Abstract
Background
First-generation or second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are commonly used in EGFR-mutation-positive advanced non-small-cell lung cancer (NSCLC) with no relevant differences in efficacy in randomised clinical trials (RCTs). Patients enrolled to RCTs may differ from NSCLC population in everyday practice. Limited real-world experience (RWE) exists on efficacy of EGFR TKIs in European patient cohorts.
Patients and methods
In this retrospective study, real-world data of all patients who started first-line EGFR TKIs between 2012 and 2016 in Poland were analysed. The main endpoints were progression-free survival (PFS) and overall survival (OS). Secondary endpoints were an objective response rate and toxicity.
Results
A total of 620 treatment-naive EGFR mutated patients with stage III/IV NSCLC were analysed with follow-up time of 24.5 months. A significantly longer median PFS (p=0.005) and higher 1-year OS rate (p=0.004) for afatinib (16.4 months and 78.2%) vs gefitinib (10.3 months and 69.1%) and erlotinib (12.1 months and 71.6%) were observed. In multivariate analysis toxicity was predictive for PFS and OS. In patients with adverse events (AEs) versus those without AEs, improved median PFS (13.6 months vs 8.8 months) and median OS (23.6 vs 15.5 months) were observed. Median OS in the group with AE of grades 3–4 and those with AE of grades 1–2 were 42.1 months and 23.4 months, respectively.
Conclusion
This study represents the largest RWE of first-line TKI therapy in a European country with longer survival of patients receiving second-generation TKI. We confirmed in everyday practice the role of toxicity as a marker of clinical benefit.
Keywords: non-small-cell lung cancer, EGFR activating mutations, EGFR tyrosine kinase inhibitors, real-world practice
Key questions.
What is already known about this subject?
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are standard of care in EGFR mutated non-small-cell lung cancer (NSCLC) patients.
In randomised clinical trials, no clinically relevant differences in efficacy between afatinib, erlotinib and gefitinib were observed; patients enrolled to clinical trials may differ from population in everyday practice.
What does this study add?
A very few publications report real-world data in large European patients cohort treated with EGFR TKIs in first line.
We report the efficacy and safety of first-and second-generation EGFR TKIs in one of the largest European patients population, who represent all treatment-naive unselected patients with advanced EGFR mutated NSCLC treated across the country.
How might this impact on clinical practice?
We confirmed in a large real-world study in European patients cohort the survival benefit of patients receiving second-generation TKI.
The observed outcomes and toxicity as a marker of clinical benefit may be relevant in everyday practice for clinicians and healthcare system providers.
This study provides real-world data in unselected EGFR mutated patients that may help in choosing the treatment options in routine practice.
Introduction
Activating epidermal growth factor receptor (EGFR) mutations are diagnosed in approximately 10% of patients with lung adenocarcinomas.1 Three first-generation or second-generation EGFR tyrosine kinase inhibitors (TKIs), erlotinib, gefitinib or afatinib, are commonly used in EGFR-mutation-positive advanced non-small-cell lung cancer (NSCLC). All these agents received regulatory approval in first-line treatment of patients with EGFR-mutation-positive advanced NSCLC based on the results of numerous randomised trials showing superiority over chemotherapy in terms of progression-free survival (PFS) and tolerance.2–6 Randomised clinical trials (RCTs) and meta-analyses found no significant or clinically relevant differences in efficacy among these agents but showed somewhat distinct toxicity profiles.4–9 Similar outcomes were found despite some differences in the method of action of first-generation (erlotinib, gefitinib) and second-generation TKIs (afatinib)—the broader spectrum of activity and irreversible mechanism of action of afatinib did not translate into its clinically meaningful higher effectiveness.10 Patients enrolled to RCTs may differ substantially from NSCLC population in everyday practice.11 Limited real-world experience exists on efficacy of EGFR TKIs in European patient cohorts. The present study was aimed to analyse the efficacy and safety of EGFR TKIs in a large cohort of patients with EGFR-mutated advanced NSCLC.
Material and methods
All EGFR TKIs are available in Poland within a nationwide therapeutic programme (TP) financed centrally by the National Health Fund. The programme was introduced in 2011. Patients with advanced NSCLC and confirmed EGFR activating common mutation (either exon 19. deletion or exon 21. substitution) receive first-line EGFR TKI if inclusion and exclusion criteria are fulfilled. At the time of analysis, osimertinib was not available in daily practice in first-line treatment. This retrospective analysis included all patients with NSCLC who started first-line EGFR TKIs reimbursed in Poland between 2012 and 2016 (first-line treatment with erlotinib and gefitinib was available from 2012 and with afatinib from 2015).
Main inclusion criteria for the programme were: EGFR-mutated stage III (ineligible for radical treatment) or stage IV disease; adenocarcinoma or NSCLC with predominance of adenocarcinoma or large-cell carcinoma component; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1. Patients with no or clinically stable brain metastases following local treatment were permitted. All patients were identified in National Health Fund TP database. Patients provided written informed consent before the treatment start. Data extracted for this analysis included patients’ demographics (age, sex, PS, date of diagnosis, toxicity, overall tumour response, date of progression and death).
The main endpoints were PFS and overall survival (OS). The PFS was defined as the time between the date of EGFR TKI initiation and progression or death, and OS was calculated from the date of treatment initiation to the date of death or last known follow-up. According to the TP protocol tumour response was evaluated every 2 months and objective response rate (ORR) was calculated according to Response Evaluation Criteria In Solid Tumours (RECIST) V.1.1. Time to the start treatment (TST) was defined as the time from date of pathological diagnosis of NSCLC to the date when first-line treatment was started. An ORR was classified based on RECIST V.1.1 criteria. Toxicity data were not systematically collected in the system of TP monitoring (entered voluntarily by treating physicians). We received all data in de-personalised form with permission of respective institutions.
Cox’s proportional hazard regression model was used to analyse the effects of investigated clinical factors (eg, age, ECOG PS, type of treatment, adverse events (AEs)) and to calculate HRs and 95% CIs for OS and PFS. The Kaplan-Meier method was used to evaluate OS and PFS. All reported p values were two sided. The proportions of patients achieving objective responses and with AEs were compared using Pearson’s χ2 test test. Calculations were performed using the Statistica V.12 software (Statsoft).
Results
Patients characteristic
A total of 620 treatment-naive patients with stage III/IV NSCLC harbouring activating EGFR mutations were analysed. Patients characteristics are summarised in table 1. Patients in the afatinib group (N=112, 18.1%) were significantly younger (median 62 years; p=0.0014) than those in the erlotinib (N=253, 40.8%) or gefitinib (N=255, 41.1%) groups (67 and 68 years, respectively), other characteristics were similarly distributed (table 1).
Table 1.
Patients characteristics
| Variable | n (%) | Afatinib | Erlotinib | Gefitinib | P value |
| 620 (100) | 112 (18.1) | 253 (40.8) | 255 (41.1) | ||
| Age | 0.0014 | ||||
| Median, years (range) | 66 (29–91) | 62 (29–86) | 67 (32–91) | 68 (31–88) | |
| <65 years | 285 (46.0) | 68 (60.7) | 114 (45.1) | 103 (40.4) | |
| ≥65 years | 335 (54.0) | 44 (39.3) | 139 (54.9) | 152 (59.6) | |
| Sex | 0.19 | ||||
| Female | 409 (66.0) | 69 (61.6) | 177 (70.0) | 163 (63.9) | |
| Male | 211 (34.0) | 43 (38.4) | 76 (30.0) | 92 (36.1) | |
| ECOG performance status | 0.85 | ||||
| 0 | 155 (25.0) | 28 (25.0) | 66 (26.1) | 61 (23.9) | |
| 1 | 465 (75.0) | 84 (75.0) | 187 (73.9) | 194 (76.1) | |
| Time from diagnosis to start treatment | 0.35 | ||||
| Median, mo (range) | 1.1 (0.9–1.4) | 1.2 (1.1–1.5) | 1.6 (1.4–1.7) | ||
| <3 months | 472 (76.1) | 92 (82.1) | 195 (77.1) | 185 (72.5) | |
| ≥3 months | 131 (21.1) | 18 (16.1) | 52 (20.6) | 61 (23.9) | |
| No data | 17 (2.7) | 2 (1.8) | 6 (2.4) | 9 (3.5) |
ECOG, Eastern Cooperative Oncology Group
Median TST was consistent across all groups. However, 131 (21%) patients started treatment more than 3 months after NSCLC diagnosis. TST varied across the year of NSCLC diagnosis from 2.3 months in 2012 to 1.1 months in 2016 (table 2).
Table 2.
Number of treated patients in the consecutive years and median time from diagnosis to the start of first-line treatment
| Year | TST median (months) | ||||
| Erlotinib | Gefitinib | Afatinib* | Total | ||
| 2012 | 2.3 | 3 | 43 | NA | 46 |
| 2013 | 1.5 | 29 | 49 | NA | 78 |
| 2014 | 1.3 | 66 | 53 | NA | 119 |
| 2015 | 1.2 | 86 | 48 | 31 | 165 |
| 2016 | 1.1 | 69 | 62 | 81 | 212 |
| Total | 253 | 255 | 112 | 620 | |
*Afatinib was not routinely available in 2012–2015.
NA, not available; TST, Time to the start treatment.
At the time of analysis, 412 of 620 (66.4%) patients completed first-line treatment. Main reason for discontinuation was disease progression in 228 patients (36.8%), clinical deterioration or death in 106 patients (17.1%), AEs in 10 patients (1.6%) and consent withdrawal in 15 patients (2.4%). Reason for treatment discontinuation was unknown in 53 patients (8.5%).
Progression-free survival
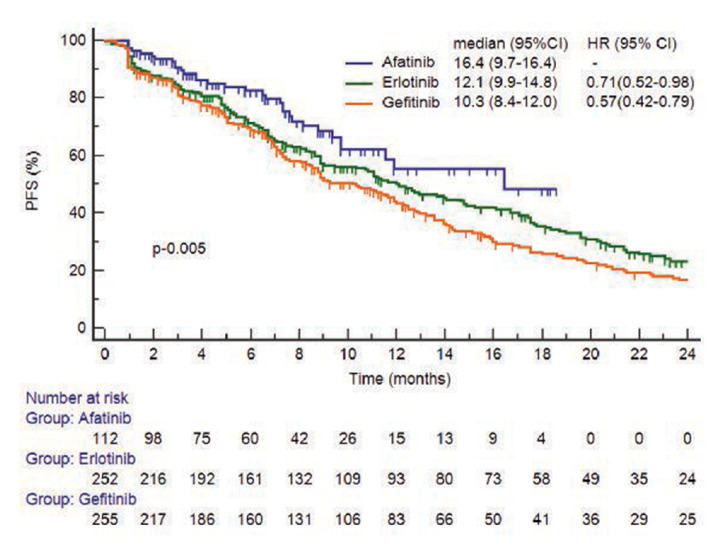
Median follow-up time for all patients was 24.5 months (95% CI 22.9 to 26.0) with cut-off date of 14 March 2017. The median PFS was 11.9 months with 35% of patients being censored. PFS was significantly longer with afatinib than with erlotinib (adjusted HR 0.71; 95% CI 0.52 to 0.98) or gefitinib (adjusted HR 0.57; 95% CI 0.42 to 0.79; p=0.005). Median PFS was 16.4 months (95% CI 9.7 to 16.4) with afatinib vs 10.3 months (95% CI 8.4 to 12.0) with gefitinib and 12.1 months (95% CI 9.9 to 14.8) with erlotinib (figure 1). The HR for PFS in gefitinib vs erlotinib group was not significantly different (HR 1.24; 95% CI 0.99 to 1.53).
Figure 1.
Kaplan-Meier curve of progression-free survival (PFS) in 619 evaluable patients.
A subsequent treatment after progression was analysed only in patients for whom osimertinib was potentially available in second line. This group included patients with disease progression who started first line treatment in 2016. In this group only 55% received further therapy of that 41% was osimertinib and 14% chemotherapy.
Overall survival
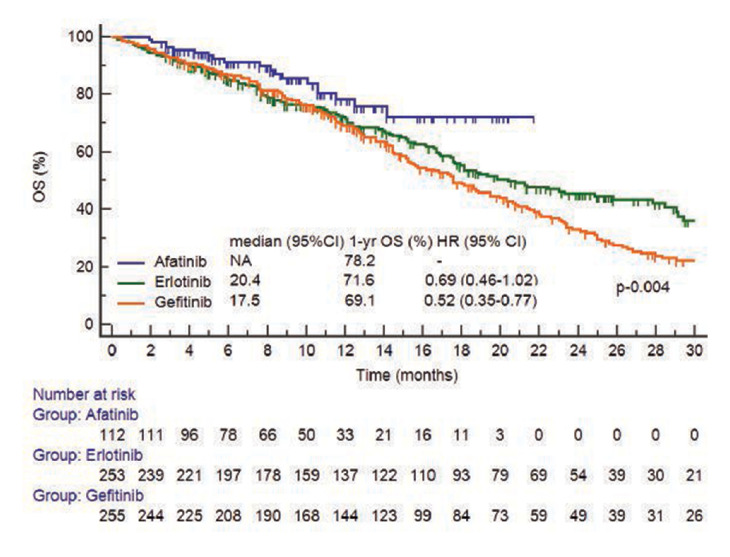
At the time of analysis 300 of 620 (48.4%) patients had died. Median OS was 19.4 months (95% CI 17.5 to 21.7) in all patients. One-year OS rate was significantly higher for afatinib (78.2%) than for gefitinib (69.1%) and erlotinib group (71.6%) (figure 2) with median OS for afatinib not reached; median OS for erlotinib—20.4 months (95% CI 17.5 to 27.8) and for gefitinib—17.5 months (95% CI 15.2 to 20.3).
Figure 2.
Kaplan-Meier curve of overall survival (OS) in all (n=620) patients. NA, not available.
Toxicity
Grade 1 or 2 AEs were reported in 347 of 620 (56.0%) patients and of grade 3 or 4 in 28 (4.5%) patients, while toxicity was unknown in 98 of 620 (15.8%) patients. In subgroup of patients with known toxicity data, AE of any grade were more frequent in the afatinib group (84.4%) than in patients given first-generation TKIs (69.0%) (p<0.01). The frequency of grades 3–4 AEs was comparable in both groups (7.3% in afatinib group and 4.9% in erlotinib/gefitinib group) (table 3). Specific definition of AE was not reported in the database.
Table 3.
Analysis of treatment related adverse events in 522 patients with toxicity data available
| Afatinib | Erlotinib/gefitinib | P value | |
| Adverse event, n (%) 522 (100) |
96 (100) | 426 (100) | |
| None | 15 (15.6) | 132 (31.0) | <0.01 |
| Any grade | 81 (84.4) | 294 (69.0) | |
| Grade | |||
| 1–2 | 74 (77.1) | 273 (64.1) | |
| 3–4 | 7 (7.3) | 21 (4.9) | NS |
| Permanent discontinuation | 1 | 6 | NS |
NS, not significant
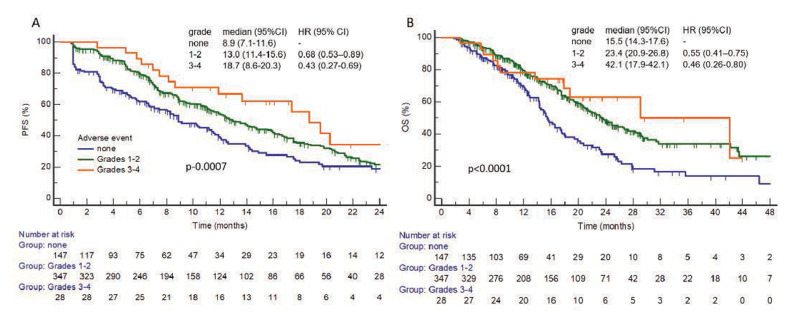
Toxicity of any grade was predictive for longer PFS and OS. In patients with AE of any grade PFS was 13.6 months (95% CI 11.9 to 16.0) vs 8.9 months (95% CI 7.1 to 11.6) in patients without any toxicity (adjusted HR 0.66; 95% CI 0.51 to 0.86; p=0.0006) and OS was 23.6 months (95% CI 21.2 to 28.3) vs 15.5 months (95% CI 14.3 to 17.6) (figure 3). For patients with grades 3–4 AEs median PFS and OS were, respectively, 18.7 months (95% CI 8.6 to 20.3) and 42.1 months (95% CI 17.9 to 42.1) vs 13.0 months (95% CI 11.4 to 15.6) and 23.4 months (95% CI 20.9 to 26.8) in the group with AE of grades 1–2, respectively.
Figure 3.
(A) Kaplan-Meier curve of progression-free survival (PFS) and (B), Kaplan-Meier curve of overall survival (OS) according to toxicity grade in 522 patients with known toxicity data.
Response
Response based on RECIST criteria was evaluable in 524 of 620 patients (84.5%). ORR was similar among the three groups: afatinib (50.0%), erlotinib (52.8%) and gefitinib (56.5%).
Discussion
The results of randomised trials led to registration of erlotinib, gefitinib and afatinib in the first-line treatment in NSCLC patients harbouring EGFR activating mutations. Patients enrolled to RCTs and treated in daily practice differ from each other. The greatest disparities between trials and the clinical practice population are observed in patients with lung cancer and industry-funded trials with targeted therapies.11 Real-world treatment efficacy based on surrogate endpoints (PFS, ORR) in NSCLC is 18% lower than observed in RCTs.12 Therefore, the real-world evidence complement clinical trials by comparing the generalisability of the trial population with the real-world population of interest.13
EGFR mutations are more frequent in Asian population, and there are significantly less data on first line TKI therapy outcomes in the European population.14 15 In EURTAC study, 86 patients received erlotinib in the first line, while in Lux Lung 3 trial only 64 of 230 enrolled to afatinib were non-Asians.4 5 To our knowledge, this retrospective analysis is the largest real-world data analyses of TKI treatment in European population with 620 patients treated in the first-line setting. In the Spanish retrospective study included 187 patients, but no patients were treated with afatinib in the first or second line.16 In our study, we observed longer PFS with the second-generation TKI afatinib—median PFS reached 16.4 months compared with both first-generation TKIs (PFS 11.2 months) even after adjustment for other potentially confounding factors. This findings are similar to those from ARCHER1050 study (comparison dacomitinib and gefitinib) that showed 5.5 months PFS benefit for the second-generation agent dacomitinib.17 In our analysis, the HR for PFS in afatinib group versus first-generation TKIs (HR 0.67; 95% CI 0.49 to 0.92) was similar to that reported in LuxLung 7 trial (HR 0.73; 95% CI 0.58 to 0.92) when afatinib was compared with gefitinib.18Our results show better 1-year OS rate with afatinib than gefitinib but not erlotinib. We did not observe any significant difference between PFS with erlotinib and gefitinib. This is consistent with findings in meta-analysis including 17.621 patients from eight randomised studies and 82 cohort studies (HR 0.99; 95% CI: 0.93 to 1.06).19
The observed OS and PFS benefit in favour of afatinib may be biased due to patient selection, shorter follow-up time in afatinib group, and higher number of censored observations. Afatinib was given less frequently in elderly patients, which may be due to toxicity concerns. This is consistent with the results of other observational studies with potentially more toxic treatment given less frequently to the elderly.20 21 In our study, PFS in all first-line treated patients was 11.6 months which is consistent to that reported in phase III studies in European population. Observed OS of 19.4 months is comparable to that reported in other European retrospective studies and slightly shorter than 20–28 months in randomised phase III studies.5 16 22 However, these results may be biased due to different patients characteristic and unknown proportion of patients harbouring del19 and L858R mutation. Regarding this finding we did not performed comparative analysis between our results and other trials.
Recently observed OS improvements in EGFR mutated NSCLC patients are driven by the wider use of new third-generation TKI osimertinib.23 24 However, the benefits of different sequential EGFR TKI regimens, especially those involving second-generation and third-generation agents, have remained uncertain.25 The impact of subsequent or first-line treatment with osimertinib was not analysed in this study, because osimertinib was not routinely available at the study cut-off date. Osimertinib has become routinely available in Poland only for second line treatment starting from November 2017. Due to low number of patients who may have received osimertinib in second line, it is unlikely that it affected the OS.
Erlotinib and gefitinib have been reimbursed in the first-line setting in Poland in 2012 and afatinib in 2015. We found that despite unified inclusion and exclusion criteria for TP protocol median TST improved over the time from 2.3 months in 2012 to 1.1 month in 2016. This observation suggests that molecular testing algorithm at national level should have been implemented in routine clinical practice together with drug availability and reimbursement policy.
We noted that frequency of grades 3–4 AEs for afatinib, gefitinib and erlotinib were comparable with absolute difference of about 2%. Discontinuation rate due to toxicity was less than 2% of patients which is lower than reported in prospective studies.4 18 26 27 In patients receiving EGFR TKIs, association between severity of skin toxicity and clinical efficacy was reported in several studies.28 29 Meta-analysis of 17 prospective and 7 retrospective studies found significant and strong prognostic value of skin rash.30 The explanation for this association remains unknown, although variability in pharmacodynamics of EGFR TKIs may lead to higher drug concentrations, and to better target inhibition in the tumour at the expense of skin toxicity. In our study, patients with grade 3 or 4 toxicity had an impressive median OS of 42.1 months that is doubled to group with grade 1 or 2 AEs and almost tripled to those without any toxicity.
Our study has several limitations. First, our treatment effectiveness estimates for particular EGFR TKIs may be confounded by the patient selection bias. Another limitation is that TP database does not contain some important information like exact tumour genotype (mutation subtype), toxicity profile or information about subsequent treatments in all patients. In TP database no information about baseline status of brain metastases were entered. This limitations might have affected the reported outcomes.
Although the study was not designed to have sufficient power for testing interaction, population included in our analysis was homogeneous because all patients treated within TP protocol had to complete unified inclusion and exclusion criteria. The limitations should be balanced with the strength of our study, such as large number patients treated with EGFR TKIs in real-world setting in European population.
Conclusions
In conclusion, this study represents the largest real-world dataset with outcome of advanced EGFR-mutated NSCLC patients given first-line TKI therapy in a European country. Despite the limitations, our results demonstrated favourable survival in patients receiving second-generation TKI, with slightly increased toxicity. We also confirmed in daily practice the role of toxicity as an important marker of clinical benefit.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AP reports personal fees and non-financial support from Roche, personal fees and non-financial support from Bristol Myers Squibb, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Boehringer Ingelheim, personal fees from MSD, personal fees and non-financial support from Astra Zeneca, personal fees from Takeda, outside the submitted work; DK reports and Advisory Board and Consultancy: Boehringer-Ingelheim, Roche, Astra Zeneca, Bristol-Myers Squibb, MSD, Amgen, Pfizer, Takeda, Merck, Novartis. RD reports personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Roche, personal fees from Bristol-Myers Squibb, personal fees from MSD, personal fees from Pfizer, personal fees from Celon Pharma, personal fees from Boehringer-Ingelheim, personal fees from FoundationMedicine, personal fees from Takeda, outside the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Data in depersonalised form obtained from therapeutic programme monitoring system.
References
- 1.Krawczyk P, Ramlau R, Chorostowska-Wynimko J, et al. . The efficacy of EGFR gene mutation testing in various samples from non-small cell lung cancer patients: a multicenter retrospective study. J Cancer Res Clin Oncol 2015;141:61–8. 10.1007/s00432-014-1789-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC-H, Wu Y-L, Schuler M, et al. . Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu Y-L, Chen G, et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 4.Sequist LV, Yang JC-H, Yamamoto N, et al. . Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, et al. . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu Y-L, Thongprasert S, et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 7.Haaland B, Tan PS, de Castro G, et al. . Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol 2014;9:805–11. 10.1097/JTO.0000000000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haspinger ER, Agustoni F, Torri V, et al. . Is there evidence for different effects among EGFR-TKIs? Systematic review and meta-analysis of EGFR tyrosine kinase inhibitors (TKIs) versus chemotherapy as first-line treatment for patients harboring EGFR mutations. Crit Rev Oncol Hematol 2015;94:213–27. 10.1016/j.critrevonc.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Popat S, Mok T, Yang JC-H, et al. . Afatinib in the treatment of EGFR mutation-positive NSCLC--a network meta-analysis. Lung Cancer 2014;85:230–8. 10.1016/j.lungcan.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Ninomiya T, Takigawa N, Ichihara E, et al. . Afatinib prolongs survival compared with gefitinib in an epidermal growth factor receptor-driven lung cancer model. Mol Cancer Ther 2013;12:589–97. 10.1158/1535-7163.MCT-12-0885 [DOI] [PubMed] [Google Scholar]
- 11.Ludmir EB, Mainwaring W, Lin TA, et al. . Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol 2019. 10.1001/jamaoncol.2019.2055. [Epub ahead of print: 03 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakdawalla DN, Shafrin J, Hou N, et al. . Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health 2017;20:866–75. 10.1016/j.jval.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Bartlett VL, Dhruva SS, Shah ND, et al. . Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open 2019;2:e1912869. 10.1001/jamanetworkopen.2019.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y-L, Yuan J-Q, Wang K-F, et al. . The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985–93. 10.18632/oncotarget.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr KM, Dafni U, Schulze K, et al. . Prevalence and clinical association of gene mutations through multiplex mutation testing in patients with NSCLC: results from the ETOP Lungscape project. Ann Oncol 2018;29:200–8. 10.1093/annonc/mdx629 [DOI] [PubMed] [Google Scholar]
- 16.Arriola E, García Gómez R, Diz P, et al. . Clinical management and outcome of patients with advanced NSCLC carrying EGFR mutations in Spain. BMC Cancer 2018;18:106. 10.1186/s12885-018-4004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y-L, Cheng Y, Zhou X, et al. . Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (Archer 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454–66. 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]
- 18.Park K, Tan E-H, O'Byrne K, et al. . Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Hackshaw A, Feng Q, et al. . Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer 2017;140:2805–19. 10.1002/ijc.30691 [DOI] [PubMed] [Google Scholar]
- 20.Lau SC, Chooback N, Ho C, et al. . Outcome differences between first- and second-generation EGFR inhibitors in advanced EGFR mutated NSCLC in a large population-based cohort. Clin Lung Cancer 2019;20:e576–83. 10.1016/j.cllc.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Arnold BN, Thomas DC, Rosen JE, et al. . Lung cancer in the very young: treatment and survival in the National cancer data base. J Thorac Oncol 2016;11:1121–31. 10.1016/j.jtho.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 22.Sluga R, VAN DEN Borne BEEM, Roepman P, et al. . Utilization of molecular testing and survival outcomes of treatment with first- or second-line tyrosine kinase inhibitors in advanced non-small cell lung cancer in a Dutch population. Anticancer Res 2018;38:393–400. 10.21873/anticanres.12235 [DOI] [PubMed] [Google Scholar]
- 23.Ramalingam SS, Vansteenkiste J, Planchard D, et al. . Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41–50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 24.Soria J-C, Ohe Y, Vansteenkiste J, et al. . Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 25.Shah R, Lester JF. Tyrosine kinase inhibitors for the treatment of EGFR Mutation-Positive non-small-cell lung cancer: a clash of the generations. Clin Lung Cancer 2020;21:e216–28. 10.1016/j.cllc.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Urata Y, Katakami N, Morita S, et al. . Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol 2016;34:3248–57. 10.1200/JCO.2015.63.4154 [DOI] [PubMed] [Google Scholar]
- 27.Wu Y-L, Zhou C, Hu C-P, et al. . Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 28.Liu H-bing, Wu Y, Lv T-feng, et al. . Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2013;8:e55128. 10.1371/journal.pone.0055128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudek AZ, Kmak KL-, Koopmeiners J, et al. . Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic non-small cell lung cancer. Lung Cancer 2006;51:89–96. 10.1016/j.lungcan.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Petrelli F, Borgonovo K, Cabiddu M, et al. . Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer 2012;78:8–15. 10.1016/j.lungcan.2012.06.009 [DOI] [PubMed] [Google Scholar]