Abstract
Background
A substantial number of persons living with HIV (PLWH) in Nigeria do not experience durable viral suppression on first-line antiretroviral therapy (ART). Understanding risk factors for first-line treatment failure informs patient monitoring practices and distribution of limited resources for second-line regimens. We determined predictors of immunologic and virologic failures in a large ART delivery program in Abuja, Nigeria.
Methods
A retrospective cohort study was conducted at the University of Abuja Teaching Hospital, a tertiary health care facility, using data from February 2005 to December 2014 in Abuja, Nigeria. All PLWH aged ≥ 15 years who initiated ART with at least 6-month follow-up and one CD4 measurement were included. Immunologic failure was defined as a CD4 decrease to or below pre-ART level or persistent CD4 < 100 cells per mm3 after 6 months on ART. Virologic failure (VF) was defined as two consecutive HIV-1 RNA levels > 1000 copies/mL after at least 6 months of ART and enhanced adherence counselling. HIV drug resistance (Sanger sequences) was analyzed using the Stanford HIV database algorithm and scored for resistance to common nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Univariate and multivariate log binomial regression models were used to estimate relative risks (RRs) and 95% confidence intervals (CIs).
Results
Of 12,452 patients followed, a total of 5928 initiated ART with at least 6 months of follow-up and one CD4 measurement. The entry point for 3924 (66.2%) was through the program’s own voluntary counseling and testing (VCT) center, while 1310 (22.1%) were referred from an outside clinic/program, 332 (5.6%) in-patients, and 373 (6.3%) through other entry points including prevention of mother to child transmission (PMTCT) and transferred from other programs. The mean CD4 at enrollment in care was 268 ± 23.7 cells per mm3, and the mean HIV-1 RNA was 3.3 ± 1.3.log10 copies/mL. A total of 3468 (80.5%) received nevirapine (NVP) and 2260 (19.5%) received efavirenz (EFV)—based regimens. A total of 2140 (36.1%) received tenofovir (TDF); 2662 (44.9%) zidovudine (AZT); and 1126 (19.0%) stavudine (d4T). Among those receiving TDF, 45.0% also received emtricitabine (FTC). In a multivariate model, immunologic failure was more common among PLWH with female gender as compared to male [RR (95% CI) 1.22 (1.07–1.40)] and less common among those who entered care at the program’s VCT center as compared to other entry points [0.79 (0.64–0.91)], WHO stage 3/4 as compared to 1/2 [0.19 (0.16–0.22)], or CD4 200 + cells per mm3 as compared to lower [0.19 (0.16–0.22)]. Virologic failure was more common among PLWH who entered care at the program’s VCT center as compared to other entry points [RR (95% CI) 1.45 (1.11–1.91) and those with CD4 < 200 cells per mm3 at entry into care as compared to higher [1.71 (1.36–2.16)]. Of 198 patient-derived samples sequenced during virologic failure, 42 (21%) were wild-type; 145 (73%) carried NNRTI drug resistance mutations; 151 (76.3%) M184I/V; 29 (14.6%) had ≥ 3 TAMs, and 37 (18.7%) had K65R, of whom all were on TDF-containing first-line regimens.
Conclusions
In this cohort of Nigerian PLWH followed for a period of 9 years, immunologic criteria poorly predicted virologic failure. Furthermore, a subset of samples showed that patients failing ART for extended periods of time had HIV-1 strains harboring drug resistance mutations.
Background
In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) released the ambitious 95–95–95 targets to end the HIV epidemic globally by 2030, which included mandates that 95% of all diagnosed persons living with HIV (PLWH) should be on antiretroviral therapy (ART) and 95% of those should have plasma HIV-1 RNA suppression [1]. The Government of Nigeria, with support from the US President’s Emergency Plan for AIDS Relief (PEPFAR), The Global Fund to Fight AIDS, Tuberculosis and Malaria and other international and domestic donors, has scaled up ART services in Nigeria in an effort to achieve these targets [2]. From 2006 to 2009, the number of Nigerian PLWH initiated on ART increased from 90,008 [3] to an estimated 300,000 [4]. At the end of 2014, a total of 747,382 or 50% of the estimated 1.5 million PLWH in need of ART were receiving therapy in Nigeria [5].
The Institute of Human Virology Nigeria (IHVN), a PEPFAR implementing partner since 2005, has been cumulatively responsible for screening over 6.6 million people and providing treatment to over 240,000 PLWH. In PEPFAR 2.0, IHVN accounted for 19% of all HIV testing services, 19% of patients receiving treatment, and 20% of women receiving prevention of mother-to-child transmission (PMTCT) prophylaxis in Nigeria. In addition to its 1455 treatment and PMTCT sites, IHVN supported the redistribution of its sites to new implementing partners during the US Government (USG) rationalization policy. IHVN is at the forefront of quality laboratory services through its regional training centers, international certification initiative and in establishment of a subnational viral load and early infant diagnosis (EID) network.
Nigeria adopted in 2015 a ‘test and treat’ policy of ART initiation in all PLWH regardless of CD4, clinical stage, age or population [6]. Although additional work is needed to completely achieve this goal, efforts have been made to scale-up treatment access and the number of PLWH receiving ART almost doubled between 2015 and 2018 [7]. The vast majority of PLWH initiating ART are started on TDF + 3TC + DTG as the preferred first line ART regimen with efforts to maintain this regimen for as long as possible to minimize the costs, toxicities, and inconveniences of second-line regimens [8].
Unfortunately, emergence of resistance to tenofovir disoproxyl fumarate (TDF), a key component of regimens for people starting HIV treatment, has been a particular concern that threatens the efficacy of first-line TDF-containing regimens [9], particularly in those with prior exposure to thymidine analogues before initiation of TDF-containing regimens [10]. Viral load (VL) monitoring is associated with lower prevalence of drug resistance in low and middle income countries (LMIC) [11] and is recommended as the preferred strategy for monitoring ART effectiveness [12]. The main goal is to not only reduce viral failure (VF) and resistance in those on ART, but also to reduce the community-level burden of pre-treatment drug resistance (PDR), which has been rising sharply in sub-Saharan countries [13]. In this study we investigated factors associated with virologic and immunologic failures among adult patients attending a large clinic in Abuja, Nigeria, and characterized HIV-1 drug resistance in this population.
Materials and methods
Study design and population
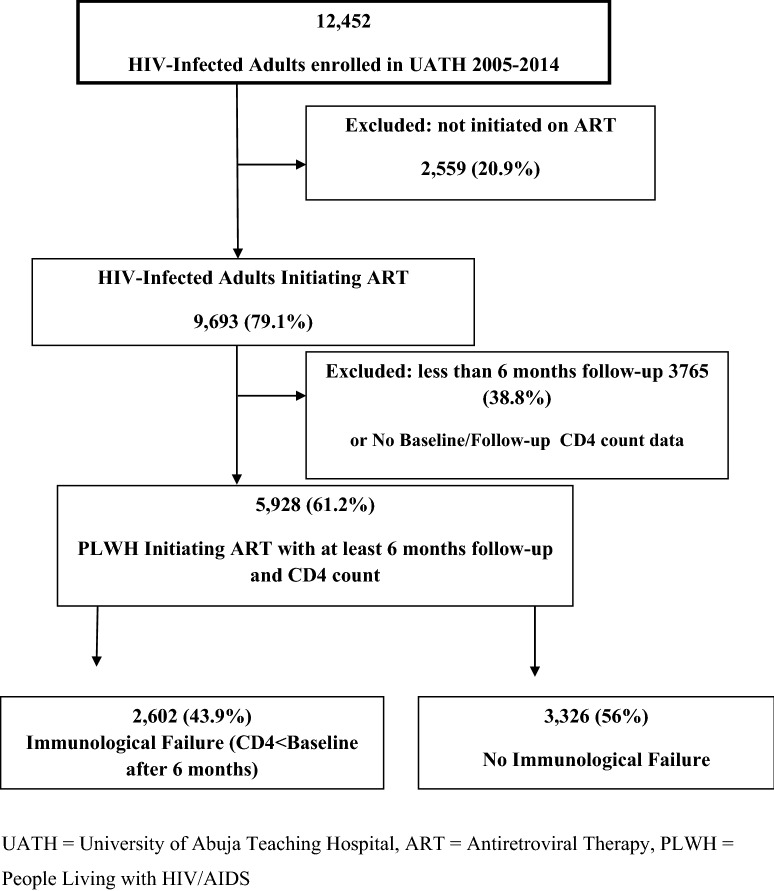
This was a retrospective cohort study using data from adult and adolescent PLWH enrolled in a large ART program funded by PEPFAR at the University of Abuja Teaching Hospital (UATH), a tertiary health care facility supported by IHVN in the North Central region of Nigeria. A total of 12,456 PLWH were enrolled into HIV care from February 2005 through December 2014. Participants included in the study were aged ≥ 15 years at enrollment with a minimum of 6 months on ART, CD4 measurements at entry into care, and a viral load test at least 6 months after ART initiation (targeted viral load monitoring until 2013) (Fig. 1).
Fig. 1.
Flowchart highlighting enrolled patient population. UATH University of Abuja Teaching Hospital, ART antiretroviral therapy, PLWH People living with HIV/AIDS
Between 2003 and 2010, PLWH at World Health Organization (WHO) stage 4, WHO stage 3 with CD4 ≤ 350 cells/mm3 and those with CD4 ≤ 200 cells/mm3 irrespective of WHO staging were considered eligible for ART. After 2010, all PLWH with CD4 ≤ 350 cells/mm3 irrespective of WHO staging and those at WHO Clinical stage 3 or 4 were eligible for ART [14].
Clinical procedures and data collection
ART eligibility was based on the Nigerian National guidelines for HIV and AIDS treatment and care among adolescents and adults. At the beginning of program implementation, the most common first-line ART included stavudine (d4T), lamivudine (3TC), and nevirapine (NVP). In late 2006, the increased recognition of the toxicity and inferior efficacy of regimens containing d4T prompted the revision of international guidelines, with eventual removal of d4T from recommended first-line regimens. In 2008–2009, the introduction of generic tenofovir (TDF) equivalents and the fixed-dose combination (FDC) with emtricitabine (FTC) and efavirenz (EFV) further expanded usage of TDF in lieu of d4T [9, 15].
PLWH were identified and enrolled in care either through external referrals from other clinics and voluntary counselling testing (VCT) or from internal referrals, from the HIV Testing Services (HTS) center, inpatient hospital, or other clinics within the facility. At clinic enrollment, patients were interviewed by clinic staff to obtain complete intake records. Medical and social histories were obtained, physical examinations were performed, and blood was taken for laboratory evaluations such as CD4 assessment, viral load quantification, and hepatitis B screening. Psychosocial and drugs adherence counseling was performed by trained staff. Adherence was assessed by self-report at each visit.
Clinicians decided whether or not to initiate ART for individual PLWH in consideration of local guidelines. PLWH who did not meet CD4-based criteria for ART initiation at the time of their enrollment into care were scheduled for a follow-up visit 6 months later for another review. Those with CD4 counts that qualified for ART initiation were either started on ART immediately or referred for intensive treatment preparation before ART initiation, depending on guidelines in effect at the time. After ART initiation, patients were scheduled for follow-up visits 2–4 weeks later and then every 2 to 3 months thereafter at the discretion of the physician. At each follow-up visit, patients underwent a history, physical and relevant laboratory investigations and these processes were documented on standardized clinical care cards or for routine clinical care management. Specific clinical and laboratory data from these forms were entered by research assistants into CAREWare (JPROG, New Orleans, LA, USA), an electronic medical record system adapted at supported site and exported into SAS for statistical analysis.
Laboratory analysis
All laboratory tests were performed on-site at IHVN Asokoro Reference Laboratory designated by the Federal Ministry of Health (FMoH) to anchor the National HIV drug resistance in Nigeria, and one of the African Society for Laboratory Medicine collaborating centers in Africa. During the study period, CD4 cell counts were the mainstay for laboratory monitoring in Nigeria [2]. Patients on ART were expected to receive 6-monthly CD4 counts to evaluate progress on ART. CD4 count measurement was performed using laser-based CD4 T-lymphocyte enumeration (Cyflow SL, Partec, Munster, Germany).
Before 2010, VL quantification was performed using the Roche Amplicor 1.5 (Cobas Amplicor; Roche Diagnostics, Switzerland) with a detection limit of 400 copies/mL. Subsequently, from 2011 to 2014, VL was performed using the Roche Cobas AmpliPrep TaqMan (Cobas Amplicor; Roche Diagnostics, Basel, Switzerland) with a detection limit of 20 copies/mL [16]. Targeted viral load (VL) approach was implemented and second-line therapy was not yet widely available at the time of this study.
ART failure
Immunologic failure was defined as having a decrease in CD4 count to below the preART level or persistent CD4 < 100 cells per mm3 after 6 months on ART. Virologic failure was defined as 2 consecutive VL > 1000 copies/mL after at least 6 months of ART and intensive adherence counselling [17]. Adherence was assessed by self-report.
HIV-1 genotyping
The cDNA HIV pol sequences from a subset of plasma samples (n = 198) from patients with a viral load ≥ 1000 copies/ml were reverse transcribed from viral RNA extracted from previously-unthawed cryopreserved plasma, with Sanger sequencing as described previously [18, 19].
Sequencing electropherograms were visually inspected using Sequencher 5.4 (Gene Codes Corp., Ann Arbor, Michigan, USA) at two independent laboratories to verify that each nucleotide base was covered at least by 3 reads, one of which had to be in the opposite direction as the other two. Multiple sequence alignment was performed using MAFFT v7 and manually curated using Se-Al. A combination of tools such as the HIV-1 Genotyping Tool at the National Center for Biotechnology information, Jumping Profile HMM Tool at GLOBICS, REGA HIV Subtyping Tool at BIOAFRICA, and Maximum Likelihood phylogenetic analysis were used for HIV subtyping. If the HIV-1 subtype(s) for a particular sequence was similar among all tools used, a final subtype result was assigned. If the results were different, neighbor joining phylogenetic trees with relevant HIV-1 reference subtype and circulating recombinant form (CRF) sequences were constructed at various breakpoints and over the span of the whole sequence to determine the genetic relatedness of the sample to reference sequences [19].
Statistical methods
Study participant characteristics at enrollment were reported as percentages or means with standard deviations (SDs). The percentage of participants experiencing immunologic and virologic failure was calculated among all patients enrolled in the treatment program at UATH. Univariate and multivariate analyses were performed using log binomial models to estimate relative risks (RRs) and 95% confidence intervals (CIs) for associations of factors of interest with immunologic and virologic failure. All available plausible predictors were included in the multivariate models if they had a p value of < 0.20 in univariate analysis. The criterion for significance for all analyses was a 2-sided P value of < 0.05. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina).
Results
Cohort characteristics
Between February 2005 and December 2014, 12,452 PLWH were followed at UATH. Of these, 5928 initiated ART, had at least a 6-month follow-up visit, at least one CD4 after ART initiation, and were included in the analyses, including 3788 (64%) females with a mean age (SD) of 34.5 (8.6) years. A total of 3610 (61%) patients were married, 3569 (67%) had at least a secondary education and 2075 (35%) were employed. The mean (SD) weight was 59.6 (17.5) kg and body mass index (BMI) was 23.8 (7.0) kg/m2 and 1126 (19%) had BMI < 18.5 kg/m2. The entry point or mode of enrollment for 3924 (66%) patients was through the UATH VCT program, 1310 (22.1%) from referral by an outside clinic/program, 332 (5.6%) were in-patients and 308 (5.2%) were enrolled through other entry points including prevention of mother to child transmission (PMTCT). Mean (SD) CD4 was 268 (237) cells/mm3, and 3058 (52%) patients had CD4 < 200 cells/mm3 while 5221 (88%) individuals had CD4 < 500 cells/mm3. The mean (SD) VL was 3.3 (1.3) log10copies/ml. Demographic characteristics at the time of ART initiation are shown in Table 1.
Table 1.
Demographic and clinical characteristics of PLWH at enrollment into the University of Abuja Teaching Hospital HIV Treatment Program
| Characteristics | N = 5928 |
|---|---|
| Gender, n (%) | |
| Male | 36.1 |
| Female | 63.9 |
| Age, mean ± SD years | 34.5 ± 8.6 |
| Age, n (%) | |
| 15–24 years | 8.7 |
| 25–30 years | 23.0 |
| 30–34 years | 23.3 |
| 35–39 years | 19.6 |
| 40–44 years | 11.9 |
| 45–49 years | 7.4 |
| 50+ years | 6.0 |
| Service entry point, n (%) | |
| In-patient | 5.6 |
| Outside clinic/program | 22.1 |
| PMTCT | 1.7 |
| VCT | 66.2 |
| Other | 3.5 |
| ART regimen, n (%) | |
| D4T, 3TC, NVP | 17.7 |
| D4T, 3TC, EFV | 1.3 |
| AZT, 3TC, NVP | 40.8 |
| AZT, 3TC, EFV | 1.9 |
| TDF, XTC, NVP | 22.0 |
| TDF, XTC, EFV | 14.1 |
| Others | 2.1 |
| WHO stage, n (%) | |
| 1 | 39.7 |
| 2 | 24.4 |
| 3 | 32.0 |
| 4 | 3.9 |
| Weight, mean ± SD kg | 59.6 ± 17.5 |
| BMI, mean ± SD kg/m2 | 23.8 ± 7.0 |
| BMI, n (%) | |
| < 17 kg/m2 | 12.1 |
| 17–18.4 kg/m2 | 7.3 |
| 18.5–24.9 kg/m2 | 49.7 |
| 25–29.9 kg/m2 | 20.8 |
| 30+ kg/m2 | 10.1 |
| CD4 count, mean ± SD cells/mm3 | 268 ± 23.7 |
| CD4 Count, n (%) | |
| < 200 cells/µL | 52.2 |
| 200+ cells/µL | 88.2 |
| log10 viral load, mean ± SD copies/mL | 3.3 ± 1.3 |
PMTCT prevention of mother-to-child transmission, VCT voluntary counselling and testing, Mean ± SD mean and standard deviation, n(%) number and percentage, D4T Stavudine, 3TC Lamivudine, NVP nevirapine, EFV efavirenz, AZT zidovudine, TDF tenofovir, XTC emtricitabine, ART antiretroviral therapy, WHO World Health Organisation, BMI body mass index
Predictors of immunologic failure
In a multivariate model, immunologic failure was more common amongst patients receiving NVP–based regimens [RR (95% CI) 1.21 (0.99–1.45)] and of female gender [RR (95% CI) 1.22 (1.07–1.40)]. Immunologic failure was less likely among patients identified and enrolled either through VCT [RR (95% CI) 0.79 (0.64–0.91)], at WHO stage 1 or 2 [RR (95% CI) 0.76 (0.60–0.96)] and CD4 < 200, cells/mm3 [RR (95% CI) 0.19 (0.16–0.22)] (Table 2).
Table 2.
Univariate and multivariate analysis of predictors of immunologic failure
| Characteristics | Unadjusted RR PR (95% CI) |
p-value1 | Adjusted PRR (95% CI) |
p-value2 |
|---|---|---|---|---|
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.98 (0.88–1.09) | 0.17 | 1.22 (1.07–1.40) | 0.005 |
| Occupation | ||||
| Employed | 0.92 (0.80–1.05) | 0.19 | 1.18 (0.95–1.46) | 0.14 |
| Others | 1.00 | 1.00 | ||
| Service entry point | ||||
| Voluntary counseling and testing | 0.78 (0.70–0.88) | < 0.001 | 0.79 (0.64–0.91) | 0.002 |
| Others | 1.00 | 1.00 | ||
| NVP containing regimen | ||||
| Yes | 1.35 (1.18–1.54) | < 0.001 | 1.21 (0.99–1.45) | 0.23 |
| No | 1.00 | 1.00 | ||
| WHO stage | ||||
| 1 or 2 | 1.00 | 0.14 | 1.00 | 0.013 |
| 3 or 4 | 0.97 (0.84–1.19) | 0.76 (0.60–0.96) | ||
| CD4 cell count, cells/mm3 | ||||
| 200 + | 0.22 (0.20–0.24) | 0.19 (0.16–0.22) | ||
| < 200 | 1.00 | < 0.001 | 1.00 | < 0.001 |
1Prevalence RatioRelative risk (PRRR), 95% confidence interval (CI) and p-values were calculated using log binomial regression
2Multivariate models included all predictors significant at p value < 0.20
Predictors of virologic failure
Factors that were independently associated with virologic failure in a multivariable model included CD4 < 200 cells/mm3 at ART initiation [1.71 (1.36–2.16)] and HIV care enrollment via UATH VCT [RR (95% CI): 1.45 (1.11–1.91)] versus other service entry points (Table 3).
Table 3.
Univariate and multivariate analysis of predictors of virological failure
| Characteristics | Unadjusted RR PR (95% CI) |
p-value1 | Adjusted PRR (95% CI) |
p-value2 |
|---|---|---|---|---|
| Age categories | ||||
| < 30 | 1.00 | 1.00 | ||
| 30–39 | 0.57 (0.42–0.77) | < 0.001 | 0.45 (0.31–0.67) | < 0.001 |
| 40–49 | 0.56 (0.41–0.76) | < 0.001 | 0.42 (0.28–0.63) | < 0.001 |
| 50 + | 0.37 (0.26–0.54) | < 0.001 | 0.23 (0.14–0.39) | < 0.001 |
| Marital status | ||||
| Married | 0.73 (0.60–0.91) | 0.004 | 0.71 (0.55–0.97) | 0.021 |
| Others | 1.00 | .00 | ||
| Service entry point | ||||
| Voluntary counseling and testing | 1.61 (1.25–2.07) | < 0.001 | 1.45 (1.11–1.91) | 0.007 |
| Others | 1.00 | 1.00 | ||
| WHO stage | ||||
| 1 or 2 | 1.00 | < 0.001 | 1.00 | 0.003 |
| 3 or 4 | 0.52 (0.41–0.74) | 0.63 (0.46–0.85) | ||
| CD4 cell count < 200, cells/mm3 | ||||
| < 200 | 1.43 (1.16–1.76) | < 0.001 | 1.71 (1.36–2.16) | < 0.001 |
| 200 + | 1.00 | 1.00 | ||
1 Relative riskPrevalence Ratio (PRR), 95% confidence interval (CI) and p-values were calculated using log binomial regression
2 Multivariate models included all predictors significant at p value < 0.20
Antiviral drug–resistance mutations and HIV subtypes
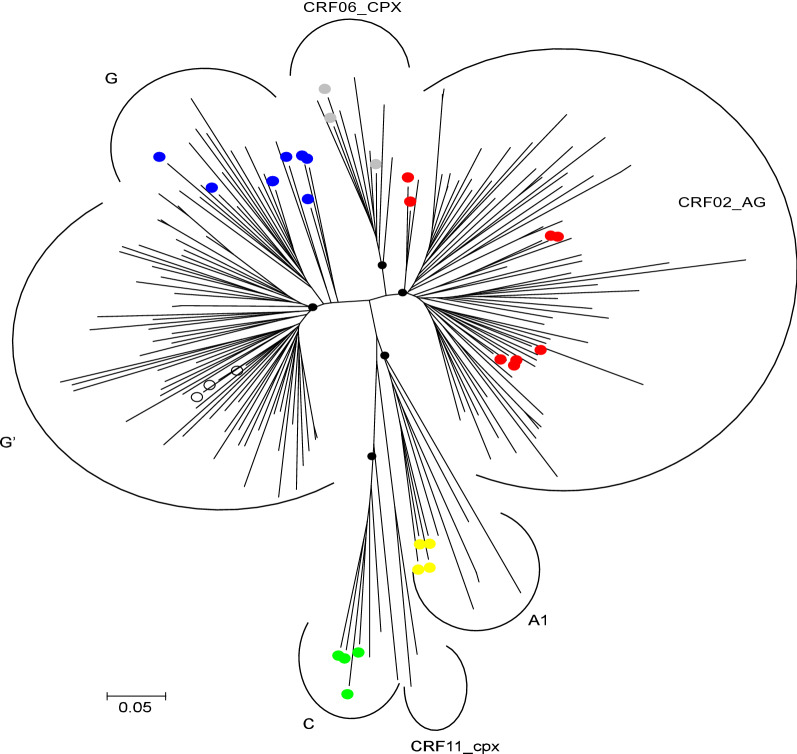
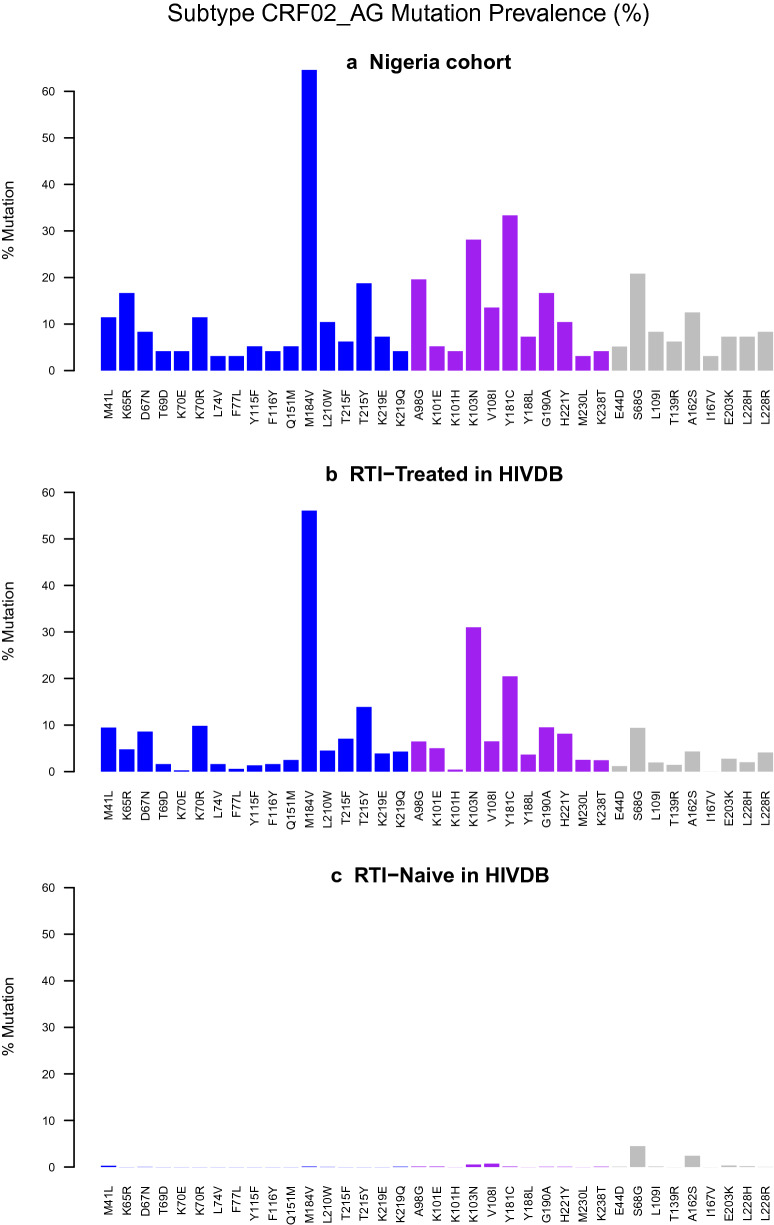
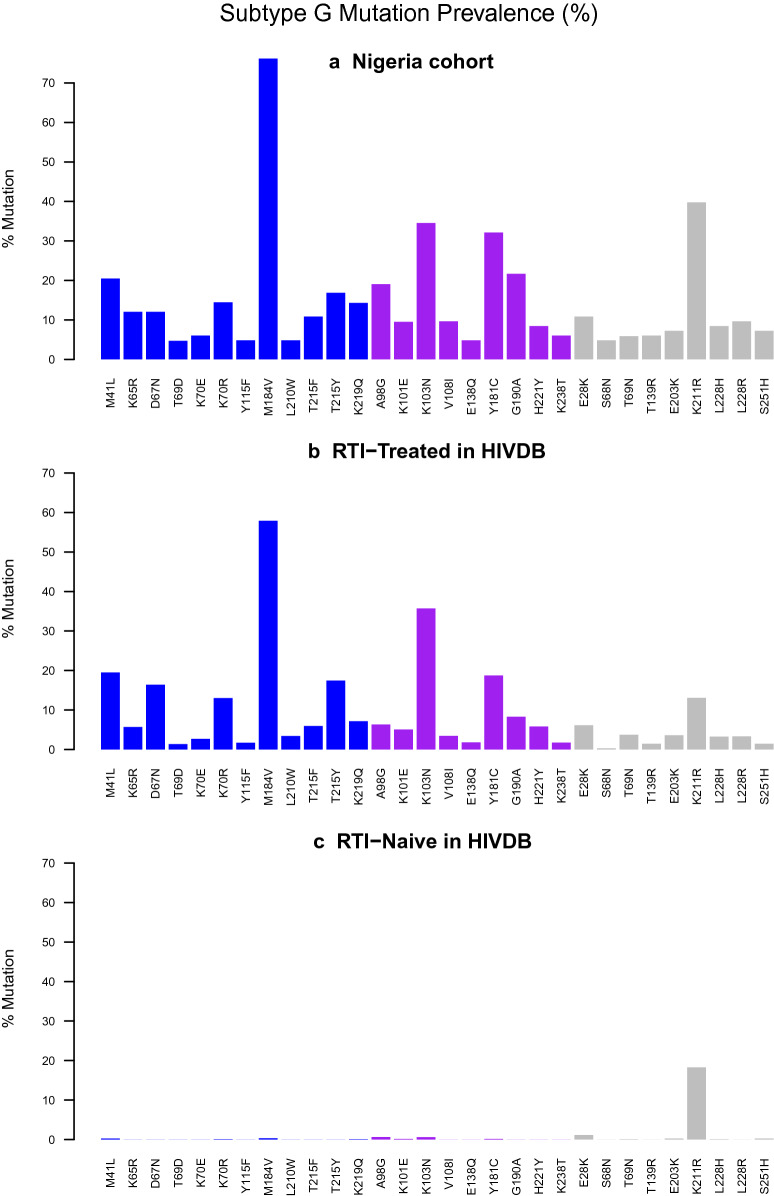
Of the 198 patient-derived HIV-1 sequences obtained from individuals with evidence of virologic failure, CRF02_AG HIV-1 subtype was the most prevalent (n = 96, 48.5%), followed by subtype G (n = 83, 42%) and other subtypes or CRFs (n = 179, 9.5%) (Fig. 2). CRF02_AG viruses harbored a total of 226 RT mutations, defined as amino acid changes relative to CRF02_AG consensus amino acid sequence (Los Alamos HIV Database). For subtype G sequences, there were 227 RT mutations compared to subtype G consensus amino acid sequence (Los Alamos HIV Database). For each of the 226 mutations present in 96 CRF02_AG sequences, we compared its frequency to the prevalence in treatment-naive subtype CRF02_AG patients in the Stanford HIV Drug Resistance Database (HIVDB). Similar analysis was done with the subtype G sequences. We created three plots for subtype CRF02_AG: For the 226 mutations, Fig. 3 shows its prevalence in CRF02_AG Nigerian samples, ART-experienced CRF02_AG patients in HIVDB and ART -naive CRF02_AG patients in HIVDB. Thirty-seven of the 226 mutations occurred significantly more frequently in CRF02_AG Nigerian samples compared to CRF02_AG treatment-naive samples in HIVDB (adjusted Fisher’s exact test p value < 0.01). Those 37 mutations are shown in Fig. 3 and 30 mutations occurring significantly more frequently in Nigerian subtype G patients compared to treatment-naive subtype G patients in HIVDB shown in Fig. 4.
Fig. 2.
HIV-1 phylogenetic tree was derived from an alignment of pol sequences. HIV-1 reference strains are highlighted
Fig. 3.
(1) For the 226 mutations, this figure shows its prevalence in CRF02_AG Nigerian patients (a), in treated CRF02_AG patients in HIVDB (b) and in treatment-naive CRF02_AG patients in HIVDB (c). NRTI DRMs are shown in blue, NNRTI DRMs are shown in purple and the remaining mutations are shown in grey. (2) 146 of the 226 mutations occurred ≥ 2 patients in CRF02_AG Nigerian patients. (3) 37 of the 226 mutations occurred significantly more frequently in CRF02_AG Nigerian patients compared to CRF02_AG treatment-naive patients in HIVDB (adjusted Fisher’s exact test p value < 0.01). Those 37 mutations are shown in this figure. NRTI nucleoside reverse transcriptase inhibitor, NNRTI non-nucleoside reverse transcriptase inhibitor, DRM drug resistant mutation, CRF circulating recombinant form, HIVDB HIV data base, RTI reverse transcriptase inhibitor
Fig. 4.
Thirty (n = 30) mutations occurring significantly more frequently in Nigerian subtype G patients compared to treatment-naive subtype G patients in HIVDB are shown in this figure. NRTI DRMs are shown in blue, NNRTI DRMs are shown in purple and the remaining mutations are shown in grey. 30 mutations occurring significantly more frequently in Nigerian subtype G patients compared to treatment-naive subtype G patients in HIVDB shown in this figure
Discussion
Our study evaluated immunologic and virologic failure in a large African HIV treatment program. We found that predictors of immunologic failure included female gender, entry into care via VCT, and low CD4. Predictors of virologic failure included young age, unmarried status, entry into care via VCT, WHO stage 3/4, and low CD4. Immunologic criteria poorly predicted virologic failure.
Although some studies have examined predictors of immunologic failure among patients failing first-line regimens, there is a paucity of data from sub-Saharan Africa [20–25]. In a retrospective multi-site study evaluating long-term outcomes of patients receiving ART in a treatment program in Nigeria, authors indicated that older age, ART regimen, lower CD4 count, higher VL and inadequate adherence were predictors of virologic failure, while female sex and lower baseline CD4 were predictive of immunologic failure [14]. Our findings in North Central Nigeria are consistent with that prior work with respect to the impact of gender and age on risk of immunologic failure as well as the impact of age and CD4 at ART initiation on risk of VF.
Many PLWH in sub-Saharan Africa are diagnosed late in the disease course, with low CD4 counts [26]. In this study, more than 50% of participants had CD4 counts < 200 cells/mm3 at entry into care. This may be related to patient-level factors such as lack of awareness of their HIV status or barriers to accessing care such as stigma [27, 28], high costs or distance from clinics [29].
Consistent with our findings, data from a large cohort study conducted in East Africa and Nigeria showed that younger age, single marital status and female gender were independently associated with virologic failure and immunologic failure [25]. In addition, at a tertiary hospital such as UATH, the clinicians who manage patients are largely resident doctors who rotate through various departments, including the HIV treatment clinic, with variable supervision. Instability and attrition of trained clinicians may result in suboptimal care that creates additional barriers to engagement and retention of PLWH (Ndembi et al. unpublished data).
Similar to previous studies [18, 30–33], we observed that HIV-infected individuals in this study were predominantly infected with CRF02_AG and subtype G. Although polymorphisms were detected in different proportions among distinct subtypes, the prevalence of a particular HIV-1 variant in one subtype rarely differed by more than tenfold compared with the prevalence of that variant in a different subtype. Interestingly, a significantly higher rate of K65R mutation was observed among patients infected predominantly with CRF02_AG (16.7% of K65R) and G (12.0%) HIV-1 strains on first-line ART in this West African setting. Our findings on subtype variability are similar to those from other published studies of drug resistance in patients with subtypes G and CRF02_AG [34]. Lack of routine VL monitoring limits the early detection of VF, thus promoting the accumulation of drug-resistance-associated mutations [5, 35, 36]. This may account for the high prevalence of NRTI and NNRTI resistance mutations in cohorts from resource-limited settings [9, 11, 14, 37].
Based on the reported high virological responses to d4T-containing first-line ARV regimens, it is expected that the majority of adherent adult patients receiving a first-line d4T-containing regimen will be virologically suppressed at the time of d4T switched. Because of its favorable toxicity profile, TDF will usually be preferable to AZT in these patients. TDF will retain residual activity against emerging drug-resistant variants.
Conclusion
In this cohort of Nigerian PLWH, there was a high burden of drug resistance among patients failing ART for extended periods of time. Proactive identification of participants at risk for virologic failure is needed to optimize HIV care outcomes and decrease the risk of acquired drug resistance. Immunologic criteria poorly predicted virologic failure, underscoring the need for consistent resources to support routine viral load monitoring in Nigeria and other resource-limited settings. As ART coverage increases globally, HIV drug resistance remains one of the main obstacles to achieving the 95–95–95 treatment targets.
Acknowledgements
This program evaluation has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of U2G GH002099-01, PA GH17-1753 (ACHIEVE) and NIH R01 AI147331-01(PI: Nicaise Ndembi). The authors thank James Okuma for his contribution.
Disclaimer
This work was primarily funded by PEPFAR. The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all the data related to this analysis and independently made the decision to submit the findings for publication. The findings and conclusions in this publication are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the U.S. Army, Department of Defense or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
Authors’ contributions
NN and PD conceived the analysis for the manuscript. Data collection and management was facilitated by NN, FM-I, MT, JJ, AA, PA, AA, MEQ-M, TAC, RG, S-YR, RWS, WB, MEC, and PD conducted the data analysis with input from NN and PD drafted the manuscript and NN, FM-I, MT, JJ, AA, PA, AA, MEQ-M, TAC, RG, S-YR, RWS, WB, MEC, and PD provided critical review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by the US CDC PEPFAR U2G GH002099-01, PA GH17-1753 (ACHIEVE) and NIH R01 AI147331-01 (PI: Nicaise Ndembi).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, [NN], upon reasonable request.
Ethics approval and consent to participate
This study was reviewed and approved by the National Health Research and Ethics Committee of Nigeria (NHREC Approval # NHREC/01/01/2007—8/12/2019C) and the University of Maryland Baltimore Institutional Review Board (UMB CICERO #HP00066914).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. UNAIDS Issues New Fast-Track Strategy to End AIDS by 2030. Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed 10 Sept 2019.
- 2.Dalhatu I, et al. Outcomes of Nigeria’s HIV/aids treatment program for patients initiated on antiretroviral treatment between 2004–2012. PLoS ONE. 2016;11(11):e0165528. doi: 10.1371/journal.pone.0165528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NACA. National Agency for the Control of AIDS (NACA). Nigeria UNGASS Report In: Strategic planning and research. NACA, Abuja; 2007. https://naca.gov.ng/wp-content/uploads/2016/11/NACA-2011-ANNUAL-REPORT-FINAL-DRAFT_06062012-2011REPORT.pdf. Accessed 10 Sept 2019.
- 4.UNAIDS. UNAIDS. Epidemiological factsheet Geneva: UNAIDS; 2019. https://www.unaids.org/en/regionscountries/countries/nigeria. Accessed 10 Sept 2019.
- 5.WHO. Global update on HIV treatment 2013. Glob. Updat. HIV Treat. 2013 results, impact Oppor., no. June, p. 7; 2013.
- 6.Stafford KA, et al. Evaluation of the clinical outcomes of the test and treat strategy to implement treat all in Nigeria: results from the Nigeria multi-center ART study. PLoS ONE. 2019;14(7):e0218555. doi: 10.1371/journal.pone.0218555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. New survey results indicate that Nigeria has an HIV prevalence of 1.4%—Nigeria | ReliefWeb; 2019. https://reliefweb.int/report/nigeria/new-survey-results-indicate-nigeria-has-hiv-prevalence-14. Accessed 10 Sept 2019.
- 8.Haas AD, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV. 2015;2(7):e271–e278. doi: 10.1016/S2352-3018(15)00087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The TenoRes Study Group Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16(5):565–575. doi: 10.1016/S1473-3099(15)00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregson J, et al. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: a retrospective multi-centre cohort study. Lancet Infect Dis. 2017;17(3):296–304. doi: 10.1016/S1473-3099(16)30469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9(7):409–417. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 12.WHO . WHO | HIV drug resistance report 2019. Geneva: WHO; 2019. p. 68. [Google Scholar]
- 13.Gupta RK, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FMOH. National Guidelines for HIV Prevention Treatment and Care. Abuja; 2016.
- 15.Meloni ST, et al. Long-term outcomes on antiretroviral therapy in a large scale-up program in Nigeria. PLoS ONE. 2016;11(10):e0164030. doi: 10.1371/journal.pone.0164030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abubakar A, et al. Improved performance of COBAS AmpliPrep/COBAS TaqMan Version 2.0 assay over amplicor monitor version 1.5 in the quantification of HIV-1 RNA viral load in Abuja, Nigeria. Curr HIV Res. 2015;13(4):262–267. doi: 10.2174/1570162x13666150121112139. [DOI] [PubMed] [Google Scholar]
- 17.Kazooba P, Mayanja BN, Levin J, Masiira B, Kaleebu P. Virological failure on first-line antiretroviral therapy; associated factors and a pragmatic approach for switching to second line therapy-evidence from a prospective cohort study in rural South-Western Uganda, 2004–2011. Pan Afr Med J. 2018;29:191. doi: 10.11604/pamj.2018.29.191.11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etiebet MAA, et al. Tenofovir-based regimens associated with less drug resistance in HIV-1-infected Nigerians failing first-line antiretroviral therapy. Aids. 2013;27(4):553–561. doi: 10.1097/QAD.0b013e32835b0f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayondo JK, et al. Intrapatient evolutionary dynamics of human immunodeficiency virus type 1 in individuals undergoing alternative treatment strategies with reverse transcriptase inhibitors. AIDS Res Hum Retroviruses. 2015;31(7):749–756. doi: 10.1089/aid.2015.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawizza HE, et al. Immunologic criteria are poor predictors of virologic outcome: implications for hiv treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palladino C, et al. Predictors of attrition and immunological failure in HIV-1 patients on highly active antiretroviral therapy from different healthcare settings in Mozambique. PLoS ONE. 2013;8(12):e82718. doi: 10.1371/journal.pone.0082718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anude CJ, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis. 2013;13(1):113. doi: 10.1186/1471-2334-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Ndagijimana Ntwali JD, et al. Viral load detection and management on first line ART in rural Rwanda. BMC Infect Dis. 2019;19(1):8. doi: 10.1186/s12879-018-3639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox MP, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. JAIDS J Acquir Immune Defic Syndr. 2012;60(4):428–437. doi: 10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiweewa F, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS ONE. 2019;14(2):e0211344. doi: 10.1371/journal.pone.0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agaba P, et al. Patients who present late to HIV care and associated risk factors in Nigeria. HIV Med. 2014;15(7):396–405. doi: 10.1111/hiv.12125. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Hart C, et al. The synergistic impact of sexual stigma and psychosocial well-being on HIV testing: a mixed-methods study among Nigerian men who have sex with men. AIDS Behav. 2018;22(12):3905–3915. doi: 10.1007/s10461-018-2191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowell TA, et al. Stigma, access to healthcare, and HIV risks among men who sell sex to men in Nigeria. J Int AIDS Soc. 2017;20(1):21489. doi: 10.7448/IAS.20.01.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May M, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet (London, England) 2010;376(9739):449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lihana RW, et al. Update on HIV-1 diversity in Africa: a decade in review. AIDS Rev. 2012;14(2):83–100. [PubMed] [Google Scholar]
- 31.Li Y, et al. Genetic clustering analysis for HIV infection among men who have sex with men in Nigeria: implications for intervention. AIDS. 2019 doi: 10.1097/qad.0000000000002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billings E, et al. New subtype B Containing HIV-1 circulating recombinant of sub-Saharan Africa origin in Nigerian men who have sex with men. J Acquir Immune Defic Syndr. 2019;81(5):578–584. doi: 10.1097/qai.0000000000002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemelaar J, et al. Global and regional molecular epidemiology of HIV-1, 1990-2015: a systematic review, global survey, and trend analysis. Lancet Infect Dis. 2019;19(2):143–155. doi: 10.1016/s1473-3099(18)30647-9. [DOI] [PubMed] [Google Scholar]
- 34.Rhee S-Y, et al. HIV-1 protease, reverse transcriptase, and integrase variation. J Virol. 2016;90(13):6058–6070. doi: 10.1128/JVI.00495-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boender TS, et al. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2015;61(9):1453–1461. doi: 10.1093/cid/civ556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodall RL, et al. Rapid accumulation of HIV-1 thymidine analogue mutations and phenotypic impact following prolonged viral failure on zidovudine-based first-line ART in sub-Saharan Africa. J Antimicrob Chemother. 2017;72(5):1450–1455. doi: 10.1093/jac/dkw583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inzaule SC, et al. Previous antiretroviral drug use compromises standard first-line HIV therapy and is mediated through drug-resistance. Sci. Rep. 2018;8(1):15751. doi: 10.1038/s41598-018-33538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [NN], upon reasonable request.