Abstract
Continuous positive airway pressure (CPAP) remains the major treatment option for obstructive sleep apnea (OSA). The American Thoracic Society organized a workshop to discuss the importance of mask selection for OSA treatment with CPAP. In this workshop report, we summarize available evidence about the breathing route during nasal and oronasal CPAP and the importance of nasal symptoms for CPAP outcomes. We explore the mechanisms of air leaks during CPAP treatment and possible alternatives for leak control. The impact of nasal and oronasal CPAP on adherence, residual apnea–hypopnea index, unintentional leaks, and pressure requirements are also compared. Finally, recommendations for patient and partner involvement in mask selection are presented, and future directions to promote personalized mask selection are discussed.
Keywords: obstructive sleep apnea, continuous positive airway pressure, masks
Contents
Overview
Introduction
Methods
Results
Breathing Route and Upper-Airway Patency
Management of Nasal Symptoms and Impact on Mask Selection
Current Evidence Comparing Nasal and Oronasal Masks for OSA Treatment
Mechanisms of Oral Leak during Nasal CPAP and Management
Monitoring Adherence and Adverse Effects
Patient Participation in Mask Selection
Mask-Fitting Considerations
Future Perspectives
Overview
Continuous positive airway pressure (CPAP) remains the most prescribed treatment for obstructive sleep apnea (OSA). CPAP masks play an important role in the outcomes of CPAP treatment for OSA. The American Thoracic Society organized a workshop to discuss the importance of mask selection for OSA treatment with CPAP. The major conclusion was that nasal CPAP should be the initial option for most patients. Additional conclusions of the workshop were the following:
-
•
Breathing route and airway patency: although mouth breathing is common among OSA patients, a nasal mask is usually the best option for most patients. Even though many patients do well with oronasal CPAP, it may compromise airway patency. Patients using oronasal CPAP may experience high leak, experience residual respiratory events, and require high levels of CPAP. Switching to a nasal mask should be considered.
-
•
Management of nasal symptoms and impact on mask selection: nasal symptoms are common among patients with OSA and may compromise CPAP adherence. Humidification, nasal steroids, and nasal surgery are considered the main pillars of the management of nasal symptoms. Controlling nasal symptoms should be implemented both before and during CPAP use and may improve nasal CPAP adherence.
-
•
Current evidence comparing nasal and oronasal masks for OSA treatment: most studies suggest that nasal CPAP results in better adherence, lower residual apnea–hypopnea index (AHI), and higher therapeutic levels as compared with oronasal CPAP. However, oronasal masks can be effective for many patients with OSA.
-
•
Mechanisms of oral leak during nasal CPAP and management: oral leak may provoke patient discomfort, decrease CPAP efficacy, and impair adherence. Excessive leaks may occur through the mask because of an inappropriate mask seal or through the mouth. Two different mechanisms can be observed during nasal CPAP-associated mouth leak: leak due to mouth opening and expiratory leak due to palatal prolapse and expiratory flow limitation. Risk factors for oral leaks include nasal obstruction, aging, obesity, and male sex. Potential interventions include review of the mask seal, treatment of nasal obstruction, chinstraps, and heated humidification. Switching from nasal to oronasal CPAP needs to be closely monitored because of the risk of upper-airway narrowing and requirement of higher CPAP levels.
-
•
Monitoring adherence and adverse effects: monitoring for residual AHI, leak, and adherence should be combined with patient complaints and obtained periodically. Active troubleshooting is important, especially during the first week of therapy.
-
•
Patient participation in mask selection: patients and partners should be involved in the selection of CPAP masks and should receive adequate support under a proactive self-management model.
-
•
Mask-fitting considerations: time and effort should be spent during initial mask fitting, while avoiding too many mask options that can be confusing for the patient.
Introduction
The health benefits of CPAP for OSA depends on its effectiveness and patient compliance. Although nasal CPAP is still the most frequently used CPAP interface, there has been an increasing trend in oronasal CPAP prescription. However, there is emerging literature demonstrating that clinical outcomes of using nasal and oronasal CPAP for OSA treatment may differ (1).
The primary objectives of this workshop were to discuss the importance of mask selection for OSA treatment with CPAP. Adherence and effectiveness of CPAP according to interface type were reviewed. Strategies to develop personalized selection of interfaces that include facial anatomy, nasal symptoms, and patient preferences were discussed. The long-term goal of this initiative is to promote individualized mask selection for treatment of OSA with CPAP that will likely improve patient outcomes, including higher effectiveness, higher adherence, and more comfort with therapy.
Methods
Clinicians and researchers were invited for this workshop on the basis of their recognized expertise and contributions in mask selection and CPAP adaptation for patients with OSA. The most relevant topics pertaining to the different mask choices for OSA treatment were selected by the chair and distributed to the participants. The literature was reviewed and presented by the participants according to their field of expertise. Discussion followed each presentation to reach expert agreement on the current knowledge and future directions.
Results
Breathing Route and Upper-Airway Patency
Mouth breathing may compromise airway patency by narrowing the upper airway because of posterior mandibular displacement (2) and by increasing upper-airway surface tension (3). Patients with OSA spend as much as 59% of the total sleep time breathing through the oronasal route (4). Oronasal breathing during sleep is associated with aging, nasal obstruction, OSA severity, neck circumference, and obesity (4–7). However, the exact mechanisms leading to mouth breathing during sleep among subjects with OSA are not completely understood. Jaw opening may occur during inspiration because of the activation of submental muscles and tracheal tug (8). Masseter inspiratory activity is believed to counterbalance jaw-opening forces. During obstructive apneas, dynamic jaw movement progressively increases toward the end of the apnea as respiratory effort and tracheal tug also increases (8), possibly promoting mouth breathing.
Mouth opening and oral air leak are potential adverse effects of CPAP, especially in patients with nasal obstruction (9). In a study that objectively detected nasal and oral breathing, patients with oral breathing were less adherent to nasal CPAP (10). To avoid CPAP intolerance, patients reporting oronasal breathing often receive an oronasal CPAP mask even before any symptom arises. However, the self-reported breathing route does not predict the objectively measured breathing track (4). In addition, the majority of patients with OSA with oronasal breathing switch to nasal breathing while on nasal CPAP (10).
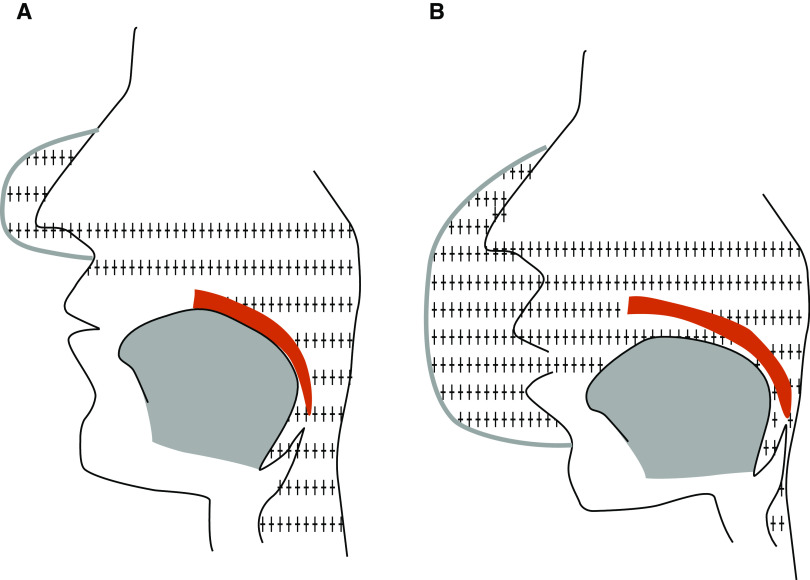
Initial evidence that oronasal CPAP might not be as effective as nasal CPAP came from a study that measured pharyngeal collapsibility during nasal and oronasal CPAP (11). Increasing levels of CPAP were not able to open the airway in any patients during oronasal CPAP. The mechanisms through which oronasal CPAP may impair upper-airway patency are incompletely understood. Oronasal CPAP may reduce the airway splinting effect because of potential neutralization of the intraluminal positive pressure applied through the nose by the positive pressure coming through the mouth (Figure 1). A study that compared upper-airway patency on nasal and oronasal CPAP showed that the airway narrows when treatment is switched from nasal to oronasal CPAP, even while oral airflow was zero. Interestingly, when the mouth was taped shut, airway narrowing no longer occurred when treatment was switched from nasal to oronasal CPAP (12). Oronasal CPAP may also posteriorly displace the mandible (13). Taken together, the effects of oronasal CPAP on the upper airway may differ between individuals and may depend on the site of obstruction or OSA endotype.
Figure 1.
Potential effect of oronasal continuous positive airway pressure (CPAP) on upper-airway patency. (A) Nasal CPAP splints the upper airway and pushes the soft palate against the tongue. (B) Oronasal CPAP may neutralize the splinting effect of nasal CPAP because of the transmission of positive pressure to the mouth.
Management of Nasal Symptoms and Impact on Mask Selection
Nasal symptoms are commonly reported by CPAP users and are common reasons for CPAP intolerance. Nasal congestion and rhinorrhea are reported by up to 45–69% of CPAP users (14, 15). Nasal congestion, mouth leak, and removing the CPAP mask at night are significantly associated with a decreased CPAP adherence (16).
An increased nasal resistance measured by anterior rhinomanometry before CPAP initiation significantly impairs patients’ initial acceptance of CPAP (17). This finding was also supported by the results of a study by Morris and colleagues, who could demonstrate that the cross-sectional area at the level of the inferior turbinate differed significantly between responders and nonresponders to CPAP (18). On the basis of acoustic reflection measurements, it has been shown that CPAP use was significantly lower in patients with a small minimal cross-sectional area (19).
Humidification, nasal steroids, and nasal surgery are considered the main pillars of the management of nasal symptoms during CPAP
On the basis of a blinded randomized controlled trial comparing CPAP treatment with CPAP plus humidification and CPAP with nasal steroid therapy with fluticasone, it has been demonstrated that heated humidification, and not nasal steroid therapy, was capable of decreasing the incidence of nasal side effects in patients with OSA initiating CPAP (20). It has been suggested that only patients with allergic rhinitis might benefit from intranasal fluticasone during CPAP initiation (21).
A cost-effectiveness analysis of nasal surgery to increase CPAP adherence in patients with OSA and nasal obstruction pointed out that nasal surgery is cost-effective in almost every patient with OSA and nasal obstruction, that turbinate reduction can be cost-positive in the short term, and that septoplasty can have a higher cost/benefit outcome after a longer time span (22).
Nasal symptoms should be adequately treated before CPAP initiation. The use of nasal corticosteroid therapy is probably only useful in selected patients, whereas nasal surgery leads to increased CPAP use, increased CPAP tolerance, and a reduced CPAP level and, therefore, should be regarded as being cost-effective as adjuvant therapy.
Current Evidence Comparing Nasal and Oronasal Masks for OSA Treatment
The initial description of CPAP (23) involved nasal positive pressure, believed to push the soft palate and tongue anteriorly, preventing oropharyngeal lumen obliteration. It was believed that pressure applied at both the nose and mouth could not effectively restore airway patency (11, 24). Subsequent experience showed that oronasal interfaces could be used successfully (25, 26). Advantages and disadvantages of nasal and oronasal masks are described in Table 1.
Table 1.
Advantages and disadvantages of nasal and oronasal CPAP masks
| Mask | Advantages | Disadvantages |
|---|---|---|
| Nasal | More comfortable | Risk of mouth leak |
| Lower overall leak | ||
| Lower therapeutic pressure requirement | ||
| Higher adherence | ||
| Lower cost | ||
| Less risk of aspiration | ||
| Lower risk of CO2 rebreathing | ||
| Lower risk of aerophagia | ||
| Oronasal | Better control of mouth leak | Less comfortable |
| Better control of REM-associated leak | Higher overall leak | |
| Higher therapeutic pressure requirement | ||
| Lower adherence | ||
| Higher cost | ||
| Higher risk of aspiration | ||
| Higher risk of CO2 rebreathing | ||
| Higher risk of aerophagia |
Definition of abbreviations: CPAP = continuous positive airway pressure; REM = rapid eye movement.
Although both types of interfaces are used clinically, the debate over the effectiveness of oronasal masks continues. Recent meta-analyses have found lower pressure (1) and residual AHI (1, 27) with nasal CPAP but have found no difference in sleepiness (27) and have found higher adherence with nasal masks in nonrandomized studies, but not in randomized trials (1, 27). Overall, in unselected patients with OSA, any effective pressure differences are probably not clinically relevant (28, 29). However, in specific individuals, differences can be marked (13), particularly in more severe OSA (30). When pressure or adherence were similar, satisfaction was higher with nasal interfaces. In studies in which patients underwent nasal CPAP titration and were then randomized to several weeks each of nasal versus oronasal CPAP, nasal interfaces resulted in better adherence and reduced sleepiness (31) or in a lower residual AHI (32). A potential additional advantage of nasal masks is the reduced risk for aspiration of gastric content and lower risk of CO2 rebreathing with failure of the CPAP flow generator.
A large prospective study based on a national registry found lower adherence and higher pressure levels with oronasal interfaces (33). Several side effects were more prevalent with oronasal interfaces, but as oronasal interfaces are usually the second-line therapy, these patients may inherently have been more difficult to treat.
Clear predictors of different effectiveness of nasal versus oronasal masks have not been identified. Several studies have found air leak to be lower with nasal interfaces (28, 29, 34), yet this was not the cause for ineffective oronasal CPAP in a case series (13). Body habitus, airway shape, and cephalometric measurements were not predictive (13, 29). Higher patient age (32) and OSA severity (30) may be associated with oronasal mask ineffectiveness. Position may be relevant in some cases: oronasal CPAP appeared ineffective exclusively in the supine position in a case report (35).
In summary, although oronasal masks can be effective for OSA correction, nasal masks may be more advantageous and should be the first-line therapy in most cases. If titration is performed with a nasal mask, pressure may need to be adjusted if converting to an oronasal mask. There are currently no validated methods for predicting which interface type would be most beneficial for a given patient. Although this discussion focused on nasal versus oronasal interfaces, it should be remembered that intranasal and oral interfaces are also available.
Mechanisms of Oral Leak during Nasal CPAP and Management
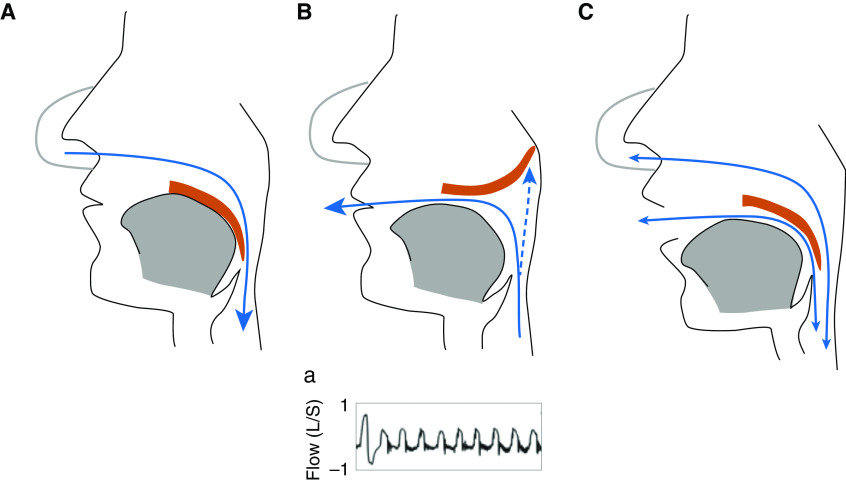
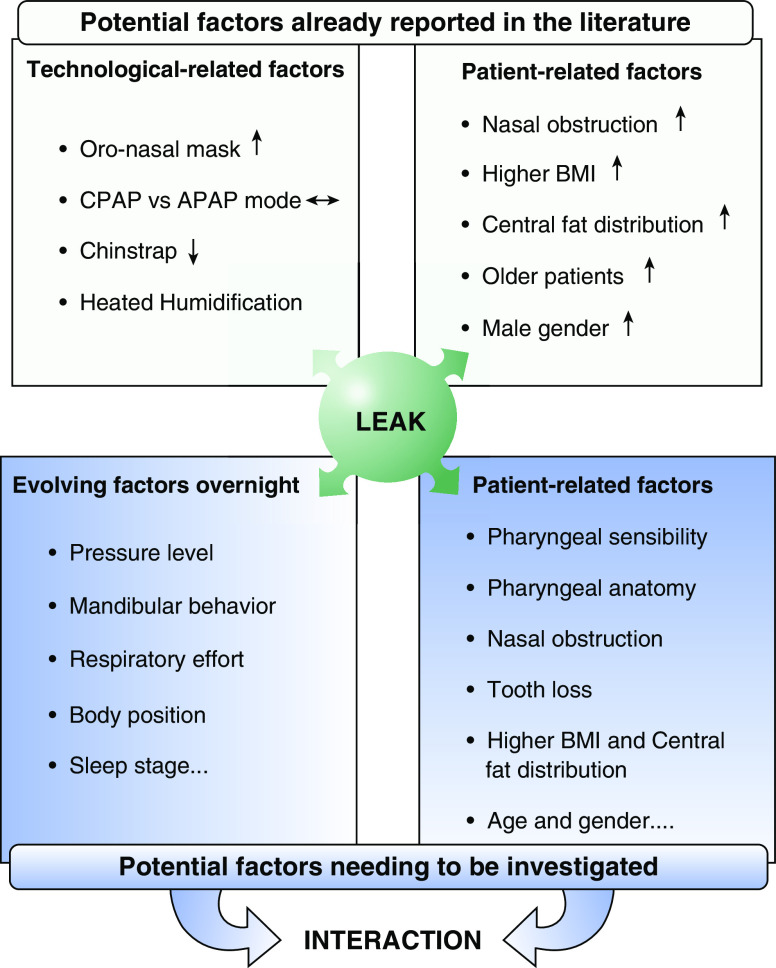
Concerns and priorities related to oral leaks during CPAP treatment may vary on the basis of patients, physician, or caregiver perspectives. If patients’ concerns are more focused on noise, oropharyngeal dryness, or perturbation of sleep, physicians are more concerned about failure of upper-airway patency control or CPAP-device misfunction due to leaks. However, at the end, both will lead to a decrease in CPAP compliance because of the discomfort of treatment or inefficacy. Two types of oral leaks can be identified. The most common is related to jaw opening and loss of soft palate–tongue seal (Figure 2C). Oral leak may also occur because of the prolapse of the soft palate during expiration, causing obstruction of the nasopharynx and air leak through the mouth (36) (Figure 2B). This particular type of mouth leak can be detected by the analysis of airflow shape (Figure 2Ba), which can be retrieved from CPAP data cards. Mouth leak may increase unidirectional nasal flow and induce an increase in nasal resistance because of nasal congestion (37). Mechanisms of oral leaks depend on head position and stability of the jaw, which is highly related to teeth congruence. Physicians and caregivers should be aware that leaks may change over time because of modifications of pressure levels or jaw stability (e.g., teeth extraction, change in sleeping position). The way to investigate the origin and how to react regarding oral or unintentional leaks is well summarized in Figure 3, adapted from Lebret and colleagues (38). Most of the time, a simple oronasal clinical examination and a careful reading of the pressure-support-device recording will give insight into the main cause of leaks and will therefore provide the solution. In some cases, this may require recording the patient during sleep with the appropriate sensors, including an oral thermistor, mandibular-position sensor, and video recording (38). Many CPAP devices report air leak graphically, which may help to distinguish between mouth and mask leak. It has been suggested that a sawtooth pattern is indicative of mouth leak (39). In addition, several devices allow the download of breath-by-breath airflow tracings from the previous nights. Expiratory leak associated with palatal prolapse during nasal CPAP (Figure 2B) can be detected through the analysis of airflow and is characterized by the abrupt reduction of expiratory flow (36, 39). A greater understanding of the mechanisms of mouth opening is needed before appropriate responses can be mounted. Although the use of an oronasal mask is often tried to control oral leaks, several pitfalls need to be known. Indeed, these types of mask are improving comfort and efficiency of treatment in mouth-breathing patients (40). However, to maintain treatment quality when switching from a nasal to an oronasal mask, we need to be aware that the autoadjusting positive airway pressure (APAP) mean pressure level may increase to maintain low AHI, and, in some cases, residual AHI may not be controlled. This is of importance because a higher CPAP pressure may reduce adherence to treatment (33). Interestingly, the level of unintentional leaks is not decreased with oronasal mask use, as demonstrated by five randomized controlled trials (see Lebret and colleagues [38] for review), although oronasal masks reduced the risk of unintentional leaks in cases of mouth opening and rapid-eye-movement sleep. This highlights the fact that the origin of leaks should be determined before switching masks, and only mouth-breathing patients should use oronasal masks.
Figure 2.
Different mechanisms of oral leak during nasal continuous positive airway pressure (CPAP). (A) Normal nasal inspiration: observe that the tongue and soft palate are coupled. (B) Expiratory mouth leak: observe that the soft palate prolapses and blocks the nasopharynx, and air leaks through the mouth while it is closed. (a) Airflow characteristics of expiratory mouth leak: a normal breath with symmetric inspiratory (positive) and expiratory (negative) shape is followed by a series of breaths with abrupt termination of expiratory flow. Adapted by permission from Reference 36. (C) Continuous mouth leak during inspiration and expiration: observe that the mouth is open and that the soft palate–tongue coupling is lost.
Figure 3.
Mechanisms involved in CPAP unintentional leak. Adapted by permission from Reference 38. APAP = autoadjusting positive airway pressure; BMI = body mass index; CPAP = continuous positive airway pressure.
Monitoring Adherence and Adverse Effects
Adherence to CPAP affects OSA treatment efficacy and has become a critical problem when treatment alternatives are limited or ineffective. Adverse effects of CPAP treatment are associated with poorer adherence (10, 33). The best predictors of CPAP adherence include early usage (41, 42), nasal passage size (19), inferior turbinate cross-sectional area (18), nasal resistance (17), excessive air leak (10), and intolerance related to dry mouth, nasal congestion, choking sensations, and perceived inconvenience (33). OSA severity is a weak predictor of adherence (33). Technological advancements, including heated humidification, APAP, bilevel, and expiratory pressure relief were evaluated by a Cochrane Systematic Review, and only APAP was shown to significantly improve adherence (43).
Overall, early usage has been the best predictor of long-term CPAP adherence (41, 44). Remote monitoring of the CPAP device allows for early identification of low adherence and excessive air leak. However, the results of remote monitoring on adherence have been mixed so far (38, 45). Approaches to improve compliance include psychological and educational interventions. A metanalytic study (43) revealed that cognitive behavioral interventions led to the greatest improvement in adherence, whereas short-term education did not lead to significant change. Ongoing support showed a nonsignificant trend toward improved adherence. The resolution of adverse effects should also be addressed early. Adaptive servo ventilation may improve positive airway pressure (PAP) adherence among patients with persistent or treatment-emergent central sleep apnea (46). Aerophagia is more common with oronasal CPAP than with nasal CPAP (47). Among patients on an oronasal mask who report aerophagia, switching to a nasal mask should be considered.
Air leaks are common adverse effects of CPAP and are associated with poorer adherence (10). Unfortunately, each CPAP manufacturer reports air leak differently, and there is no consensus to discriminate between tolerable air leak and leak that deserves correction (48). Patient complaints and the magnitude of the reported leak should be interpreted together before implementing any correction. If oral leak is present despite addressing nasal symptoms and attempting pressure reduction, the use of a heated humidifier on the circuit may reduce nasal congestion, moisten the soft palate, and improve the soft palate–tongue seal (49). Unfortunately, chinstraps are not comfortable enough to be worn on a long-term basis, and in some patients may increase upper-airway resistance (50). Ultimately, a full-face mask can be considered if oral leak remains a significant complaint.
Patient Participation in Mask Selection
Despite recent CPAP technology advances that incorporate multiple features to improve comfort and pressure delivery, adherence to CPAP remains poor (43). Nonadherent users report negative early experiences, reinforcing a low belief in their ability to use CPAP (51). Although there is no conclusive evidence supporting that the technologic interface or level of pressure predict CPAP adherence, mask selection can have a significant impact on patients’ early experience with CPAP. Patterns of CPAP adherence are established within the first week of therapy (41, 44), and a dramatic decline of use in the first few days predicts poor long-term use (41, 52). CPAP interference with being intimate, together with being Black and having a high residual AHI, were salient predictors of poor first-week CPAP adherence (53). Socioeconomic status may also influence CPAP acceptance and adherence (54, 55). A more recent study examining couples’ experiences with CPAP further supports patient participation in mask section. Concerns with the mask were highlighted in each of the four major barriers to CPAP use: bothersome equipment causing disruptions in sleep and bedtime routine, anxiety related to CPAP use particularly in the beginning of therapy, interruptions to intimacy, and concern about image change while wearing a CPAP mask (56).
Recommendations
First and foremost, OSA and its management, including mask selection, should move from the traditional disease-focused model to a more proactive self-management model (57). Care for OSA has been heavily dependent on clinicians and is primarily delivered at the sleep center. Less motivated patients might just stop using CPAP if they perceive there is a problem that cannot be addressed by their care provider. In contrast, in the proactive self-management model, engaging patients early will build the sense of being an “active care participant” rather than a “passive care recipient.” Second, the significant other or partner of the patient needs to be involved in the mask selection and follow-up care, if possible. Third, the critical time to support, troubleshoot, and motivate CPAP use should be before treatment initiation and early within the first week. Finally, more research is needed to develop and test behavioral interventions to promote long-term self-management. When clinicians work on the mask selection with the patients, is it important to set up reasonable expectations and emphasize that it may take more than one try to find the right mask. Mobile health technologies, including wearables and apps, which hold great promise to deliver health-behavior interventions, improve communication, and improve individual tailoring (58), can be developed for both CPAP users and their partners to promote effective self-management.
Mask-Fitting Considerations
Manufacturers of CPAP devices have been innovative in offering a variety of different types of masks: nasal, nasal-pillow, cradle, nasal–oral, and low-profile nasal–oral masks. They were designed to tackle different complaints and offer comfort, sealing, and stability. Even with recent mask-fitting advances, we are still encountering issues of compliance and complaints. We have found that there are three main obstacles to overcome when introducing masks to patients. Obstacle one is dismantling preconceived notions and expectations of what PAP will and will not accomplish through patient orientation. Although some patients believe that PAP is the answer to all of their problems, others believe it will not help at all. Obstacle two is combating claustrophobia. Patients describe experiencing distinct types of claustrophobia: 1) discomfort from having a physical object on the face and 2) having a confining space to breathe into. Determining which type of claustrophobia the patient is describing will allow sleep clinicians to choose an appropriate mask. Obstacle three is the potential that oral leak may influence the overuse of oronasal masks as the initial mask choice. Durable medical equipment companies may induce the use of oronasal masks because of additional potential reasons: higher profit margins of oronasal masks as compared with nasal masks, limited mask options and the possibility of mask switching, and reduced time for patient adaptation and education.
There are a few things to consider when fitting a mask. As opposed to technicians asking the patient, “Do you breathe through your mouth at night?,” technicians should focus on whether this can be observed. This is important because many patients experience oral breathing throughout the night, as they are trying to catch their breath after apneic events. Also allow time to let the patients buy into therapy, increasing their confidence and gaining commitment. Lastly, give a reasonable number of mask options to avoid choice fatigue (59). All patients should have a trial with pressurized air for proper mask fit. Verification of leak is important; no matter how small the leak is perceived to be, the location of the leak may cause irritation. There are troubleshooting materials, such as nasal saline sprays, petroleum-free skin moisturizers, mask wipes, dry-mouth oral rinses, and PAP mask liners, to help mitigate discomfort. Durable medical equipment companies need to be easily reachable, supportive, and flexible, particularly at therapy initiation, as this period plays a crucial role in maximizing long-term adherence. Selecting a comfortable CPAP interface during initial CPAP adaptation may improve CPAP adherence. Patients who are well adapted to the mask initially chosen have better long-term adherence than those who need mask switching (60). In addition, adherence may be compromised when a discontinued mask model needs to be switched to a newer model (60), highlighting the importance of a long life cycle for masks in the market.
Decreasing reimbursements demand more efficient mask fitting. Manufacturers have been offering fit packs (masks with all sizes in one package) for a select group of masks. These fit packs may be helpful to decrease sterilization needs and telehealth fittings, which likely improves efficiency. As telehealth fittings become more common, technology can provide education, engagement, and objective data about which masks work best for patient subsets (i.e., by ethnicity or anthropomorphic characteristics).
Future Perspectives
Current evidence suggests that the nasal interface should be the first option for most patients with OSA. However, many gaps remain to be studied to optimize interface selection for patients with OSA. Many different characteristics that have been poorly addressed, including facial and nasal anatomy, preferential breathing route, site of airway collapse, obesity, race, sex and age, may influence the therapeutic outcomes of the different CPAP interfaces. A personalized-medicine approach must be considered to provide new evidence for the correct choice among the different CPAP interfaces.
It is unclear whether some patients benefit from using an oronasal interface upfront. In a randomized crossover study conducted by Goh and colleagues in Singapore, nasal, nasal-pillow, and oronasal CPAP were compared. Although nasal CPAP led to better adherence, 26% of patients had greater use on oronasal CPAP. Patients showing a better adherence to oronasal CPAP had less nasal obstruction and a proportionally increased mid-face width and chin-lower lip distance (61). Future controlled studies should consider anatomical characteristics in the selection between nasal and oronasal masks and may ultimately lead to the development of newer mask designs that better fit specific facial characteristics. Three-dimensional face scanning and three-dimensional mask printing are an interesting approach to individualize the CPAP interface according to the patient’s own facial anatomy (62).
The best option for patients with OSA and significant nasal obstruction also needs further assessment. The utility of imaging, nasal-resistance measurements, and preferential flow-route determination should be tested in controlled studies to guide the best approach for patients with OSA and nasal obstruction (i.e., nasal surgery or a trial of oronasal CPAP). In addition, the use of nasal steroids in selected patients with significant nasal symptoms should be tested.
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology.
Members of the subcommittee are as follows:
Pedro R. Genta, M.D., A.T.S.F. (Chair)1
Pam DeYoung, B.A., R.P.S.G.T.2
Matthew R. Ebben, Ph.D.3
Bradley A. Edwards, Ph.D.4,5
William Hevener, R.P.S.G.T.6
Marta Kaminska, M.D., M.Sc.7,8
Ana C. Krieger, M.D., M.P.H.2
Geraldo Lorenzi-Filho, M.D.1
Patrick Strollo, M.D.9
Renaud Tamisier, M.D., Ph.D., M.B.A.10,11
Olivier M. Vanderveken, M.D.12,13
Terri E. Weaver, Ph.D., R.N., F.A.A.N., A.T.S.F.14
Lichuan Ye, Ph.D., R.N.15
1Laboratório do Sono, Laboratório de Investigação Médica 63, Divisão de Pneumologia, Instituto do Coração (InCor), Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil; 2Division of Pulmonary, Critical Care and Sleep Medicine, University of California, San Diego, California; 3Center for Sleep Medicine, Weill Medical College, Cornell University, New York, New York; 4Sleep and Circadian Medicine Laboratory, Department of Physiology, and 5School of Psychological Sciences, Turner Institute for Brain and Mental Health, Monash University, Melbourne, Victoria, Australia; 6Clinical Initiatives Division, Sleep Data, BetterNight, San Diego, California; 7Respiratory Division and Sleep Laboratory, and 8Respiratory Epidemiology and Clinical Research Unit, McGill University, Montreal, Quebec, Canada; 9University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, Pennsylvania; 10Laboratoire Hypoxie et Physiopathologies Cardiovasculaires et Respiratoires, Institut National de la Santé et de la Recherche Médicale U1042, and 11Laboratoire de Exploration Fonctionelle Respiratoire, Pôle Thorax et Vaisseaux, Centre Hospitalier Universitaire de Grenoble, Université Grenoble Alpes, Grenoble, France; 12Department of Otorhinolaryngology, Head, and Neck Surgery, Antwerp University Hospital, and 13Faculty of Medicine and Health Sciences, Research Group on Translational Neurosciences, University of Antwerp, Antwerp, Belgium; 14College of Nursing, University of Illinois at Chicago, Chicago, Illinois; and 15School of Nursing, Bouve College of Health Sciences, Northeastern University, Boston, Massachusetts
Footnotes
Supported by the American Thoracic Society.
This official workshop report of the American Thoracic Society was approved September 2020
A complete list of American Thoracic Society Assembly on Sleep and Respiratory Neurobiology subcommittee members may be found before the beginning of the References.
Author Disclosures: M.K. served on an advisory committee and consultant for Biron; received research support from Fisher Paykel, Philips Respironics, ResMed, VitalAire. B.A.E. received research support from Apnimed, Oventus; received personal fees from Signifier Medical Technologies. T.E.W. served as a consultant for Jazz Pharmaceuticals; received royalties from UpToDate; received royalties for the use of the Functional Outcomes of Sleep Questionnaire (FOSQ) from Bayer, Cook Medical, Inspire, Jazz Pharmaceuticals, Merck, NightBalance, Nyxoah, Philips Respironics, ResMed, RWS, Stratevi, Verily Life Sciences. G.L.-F. served on an advisory committee for and has ownership or investment interests in Biologix. P.S. served as a consultant for Biologix, Enhale Medical, Inspire, Philips Respironics, Takeda; received research support from Inspire, Itamar, Jazz Pharmaceuticals, PinMed, ResMed; has an intellectual property/patent unsold for Belluscura. P.R.G., M.R.E., A.C.K., R.T., L.Y., O.M.V., P.D., and W.H. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Sleep and Respiratory Neurobiology
References
- 1.Andrade RGS, Viana FM, Nascimento JA, Drager LF, Moffa A, Brunoni AR, et al. Nasal vs oronasal CPAP for OSA treatment: a meta-analysis. Chest. 2018;153:665–674. doi: 10.1016/j.chest.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Choi JH, Shin C, Lee HM, Kwon SY, Lee SH. How does open-mouth breathing influence upper airway anatomy? Laryngoscope. 2007;117:1102–1106. doi: 10.1097/MLG.0b013e318042aef7. [DOI] [PubMed] [Google Scholar]
- 3.Verma M, Seto-Poon M, Wheatley JR, Amis TC, Kirkness JP. Influence of breathing route on upper airway lining liquid surface tension in humans. J Physiol. 2006;574:859–866. doi: 10.1113/jphysiol.2005.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nascimento JA, Genta PR, Fernandes PHS, Barroso LP, Carvalho TS, Moriya HT, et al. Predictors of oronasal breathing among obstructive sleep apnea patients and controls. J Appl Physiol (1985) 2019;127:1579–1585. doi: 10.1152/japplphysiol.00964.2018. [DOI] [PubMed] [Google Scholar]
- 5.Madronio MR, Di Somma E, Stavrinou R, Kirkness JP, Goldfinch E, Wheatley JR, et al. Older individuals have increased oro-nasal breathing during sleep. Eur Respir J. 2004;24:71–77. doi: 10.1183/09031936.04.00004303. [DOI] [PubMed] [Google Scholar]
- 6.McLean HA, Urton AM, Driver HS, Tan AK, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005;25:521–527. doi: 10.1183/09031936.05.00045004. [DOI] [PubMed] [Google Scholar]
- 7.Koutsourelakis I, Vagiakis E, Roussos C, Zakynthinos S. Obstructive sleep apnoea and oral breathing in patients free of nasal obstruction. Eur Respir J. 2006;28:1222–1228. doi: 10.1183/09031936.00058406. [DOI] [PubMed] [Google Scholar]
- 8.Hollowell DE, Suratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol (1985) 1991;71:2267–2273. doi: 10.1152/jappl.1991.71.6.2267. [DOI] [PubMed] [Google Scholar]
- 9.Lebret M, Arnol N, Contal O, Martinot JB, Tamisier R, Pepin J-L, et al. Nasal obstruction and male gender contribute to the persistence of mouth opening during sleep in CPAP-treated obstructive sleep apnoea. Respirology. 2015;20:1123–1130. doi: 10.1111/resp.12584. [DOI] [PubMed] [Google Scholar]
- 10.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126:1248–1254. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 11.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985) 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 12.Madeiro F, Andrade RGS, Piccin VS, Pinheiro GDL, Moriya HT, Genta PR, et al. Transmission of oral pressure compromises oronasal CPAP efficacy in the treatment of OSA. Chest. 2019;156:1187–1194. doi: 10.1016/j.chest.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Kaminska M, Montpetit A, Mathieu A, Jobin V, Morisson F, Mayer P. Higher effective oronasal versus nasal continuous positive airway pressure in obstructive sleep apnea: effect of mandibular stabilization. Can Respir J. 2014;21:234–238. doi: 10.1155/2014/408073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7:391–398. doi: 10.1097/00063198-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 16.Baltzan MA, Elkholi O, Wolkove N. Evidence of interrelated side effects with reduced compliance in patients treated with nasal continuous positive airway pressure. Sleep Med. 2009;10:198–205. doi: 10.1016/j.sleep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura T, Noda A, Nakata S, Yasuda Y, Soga T, Miyata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74:56–60. doi: 10.1159/000089836. [DOI] [PubMed] [Google Scholar]
- 18.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol. 2006;20:133–137. [PubMed] [Google Scholar]
- 19.Li HY, Engleman H, Hsu CY, Izci B, Vennelle M, Cross M, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28:1554–1559. doi: 10.1093/sleep/28.12.1554. [DOI] [PubMed] [Google Scholar]
- 20.Ryan S, Doherty LS, Nolan GM, McNicholas WT. Effects of heated humidification and topical steroids on compliance, nasal symptoms, and quality of life in patients with obstructive sleep apnea syndrome using nasal continuous positive airway pressure. J Clin Sleep Med. 2009;5:422–427. [PMC free article] [PubMed] [Google Scholar]
- 21.Kirtsreesakul V, Hararuk K, Leelapong J, Ruttanaphol S. Clinical efficacy of nasal steroids on nonallergic rhinitis and the associated inflammatory cell phenotypes. Am J Rhinol Allergy. 2015;29:343–349. doi: 10.2500/ajra.2015.29.4234. [DOI] [PubMed] [Google Scholar]
- 22.Kempfle JS, BuSaba NY, Dobrowski JM, Westover MB, Bianchi MT. A cost-effectiveness analysis of nasal surgery to increase continuous positive airway pressure adherence in sleep apnea patients with nasal obstruction. Laryngoscope. 2017;127:977–983. doi: 10.1002/lary.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 24.Kuna ST, Remmers JE. Neural and anatomic factors related to upper airway occlusion during sleep. Med Clin North Am. 1985;69:1221–1242. doi: 10.1016/s0025-7125(16)30984-1. [DOI] [PubMed] [Google Scholar]
- 25.Prosise GL, Berry RB. Oral-nasal continuous positive airway pressure as a treatment for obstructive sleep apnea. Chest. 1994;106:180–186. doi: 10.1378/chest.106.1.180. [DOI] [PubMed] [Google Scholar]
- 26.Sanders MH, Kern NB, Stiller RA, Strollo PJ, Jr, Martin TJ, Atwood CW., Jr CPAP therapy via oronasal mask for obstructive sleep apnea. Chest. 1994;106:774–779. doi: 10.1378/chest.106.3.774. [DOI] [PubMed] [Google Scholar]
- 27.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15:301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakker JP, Neill AM, Campbell AJ. Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: a pilot investigation of pressure requirement, residual disease, and leak. Sleep Breath. 2012;16:709–716. doi: 10.1007/s11325-011-0564-3. [DOI] [PubMed] [Google Scholar]
- 29.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep (Basel) 2011;34:951–955. doi: 10.5665/SLEEP.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebben MR, Oyegbile T, Pollak CP. The efficacy of three different mask styles on a PAP titration night. Sleep Med. 2012;13:645–649. doi: 10.1016/j.sleep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax. 1998;53:290–292. doi: 10.1136/thx.53.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebben MR, Narizhnaya M, Segal AZ, Barone D, Krieger AC. A randomised controlled trial on the effect of mask choice on residual respiratory events with continuous positive airway pressure treatment. Sleep Med. 2014;15:619–624. doi: 10.1016/j.sleep.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Borel JC, Tamisier R, Dias-Domingos S, Sapene M, Martin F, Stach B, et al. Scientific Council of The Sleep Registry of the French Federation of Pneumology (OSFP) Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One. 2013;8:e64382. doi: 10.1371/journal.pone.0064382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowland S, Aiyappan V, Hennessy C, Catcheside P, Chai-Coezter CL, McEvoy RD, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med. 2018;14:101–108. doi: 10.5664/jcsm.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nascimento JA, de Santana Carvalho T, Moriya HT, Fernandes PH, de Andrade RG, Genta PR, et al. Body position may influence oronasal CPAP effectiveness to treat OSA. J Clin Sleep Med. 2016;12:447–448. doi: 10.5664/jcsm.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azarbarzin A, Sands SA, Marques M, Genta PR, Taranto-Montemurro L, Messineo L, et al. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J. 2018;51:1701419. doi: 10.1183/13993003.01419-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE. Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med. 1996;154:182–186. doi: 10.1164/ajrccm.154.1.8680678. [DOI] [PubMed] [Google Scholar]
- 38.Lebret M, Martinot JB, Arnol N, Zerillo D, Tamisier R, Pepin JL, et al. Factors contributing to unintentional leak during CPAP treatment: a systematic review. Chest. 2017;151:707–719. doi: 10.1016/j.chest.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 39.Bachour A, Avellan-Hietanen H, Palotie T, Virkkula P. Practical aspects of interface application in CPAP treatment. Can Respir J. 2019;2019:7215258. doi: 10.1155/2019/7215258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins De Araújo MT, Vieira SB, Vasquez EC, Fleury B. Heated humidification or face mask to prevent upper airway dryness during continuous positive airway pressure therapy. Chest. 2000;117:142–147. doi: 10.1378/chest.117.1.142. [DOI] [PubMed] [Google Scholar]
- 41.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 42.Chai-Coetzer CL, Luo YM, Antic NA, Zhang XL, Chen BY, He QY, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep (Basel) 2013;36:1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009:CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–324. [PubMed] [Google Scholar]
- 45.Pépin JL, Tamisier R, Hwang D, Mereddy S, Parthasarathy S. Does remote monitoring change OSA management and CPAP adherence? Respirology. 2017;22:1508–1517. doi: 10.1111/resp.13183. [DOI] [PubMed] [Google Scholar]
- 46.Pépin JL, Woehrle H, Liu D, Shao S, Armitstead JP, Cistulli PA, et al. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14:57–63. doi: 10.5664/jcsm.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirlaw T, Hanssen K, Duce B, Hukins C. A randomized crossover trial comparing autotitrating and continuous positive airway pressure in subjects with symptoms of aerophagia: effects on compliance and subjective symptoms. J Clin Sleep Med. 2017;13:881–888. doi: 10.5664/jcsm.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, et al. ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems: the optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest. 2005;128:2151–2158. doi: 10.1378/chest.128.4.2151. [DOI] [PubMed] [Google Scholar]
- 50.Bachour A, Hurmerinta K, Maasilta P. Mouth closing device (chinstrap) reduces mouth leak during nasal CPAP. Sleep Med. 2004;5:261–267. doi: 10.1016/j.sleep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Sawyer AM, Deatrick JA, Kuna ST, Weaver TE. Differences in perceptions of the diagnosis and treatment of obstructive sleep apnea and continuous positive airway pressure therapy among adherers and nonadherers. Qual Health Res. 2010;20:873–892. doi: 10.1177/1049732310365502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5:229–240. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- 53.Ye L, Pack AI, Maislin G, Dinges D, Hurley S, McCloskey S, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21:419–426. doi: 10.1111/j.1365-2869.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. 2009;32:545–552. doi: 10.1093/sleep/32.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Platt AB, Field SH, Asch DA, Chen Z, Patel NP, Gupta R, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye L, Antonelli MT, Willis DG, Kayser K, Malhotra A, Patel SR. Couples’ experiences with continuous positive airway pressure treatment: a dyadic perspective. Sleep Health. 2017;3:362–367. doi: 10.1016/j.sleh.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stepnowsky CJ, Palau JJ, Gifford AL, Ancoli-Israel S. A self-management approach to improving continuous positive airway pressure adherence and outcomes. Behav Sleep Med. 2007;5:131–146. doi: 10.1080/15402000701190622. [DOI] [PubMed] [Google Scholar]
- 58.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah AM, Wolford G. Buying behavior as a function of parametric variation of number of choices. Psychol Sci. 2007;18:369–370. doi: 10.1111/j.1467-9280.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 60.Bachour A, Vitikainen P, Maasilta P. Rates of initial acceptance of PAP masks and outcomes of mask switching. Sleep Breath. 2016;20:733–738. doi: 10.1007/s11325-015-1292-x. [DOI] [PubMed] [Google Scholar]
- 61.Goh KJ, Soh RY, Leow LC, Toh ST, Song PR, Hao Y, et al. Choosing the right mask for your Asian patient with sleep apnoea: a randomized, crossover trial of CPAP interfaces. Respirology. 2019;24:278–285. doi: 10.1111/resp.13396. [DOI] [PubMed] [Google Scholar]
- 62.Ma Z, Drinnan M, Hyde P, Munguia J. Mask interface for continuous positive airway pressure therapy: selection and design considerations. Expert Rev Med Devices. 2018;15:725–733. doi: 10.1080/17434440.2018.1525291. [DOI] [PubMed] [Google Scholar]