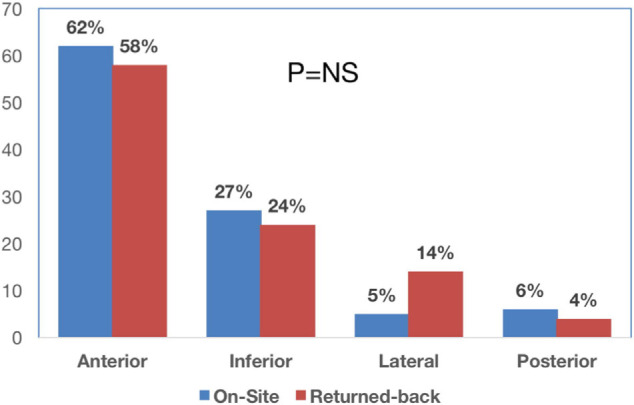
STEMI location. ACEs = adverse cardiovascular events; OS = onsite; PPCI =primary percutaneous coronary intervention; RG = returned back; STEMI: ST-elevation myocardial infarction; TIMI = thrombolysis in myocardial infarction.
3.2. Coronary angiography and PPCI
The radial approach was used in 94% of patients, whereas the femoral approach (with the use of closure device) was applied in 6% of patients. The prevalence of nonobstructive coronary artery disease, single-vessel disease, two-vessel disease, and three-vessel disease was 4.3%, 60.7%, 21%, and 14%, respectively. Among patients with obstructive coronary disease, the culprit vessel was the left anterior descending coronary artery in 57.2% patients, the right coronary artery in 23.5% patients, and the left circumflex in 15% patients. Thrombus aspiration and glycoprotein IIb/IIIa inhibitors were used in 38% and 78% patients, respectively. TIMI III flow was achieved in 94% of patients after stent deployment. The angiographic characteristics are shown in Table 2.
Table 2.
Angiographic and procedural data.
| Onsite PCI (N = 156) |
Returned back PCI (N = 350) |
p | |
|---|---|---|---|
| STEMI location | |||
| Anterior | 96 (62) | 205 (58) | 0.76 |
| Inferior | 42 (27) | 84 (24) | 0.81 |
| Lateral | 8 (5) | 49 (14) | 0.12 |
| Posterior | 10 (6) | 12 (4) | 0.7 |
| Number of vessels | |||
| Nonsignificant CAD | 6 (3) | 19 (5) | 0.89 |
| 1V-CAD | 99 (64) | 205 (59) | 0.66 |
| 2V-CAD | 37 (24) | 70 (20) | 0.75 |
| 3V-CAD | 14 (9) | 56 (16) | 0.4 |
| Radial access | 141 (90) | 334 (96) | 0.8 |
| Femoral access | 15 (10) | 16 (4) | |
| Culprit vessel | |||
| LAD | 91 (58) | 199 (57) | 0.89 |
| LCX | 21 (13) | 54 (15) | 0.87 |
| RCA | 38 (26) | 81 (23) | 0.76 |
| No PCI | 6 (3) | 19 (5) | 0.8 |
| TIMI II | 4 (2.5) | 21 (6) | 0.7 |
| TIMI III | 152 (97.5) | 329 (94) |
Data are presented as n (%).
1 V-CAD = single-vessel CAD; 2 V-CAD = two-vessel CAD; 3 V-CAD = three-vessel CAD; CAD = coronary artery disease; LAD = Left Anterior Descending artery; LCX = Left circumflex artery; PCI = percutaneous coronary intervention; RCA = Right coronary artery; STEMI = ST-elevation myocardial infarction; TIMI = thrombolysis in myocardial infarction.
3.3. Timing intervals
The first medical contact to balloon time and the onset of symptoms to balloon time were significantly longer in the RB group than in the OS group (110 ± 67 vs. 46 ± 35 min, p < 0.0001) and (366 ± 300 vs. 312 ± 120 min, p = 0.04), respectively. Conversely, procedural time and post-PPCI in-hospital stay were comparable between both groups (Table 3). Patients from the RB group were transferred back within 34 ± 23 min after the procedure completion.
Table 3.
Timing intervals.
| Onsite PCI (N = 156) |
Returned back PCI (N = 350) |
p | |
|---|---|---|---|
| Onset of symptoms to balloon time, min | 312 ± 120 | 366 ± 300 | 0.04 |
| First medical contact to balloon time, min | 46 ± 35 | 110 ± 67 | <0.0001 |
| Procedural time, min | 47 ± 32 | 45 ± 27 | 0.8 |
| Post PCI in-hospital stay, d | 3.4 ± 2 | 4.6 ± 3 | 0.057 |
Data are presented as mean ± SD.
PCI = percutaneous coronary intervention.
3.4. Adverse cardiac events
During the hospital stay, there were no differences in ACEs between the RB and OS groups: one (0.3%) versus zero (0%) (p = 0.8) for death, one (0.3%) versus one (0. 6%) (p = 0.79) for reinfarction, two (0.6%) versus three (2%) (p = 0.72) for bleeding, and two (0.6%) versus five (3%) (p = 0.64) for arrhythmias. Similarly, ACEs at 30-day follow-up were similar between groups: one (0.3%) versus one (0.6%) (p = 0.82) for death, one (0.3%) versus two (1.2%) (p = 0.68) for reinfarction, two (0.6%) versus three (2%) (p = 0.74) for bleeding, four (1.9%) versus six (3.8%) (p = 0.8) for arrhythmias, four (1.1%) versus two (1.2%) (p = 0.9) for repeat revascularization, and five (1.5%) versus seven (4.5%) (p = 0.57) for readmission, respectively (Table 4). Importantly, no ACEs were reported during transfer of the RB patients to their parent hospitals.
Table 4.
Adverse cardiac events.
| In-hospital complications
|
30-day follow-up
|
|||||
|---|---|---|---|---|---|---|
| Onsite PCI (N = 156) |
Returned back PCI (N = 350) |
p | Onsite PCI (N = 156) |
Returned back PCI (N = 350) |
p | |
| Death | 0 (0) | 1 (0.3) | 0.8 | 1 (0.6) | 1 (0.3) | 0.82 |
| Reinfarction | 1 (0.6) | 1 (0.3) | 0.79 | 2 (1.2) | 1 (0.3) | 0.68 |
| Bleeding | 3 (2) | 2 (0.6) | 3 (2) | 2 (0.6) | ||
| Minor | 2 (1.2) | 2 (0.6) | 2 (1.2) | 2 (0.6) | ||
| Major | 1 (0.6) | 0 (0) | 0.72 | 1 (0.6) | 0 (0) | 0.74 |
| Arrhythmias | 5 (3) | 2 (0.6) | 6 (3.8) | 4 (1.9) | ||
| Ventricular tachycardia/fibrillation | 1 (0.6) | 0 (0) | 1 (0.6) | 0 (0) | ||
| Atrial fibrillation | 3 (2) | 1 (0.3) | 0.64 | 4 (2.5) | 3 (1.3) | 0.8 |
| Atrioventricular block | 1 (0.6) | 1 (0.3) | 1 (0.6) | 1 (0.6) | ||
| Stroke | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Revascularization | 0 (0) | 0 (0) | – | 2 (1.2) | 4 (1.1) | 0.9 |
| Readmission | – | – | – | 7 (4.5) | 5 (1.5) | 0.57 |
Data are presented as n (%).
PCI percutaneous coronary intervention.
4. Discussion
In the present study, we demonstrated that retransferring patients after STEMI treated by PPCI is feasible in 77% of patients. Furthermore, we found that this strategy might be effective and safe in the low-risk STEMI population with very low incidence of adverse cardiac events during the in-hospital stay and 30-day follow-up.
Studies demonstrated that PPCI is a cost-effective and superior strategy when compared to fibrinolysis [16,17]. The net benefit of interventional strategy was achieved mainly because of the lower number of ACEs and higher permanent coronary reperfusion when compared with fibrinolysis [18–20]. In addition, coronary intervention results in shorter hospital stay as well as lower number of revascularization and hospitalizations during the follow-up period. This might reduce the economic burden with the extension of the cost net benefit for longer than 5 years after STEMI, along with an increase in life expectancy of about 12 months in the PPCI group [19].
The strategies of transferring patients for PPCI were analyzed in various randomized clinical trials confirming the superiority of PPCI when compared with the peripheral hospital fibrinolytic therapy, in particular when the inherent delay of interventional strategy is not excessive and is adjusted to that recommended in international clinical guidelines [5–8]. One major challenge of PPCI strategy that has yet to be resolved is the shortage of beds in critical care units when offering hospital admission for patients transferred from other non-PPCI centers. There are limited data on transferring patients back after PPCI to their referral hospitals. In one study, Margheri et al [20] described a cohort of 135 patients treated with PPCI, of whom 92 patients had been transferred from peripheral hospitals. Interestingly, 81 (88%) of 92 patients could be returned to their referral centers within 8 hours after the procedure. Nevertheless, this study was performed in the percutaneous transluminal coronary angioplasty era. Also, it was limited by a retrospective design and no specific criteria for transfer nor cardiovascular events were specified.
It is worth noting that our findings are similar to those reported in previous studies [11–13], reaffirming the safety and feasibility of retransferring patients after PCI with very low cardiac events; however, all of the previous studies excluded patients with STEMI. Furthermore, only our study used the shortest time interval (34 ± 23 min) for returning patients back to their referring centers.
It is of great importance that no clinical events were reported during the transfers, and the frequency of ACEs studied within the hospital stay and at 30-day follow-up was lower than those previously reported regarding PPCI treatment [21,22]. Certainly, the low incidence of vascular and bleeding complications can be explained by a predominant use of radial approach in our cohort. Furthermore, the low number of adverse events should also be related to the stringent selection process of our patients. Importantly, we conclude that immediate transfer of patients post PPCI is safe and feasible once an adequate flow of communication between the referring and the PCI-capable hospital is provided.
Notably, the study had been presented and won the first place prize at the Saudi Heart Association annual meeting in 2019; in addition, the abstract had been published in the Journal of the Saudi Heart Association.
4.1. Limitations of the study
This study had several limitations. First, we followed patients for 1 month only, and a longer follow-up to detect complications is anticipated. Second, we excluded high-risk patients and complicated PPCI; thus, our results cannot be generalized to all patients with STEMI. Finally, our strategy cannot be applied to other hospitals that do not have similar facilities and resources (prearranged protocol for immediate transfer, 24/7 PPCI capabilities, PPCI hospital code, non-PPCI-capable hospitals located outside 20 km from the tertiary-care center).
5. Conclusions
The immediate return of low-risk STEMI patients is feasible in the majority of cases. This strategy represents an effective and safe part of the reperfusion protocol in the low-risk STEMI population with very low incidence of adverse cardiac events.
Abbreviations
- STEMI
ST-elevation myocardial infarction
- PPCI
primary percutaneous coronary intervention
- RB
Returned back
- OS
onsite
- TIMI
Thrombolysis in Myocardial Infarction
- ACE S
Adverse cardiovascular events
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Catheter Cardiovasc Interv. 2013;82:E1–27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- 3.Grines C, Patel A, Zijlstra F, Weaver WD, Granger C, Simes RJ. Primary coronary angioplasty compared with intravenous thrombolytic therapy for acute myocardial infarction: six-month follow up and analysis of individual patient data from randomized trials. Am Heart J. 2003;145:47–57. doi: 10.1067/mhj.2003.40. [DOI] [PubMed] [Google Scholar]
- 4.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 5.Widimsky P, Groch L, Zelizko M, Aschermann M, Bednar F, Suryapranata H. Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs combined strategy for patients with acute myocardial infarction presenting to a community hospital without a Catheterization laboratory. The PRAGUE study. Eur Heart J. 2000;21:823–31. doi: 10.1053/euhj.1999.1993. [DOI] [PubMed] [Google Scholar]
- 6.Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–42. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 7.Widimsky P, Budesinsky T, Vorac D, Groch L, Zelizko M, Aschermann M, et al. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE–2. Eur Heart J. 2003;24:94–104. doi: 10.1016/s0195-668x(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 8.Grines CL, Westerhausen DR, Jr, Grines LL, Hanlon JT, Logemann TL, Niemela M, et al. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: the Air Primary Angioplasty in Myocardial Infarction study. J Am Coll Cardiol. 2002;39:1713–9. doi: 10.1016/s0735-1097(02)01870-3. [DOI] [PubMed] [Google Scholar]
- 9.Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation. 2003;108:1809–14. doi: 10.1161/01.CIR.0000091088.63921.8C. [DOI] [PubMed] [Google Scholar]
- 10.van‘t Hof AW. The challenge of reducing time to reperfusion in patients with acute ST elevation myocardial infarction. Eur Heart J. 2008;29:1793–4. doi: 10.1093/eurheartj/ehn225. [DOI] [PubMed] [Google Scholar]
- 11.Andersen JG, Kløw NE, Johansen O. Safe and feasible immediate retransfer of patients to the referring hospital after acute coronary angiography and percutaneous coronary angioplasty for patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2:256–61. doi: 10.1177/2048872613483587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen JG, Grepperud S, Klow NE, Johansen O. Effects on length of stay and costs with same-day retransfer to the referring hospitals for patients with acute coronary syndrome after angiography and/or percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care. 2016;5:375–80. doi: 10.1177/2048872615593386. [DOI] [PubMed] [Google Scholar]
- 13.Burgess SN, Nguyen VH, Xu J, Hinde Y. Validation of the RETRIEVE (REverse TRIage EVEnts) criteria for same day return of non-ST elevation acute coronary syndrome patients to referring non-PCI centres. Heart Lung Circ. 2018;27:792–7. doi: 10.1016/j.hlc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Silber S, Albertsson P, Aviles FF, Camici PG, Colombo A, Hamm C, et al. The task force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–47. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 15.Le May MR, Davies RF, Labinaz M, Sherrard H, Marquis JF, Laramee LA, et al. Hospitalization costs of primary stenting versus thrombolysis in acute myocardial infarction: cost analysis of the Canadian STAT Study. Circulation. 2003;108:2624–30. doi: 10.1161/01.CIR.0000097120.26062.FE. [DOI] [PubMed] [Google Scholar]
- 16.de Boer MJ, van Hout BA, Liem AL, Suryapranata H, Hoorntje JC, Zijlstra F. A cost-effective analysis of primary coronary angioplasty versus thrombolysis for acute myocardial infarction. Am J Cardiol. 1995;76:830–3. doi: 10.1016/s0002-9149(99)80238-0. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Grines CL, Rothbaum D, Browne KF, O’Keefe J, Overlie PA, et al. Analysis of the relative costs and effectiveness of primary angioplasty versus tissue-type plasminogen activator: the Primary Angioplasty in Myocardial Infarction (PAMI) trial. The PAMI Trial Investigators. J Am Coll Cardiol. 1997;29:901–7. doi: 10.1016/s0735-1097(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 18.Machecourt J, Bonnefoy E, Vanzetto G, Motreff P, Marliere S, Leizorovicz A, et al. Primary angioplasty is cost-minimizing compared with pre-hospital thrombolysis for patients within 60 min of a percutaneous coronary intervention center: the Comparison of Angioplasty and Pre-hospital Thrombolysis in Acute Myocardial Infarction (CAPTIM) cost-efficacy sub-study. J Am Coll Cardiol. 2005;45:515–24. doi: 10.1016/j.jacc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Selmer R, Halvorsen S, Myhre KI, Wisloff TF, Kristiansen IS. Cost-effectiveness of primary percutaneous coronary intervention versus thrombolytic therapy for acute myocardial infarction. Scand Cardiovasc J. 2005;39:276–85. doi: 10.1080/14017430510035988. [DOI] [PubMed] [Google Scholar]
- 20.Margheri M, Meucci F, Falai M, Comeglio M, Giglioli C, Chechi T, et al. Transferring patients for direct coronary angioplasty: a retrospective analysis of 135 unselected patients with acute myocardial infarction. Ital Heart J. 2001;2:921–6. [PubMed] [Google Scholar]
- 21.Antoniucci D, Valenti R, Migliorini A, Moschi G, Trapani M, Buonamici P, et al. Relation of time to treatment and mortality in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol. 2002;89:1248–52. doi: 10.1016/s0002-9149(02)02320-2. [DOI] [PubMed] [Google Scholar]
- 22.van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. doi: 10.1016/s0195-668x(02)00618-8. [DOI] [PubMed] [Google Scholar]



