Abstract
Background: In March 2020, many elective medical services were canceled in response to the coronavirus disease 2019 (COVID-19) pandemic. The daily case rate is now declining in many states and there is a need for guidance about the resumption of elective clinical services for patients with lung disease or sleep conditions.
Methods: Volunteers were solicited from the Association of Pulmonary, Critical Care, and Sleep Division Directors and American Thoracic Society. Working groups developed plans by discussion and consensus for resuming elective services in pulmonary and sleep-medicine clinics, pulmonary function testing laboratories, bronchoscopy and procedure suites, polysomnography laboratories, and pulmonary rehabilitation facilities.
Results: The community new case rate should be consistently low or have a downward trajectory for at least 14 days before resuming elective clinical services. In addition, institutions should have an operational strategy that consists of patient prioritization, screening, diagnostic testing, physical distancing, infection control, and follow-up surveillance. The goals are to protect patients and staff from exposure to the virus, account for limitations in staff, equipment, and space that are essential for the care of patients with COVID-19, and provide access to care for patients with acute and chronic conditions.
Conclusions: Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a dynamic process and, therefore, it is likely that the prevalence of COVID-19 in the community will wax and wane. This will impact an institution’s mitigation needs. Operating procedures should be frequently reassessed and modified as needed. The suggestions provided are those of the authors and do not represent official positions of the Association of Pulmonary, Critical Care, and Sleep Division Directors or the American Thoracic Society.
Keywords: COVID-19, SARS-CoV-2, pulmonary function tests, bronchoscopy, polysomnography
In March 2020, many elective medical services were canceled in response to the coronavirus disease 2019 (COVID-19) pandemic. Strategies to mitigate the spread of the virus that causes COVID-19 (i.e., severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) were undertaken across the United States in an effort to reduce the number of cases and deaths and to prevent healthcare resources from being overwhelmed. By late April 2020, the daily COVID-19 case rate was declining in many states.
The Association of Pulmonary, Critical Care, and Sleep Division Directors (APCCSDD) discussed operational aspects of COVID-19 clinical care during a weekly webinar hosted by the American Thoracic Society (ATS) throughout the pandemic. Participants foresaw the need for guidance in resuming on-site activities for patients with lung disease or sleep conditions, and it was decided that a plan would be created on the basis of consensus. The plan is guided by the realization that as community physical-distancing practices are loosened, there is a risk of virus reintroduction; therefore, ambulatory clinics must make significant efforts to mitigate the risk of viral exposure to patients and to staff until the threat of COVID-19 has receded.
The goals of the plan are to 1) protect patients and staff from exposure to the virus; 2) account for limitations in staff, equipment, and space that are essential for the care of patients with COVID-19; and 3) provide access to care for patients with acute and chronic conditions. The suggestions in this document should not be considered mandates. They are intended as a baseline from which institutions can develop their own plan, consistent with their unique circumstances. The suggestions are those of the authors and do not represent official positions of the APCCSDD or the ATS.
Methods
The ATS Chief of Guidelines and Documents solicited volunteers from the APCCSDD to serve as group leaders and create plans for restoring normal operations in outpatient pulmonary and sleep-medicine clinics, pulmonary function testing (PFT) laboratories, bronchoscopy and procedure suites, polysomnography (PSG) laboratories, and pulmonary rehabilitation (PR) facilities. Each group leader formed a working group to assist in creation of the plans. Completed plans from the individual working groups were collated into an overall plan and document. The draft was shared with all authors, who reviewed the document and provided feedback and edits. Several cycles of review and revision ensued until there was consensus that submission for peer review was warranted. The manuscript was revised to address peer-reviewer comments and then underwent several cycles of review and revision before being submitted for further consideration by the Journal.
Resuming Outpatient Clinical Services
When to Resume
The Centers for Medicare and Medicaid Services (CMS) recommend that the resumption of elective in-person services be considered only in states or regions that have satisfied the Centers for Disease Control and Prevention (CDC) gating criteria for reopening: 1) a downward trajectory of COVID-19–like cases and influenza-like illnesses over a 14-day period, 2) hospitals having capability to treat all patients without crisis care, 3) a robust testing program in place for at-risk healthcare workers, and 4) a downward trajectory of confirmed cases over a 14-day period or a downward trajectory of positive tests as a percentage of total tests over a 14-day period (1, 2). The recommendation for a downward trajectory of confirmed cases assumes that the volume of testing remains relatively constant; otherwise, variation in the daily case rate may reflect changes in testing volume rather than the infection rate. For this reason, it is important that an adequate volume of testing be achieved before determining the 14-day trajectory of the case rate, as increasing the testing volume while using the case rate for decision-making may be misleading.
It can be difficult to determine the trajectory of the case rate over 14 days if there is large day-to-day variation. In this situation, alternative approaches include 1) calculating the mean case rate over the previous 7 days and using a decrease in this measure for 14 consecutive days as indicative of a downward trajectory of the case rate or 2) using the COVID-19 hospital admission rate as a surrogate for the case rate (Figure 1). The latter has the added advantage of being less dependent on testing volume. For communities in which the case rate is already low, 14 consecutive days with a consistently low case rate is sufficient to resume elective in-person clinical visits, as long as the case rate is not trending upward.
Figure 1.
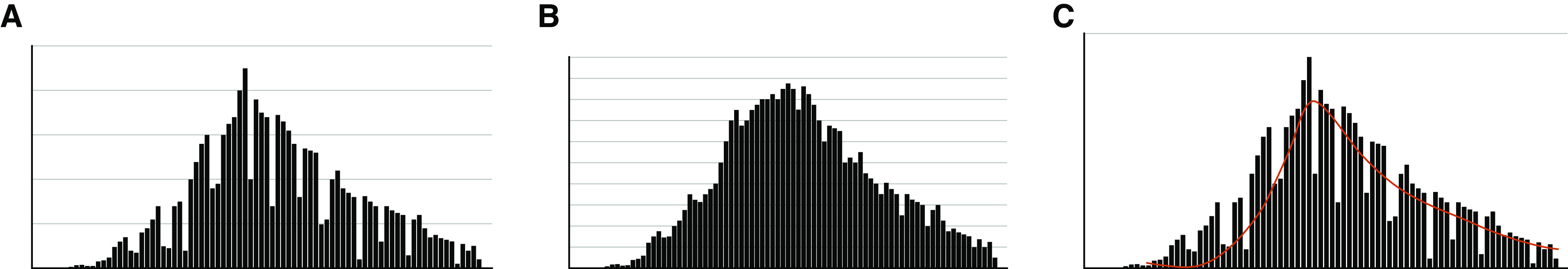
Assessment of the local new case rate. The U.S. Centers for Disease Control and Prevention and Centers for Medicare and Medicaid Services recommend a downward trajectory in the new case rate for 14 days before resuming elective medical services. (A) The trajectory of the new case rate can be variable from day-to-day. (B) Using the new coronavirus disease 2019 (COVID-19) hospitalization rate as a surrogate provides less day-to-day variability and is less dependent on testing volume. (C) The mean new case rate over the preceding 7 days (red line) also provides less day-to-day variability.
In addition to a downward trajectory or consistently low case rate, to resume elective clinical services, an institution should have the capacity to implement all of the operational strategies described below: patient prioritization, screening, diagnostic testing, physical distancing, infection control, and follow-up surveillance.
Patient Prioritization
Safely restoring outpatient pulmonary and sleep-medicine clinical services requires the prioritization of patients’ needs. During the period in which elective medical services were cancelled or reduced, many patients did not receive usual care, and a backlog developed; ideally, the resumption of in-person visits should be prioritized for the patients who need them most. The CMS Non-Emergent, Elective Medical Services, and Treatment Recommendations classify ambulatory services into tier 1, tier 2, and tier 3 on the basis of the acuity and urgency of the requested services (3). Tier 1 visits involve low-acuity services for routine specialty care, screening, preventive care, or supervised rehabilitative-therapy services. Tier 2 visits involve intermediate acuity services for established patients with new symptoms or for new patients with nonurgent symptoms. Tier 3 high-acuity services are for new patients with new symptoms or for any patient with severe symptoms (Figure 2).
Figure 2.
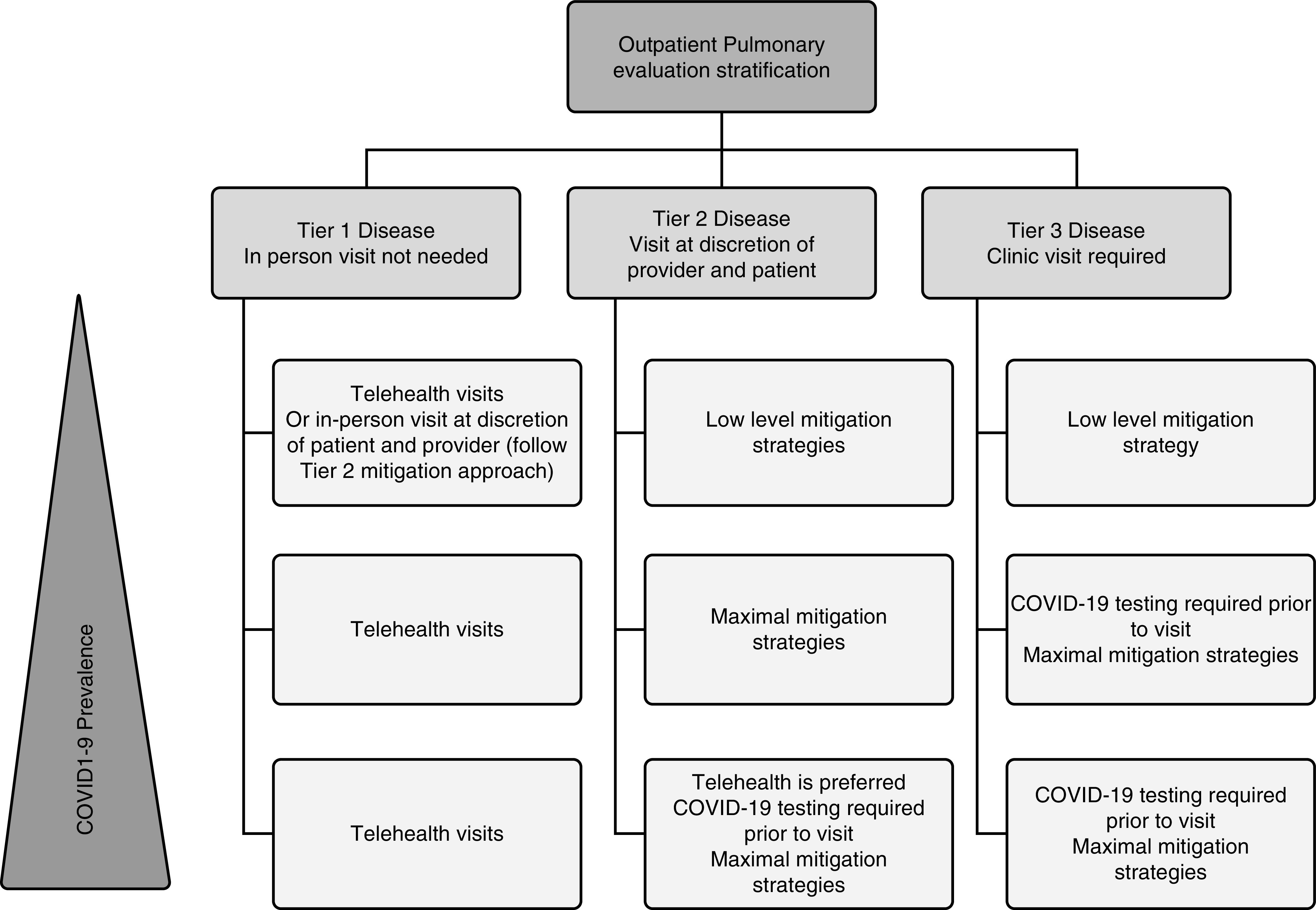
Framework for the prioritization of elective medical services on the basis of acuity. COVID-19 = coronavirus disease 2019.
This framework prioritizes outpatient services on the basis of acuity and can be tailored to institutional resources, patient and provider preferences, and community disease prevalence:
-
•
Institutional resources. Pertinent institutional resources include space, staff, and personal protective equipment (PPE). If any of these resources are in short supply, an institution may choose to resume services with fewer in-person visits than if those resources were abundant. As an example, an institution may decide to open at half-capacity because of a shortage of PPE and, therefore, may accommodate only tier 3 patients, whereas it might have opened to both tier 2 and tier 3 patients had there not been a shortage of PPE.
-
•
Provider and patient preferences. Tier 2 visits in the CMS framework incorporate provider and patient discretion. For example, providers who routinely see highly specialized pulmonary or sleep problems may be comfortable with video visits in situations in which another provider less familiar with the subspecialty problem may wish to see patients in person. Factors that may influence patient preferences include access to and familiarity with telehealth resources, balanced against the burden and increased risk of SARS-CoV-2 exposure associated with in-person visits.
-
•
Community disease prevalence. In regions with a low prevalence of COVID-19, less restrictive mitigation strategies may be appropriate. For example, tier 2 patients can be accommodated by either telehealth or in-person visits; in the context of high disease prevalence, a telehealth appointment may be preferable, whereas in the context of low disease prevalence, an in-person visit may be preferable.
Screening and Testing for SARS-CoV-2
Identification of patients infected with SARS-CoV-2 and removal of such patients from the normal flow of patient care as early as possible are essential components of an effective strategy to resume clinical operations and ensure the safety of other patients and staff.
All patients scheduled for outpatient services should undergo screening for high-risk exposures, fever, and symptoms suggestive of COVID-19 (i.e., cough, dyspnea, myalgias, anosmia, and/or dysgeusia) (4). This will ideally be done in two phases. The first phase of screening should ideally occur within 72 hours of the scheduled appointment, and no more than 7 days before the scheduled appointment, by reaching out to patients by telephone or e-mail or providing access to online check-in and screening. During the screening, patients should also be advised on institutional policies for people accompanying them to their clinic appointment, and anyone accompanying them should also be screened. Patients who are detected by screening as having symptoms consistent with COVID-19 should be referred for SARS-CoV-2 testing, ideally within 48 hours of the scheduled visit. Testing is particularly important in patients with pulmonary disease because many patients have chronic and/or acute symptoms that are difficult to distinguish from COVID-19.
The second phase of screening should occur upon arrival for their appointment (or they should undergo initial screening if they could not be reached for advanced screening) and, like the first phase, should consist of questioning about exposures to individuals with suspected or confirmed COVID-19, fever, and symptoms consistent with COVID-19. Temperature checks upon arrival are appropriate if resources permit. Facilities should take steps to reduce the risk of COVID-19 exposure and transmission by using separate spaces or buildings for care of patients with acute COVID-19 and by implementing mitigation procedures in areas used for non–COVID-19 patient care. On the basis of the results of screening plus testing, the following approaches are recommended:
-
•
No symptoms during initial screening and upon arrival, or SARS-CoV-2-negative test and no symptoms upon arrival. The patient may proceed to an area dedicated for low-risk patients to wait for their appointment.
-
•
SARS-CoV-2 negative test results but new symptoms upon arrival or SARS-CoV-2 status unknown (never tested) but new symptoms upon arrival. The patient should be directed to a dedicated waiting area for high-risk patients, where they can undergo diagnostic testing for SARS-CoV-2, or they should be sent home to quarantine until testing for SARS-CoV-2 can be performed. If the test results for SARS-CoV-2 are negative and concern for COVID-19 persists (i.e., an alternative cause of symptoms has not been identified), a second test may be performed to minimize the chance of being misled by an initial false-negative result.
-
•
SARS-CoV-2–positive test result. If appropriate, the patient should be sent home to quarantine and should contact their primary care provider regarding next steps. Their appointment may be done by telehealth while in quarantine or rescheduled to a later date. SARS-CoV-2–positive patients with acute symptoms requiring urgent evaluation should be masked and accompanied to the closest emergency department or urgent-care COVID-19 evaluation unit by personnel wearing PPE.
The decision about when to reschedule patients with COVID-19 for elective in-person visits may be extrapolated from the CDC’s guidance on when infected healthcare workers may return to work (5). Patients identified as having COVID-19 may be rescheduled for elective in-person clinical services when one of the following three situations exists: 1) they lack fever (i.e., temperature < 100°F) without fever-reducing medications, their respiratory symptoms (i.e., cough, dyspnea) have improved, and they test negative for SARS-CoV-2 from two consecutive specimens collected ≥24 hours apart; 2) they have been free from fever without fever-reducing medications for at least 24 hours, their symptoms have improved, and at least 10 days (or 10 to 20 days for patients with severe COVID-19 or who are severely immunocompromised) have passed since their symptoms first appeared; or 3) if asymptomatic, they have either tested negative for SARS-CoV-2 from two consecutive specimens collected ≥24 hours apart or at least 10 days (or 10 to 20 days for patients with severe COVID-19 or who are severely immunocompromised) have passed since their first positive SARS-CoV-2 test. The requirement for two consecutive specimens collected ≥24 hours apart is intended to minimize false-negative test results. The alternative requirement of 10 days from a positive test result or the onset of symptoms is based on limited evidence about the duration that viable virus is shed (6).
Physical Distancing
Physical distancing will remain a key approach to minimizing the recurrence of viral transmission as healthcare centers reopen, so institutions should employ strategies that facilitate physical distancing. There are numerous distancing strategies, and the higher the prevalence of COVID-19 in the community, the more strategies will be warranted. Nonpatient visitors may be restricted or prohibited. Patients may be asked to wait outside until contacted on their cell phone to come into the clinic. Fewer patients may be scheduled per day and their appointments may be spaced out to minimize the density of people in the buildings, waiting areas, and clinical areas. Waiting areas may be reconfigured to ensure that there is a minimum of 6 feet between patients (does not require physical separation between a patient and an individual accompanying the patient). Limits may be placed on the number of individuals allowed in an elevator. Patients who are able may be encouraged to use stairs instead of an elevator. Unidirectional patient flow may be designed (check in at one point, go to clinic room, then check out at another point). Similarly, hallways and stairwells may be designated for unidirectional flow.
Infection Control and PPE
Patients who are not wearing a surgical mask or suitable face covering when they arrive should be provided a surgical mask and asked to don the mask upon entry into the building. Examination rooms should be cleaned between patients. Only essential medical contact should occur (i.e., between the provider and the patient only). Other communication, such as scheduling follow-up appointments and tests, should be done electronically, either at the time of the visit or via subsequent telephone or e-mail contact with the patient.
Healthcare workers should wear a surgical mask throughout the medical facility for the entire duration of their shift. Additional protective equipment should be worn when interacting with patients, with the specific PPE depending on the risk of aerosolization. For example, healthcare workers may wear surgical masks while interviewing patients and then upgrade to surgical masks, gloves, and gowns during physical examinations, with face shields added for cough maneuvers and examinations of the nose and mouth. Vigilant PPE practices should be maintained for an extended duration, given the possibility of a second wave of COVID-19.
Follow-Up Surveillance
The patient should be instructed to contact the clinic if they develop new respiratory symptoms within 14 days of their visit and/or if COVID-19 is diagnosed. If human resources permit, institutions should reach out to patients roughly 2 weeks after their clinical service and assess if they remain symptom free, have developed symptoms of COVID-19, have undergone SARS-CoV-2 testing, and, if they have been tested, whether the results were positive or negative. Patients reporting new symptoms consistent with COVID-19 who have not been tested should be instructed to quarantine and should be referred for diagnostic testing. Those who test positive for SARS-CoV-2 should be reported to the local health department for contact tracing if such public health services are available. For those who test negative, if concern for COVID-19 remains (i.e., symptoms cannot be attributed to another cause), repeat testing may be performed ≥24 hours later to minimize the risk of false-negative results.
Assessment of Operational Success
The success, or lack thereof, of resuming pulmonary and sleep-medicine clinical services should be critically appraised periodically. Operational success can be defined as the absence of viral transmission, ability to handle the patient volume without exceeding social-distancing constraints, and lack of delays in patient flow resulting in bottlenecks. Assessment should be iterative, with the goal of gradually increasing the percentage of in-person visits.
If surveillance finds no viral transmission within the institution and the community daily case rate remains low, clinical services may be increased; however, if surveillance reveals viral transmission within the institution or the community daily case rate has increased, operating procedures should be reassessed. Before closing elective in-person clinical services, operations decision-makers should consider the potential adverse consequences of reclosure on patients and whether additional mitigation procedures are possible.
Staff Considerations
Staff should receive either COVID-19 or non–COVID-19 assignments and should not rotate through both clinical settings. They should be trained on the appropriate PPE for various tasks. Staff should also be screened for symptoms daily, and those who develop symptoms should be sent home, quarantined, and tested for SARS-CoV-2.
For staff with confirmed COVID-19, a test-based strategy may be used to determine when they can return to work if sufficient resources exist; otherwise, a non–test-based strategy should be followed. If a test-based strategy is used, staff may return to work once they lack fever (i.e., temperature < 100°F) without fever-reducing medications, have improvement of their symptoms (i.e., cough, dyspnea), and test negative for SARS-CoV-2 from two consecutive specimens collected ≥24 hours apart. Staff who are asymptomatic may return once they test negative for SARS-CoV-2 from two consecutive specimens collected ≥24 hours apart (5). If a non–test-based strategy is used, staff may return to work once they have been free from fever without fever-reducing medications for at least 24 hours, they have improvement of their symptoms, and at least 10 days (or 10 to 20 days for patients with severe COVID-19 or who are severely immunocompromised) have passed since their symptoms first appeared. Staff who are asymptomatic should wait at least 10 days (or 10 to 20 days for patients with severe COVID-19 or who are severely immunocompromised) from their first test positive for SARS-CoV-2 before returning to work (5).
The choice between a test-based strategy and a non–test-based strategy depends on institutional resources and preferences, as neither strategy is infallible. Testing may yield false-negative results, providing false reassurance and allowing an infectious healthcare worker to return to work. It may also detect prolonged shedding of inactive (rather than active) viral particles, delaying the healthcare worker’s return to work. A non–test-based strategy relies on scarce, early data regarding the duration of shedding of viable virus that has not been rigorously confirmed. Reports of outbreaks after the return of symptomatically recovered patients with COVID-19 to their living facilities suggest that a cautious approach is prudent (i.e., testing for SARS-CoV-2 from two consecutive specimens collected ≥24 hours apart to minimize false-negative results), especially for healthcare workers in high-risk settings like nursing homes and healthcare workers who care for patients who are immunocompromised. Because information about the relationship between testing results and infectivity risk is evolving, institutional guidance may change and should be followed.
Specific Pulmonary and Sleep-Medicine Services
Pulmonary Function Testing
PFT presents unique challenges during the COVID-19 pandemic. PFTs may result in high aerosol generation, which can spread droplets from an infected individual even if they are asymptomatic. Cross-contamination of equipment, testing rooms, waiting areas, and corridors may result in viral transmission to staff and other patients, which is particularly problematic for vulnerable patients. Therefore, some institutions may prefer a strategy of testing for SARS-CoV-2 in all patients who are scheduled to undergo PFTs within 72 hours of their appointment, in contrast to the approach described above, in which patients are tested for SARS-CoV-2 if they are found during screening to have symptoms consistent with COVID-19. The final strategy depends on institutional preferences, resources, and local prevalence.
It is important to be mindful of the risks versus benefits for every patient scheduled to undergo PFTs and for every procedure. In other words, how important is the lung-function test for making a diagnosis and/or decision relative to the risk of exposing staff and cross-contaminating equipment? Examples of situations that might favor performing PFTs include the evaluation of lung-transplant and lung-resection candidacy, assessment of the risk of bronchiolitis obliterans syndrome in lung or bone-marrow transplant recipients, diagnostic evaluation of patients with significant unexplained or complex dyspnea, and guidance of clinical decisions in patients with chronic lung diseases, such as cystic fibrosis and interstitial lung disease.
Once it has been determined that PFTs are indicated, the specific test must be considered because tests have varying risk of aerosolization and viral transmission (e.g., methacholine-challenge testing, exercise-challenge testing, and cardiopulmonary-exercise testing are higher risk than spirometry, 6-minute walk test, and measurement of lung volumes or diffusion capacity). Only tests deemed essential should be performed. In most situations, this will include spirometry with or without diffusing capacity of the lung for carbon monoxide. If bronchodilator testing must be performed, use of a metered dose inhaler is preferable to a nebulizer to minimize aerosol exposure. If a nebulizer must be used, a breath-actuated nebulizer is likely lower risk than a regular nebulizer. A viral/bacterial filter can be attached to the side of some nebulizers to capture the exhaled aerosol particles and limit the amount of potentially infectious aerosol that enters the room during the treatment. It is less common that lung volumes will be essential, but use of body plethysmography units might be preferable because of containment of exhaled air. Exercise testing and bronchial-challenge procedures should be postponed if possible because of their particularly high risk of high aerosol production due to high minute ventilation or coughing. For patients who need ongoing surveillance of pulmonary function, home spirometry is a reasonable option with enhanced coaching and education by video and telemedicine. However, clinicians should be aware that the results from home spirometry may be less reliable because of quality issues.
During the PFTs, appropriate precautions must be followed. For high-risk patients (e.g., those from a high-prevalence community) or high-risk aerosol-generating procedures (e.g., bronchial-challenge testing), the PFTs should be performed in a negative-pressure room, if available and as per local policy. Patients should be instructed in cough etiquette and wear a surgical mask as much as possible to avoid aerosol dispersion, and the staff should wear appropriate PPE (N95 mask, face shield, gown, and gloves). Both patients and staff should maintain a safe distance from one another and wash their hands before and after testing.
Only necessary PFT equipment should be kept within the laboratory. In-line antibacterial and antiviral filters should be used, and equipment should be disinfected according to the manufacturer’s instructions. The room should be disinfected between patients, and physical distancing should be practiced in common areas (7). Sufficient time should be allotted between patients to allow adequate room ventilation; the exact amount of time will vary depending on whether the room is under negative pressure and whether there is concomitant use of a high-efficiency particulate air (HEPA) filter or ultraviolet-light decontamination (8). The same precautions should be taken if the PFTs are performed in the context of research.
Bronchoscopy and Procedure Suites
When an institution is ready to resume outpatient clinical activities, the backlog that developed during the suspension of elective services should be addressed by prioritizing the procedures according to urgency. A priority scoring system (the Medically Necessary and Time-Sensitive instrument) was developed to assist with the prioritization of procedures (9). An example of a high-priority elective procedure is a lung mass with lymphadenopathy for diagnosis and staging, whereas a lower-priority elective procedure is surveillance of a stent or transplant or surveillance of tracheostomy changes (10).
There is no empirical evidence regarding the necessity of preprocedural testing for SARS-CoV-2. Many institutions perform preprocedural testing on all patients because of the high prevalence of asymptotic COVID-19 infections coupled with the high risk of viral transmission due to aerosolization during bronchoscopy and similar procedures. Any patient who tests positive for SARS-CoV-2 and does not have an urgent need for the procedure should have their procedure deferred. The procedure should not be rescheduled until the patient meets one of the three criteria described above for rescheduling elective in-person clinical services.
The procedure schedule should allot more time per patient than usual, so there are fewer patients and staff in the suite at any given time. In the waiting, preprocedural, and recovery areas, patients and staff should wear surgical masks, and physical distancing should be respected.
Bronchoscopy and percutaneous tracheostomy are superspreading procedures that confer a particularly high risk of viral transmission. Such procedures should be performed in a negative-pressure room. The minimal number of necessary providers should participate in a procedure, and they should be vigilant in donning and doffing PPE, including an N95 mask, face shield, gown, gloves, hat, and shoe covers. Patients should ideally recover in a negative-pressure room. Lower-risk procedures, such as pleural procedures, may be performed outside of a negative-pressure environment if a HEPA filter is available. Staff should wear a surgical mask, face shield, gown, and gloves.
Only necessary testing equipment should be kept within the procedure suite. Cleaning and decontamination should be done between procedures. This includes a standard disinfection protocol for durable, reusable video monitors and standard high-level disinfection of reusable bronchoscopes (11).
Patients who are SARS-CoV-2 positive and must have a procedure performed should have their procedure performed at the end of the workday so that the room may be terminally cleaned after the procedure. Full PPE should be used, including an N95 mask, face shield, gown, gloves, head covering, and shoe covers.
PSG Laboratories
PSG services should resume in a phased manner to allow for staff acclimation to new workflows. The first phase should involve home sleep apnea testing (HSAT) as a preferred diagnostic test for obstructive sleep apnea (this can also be deployed during an ongoing COVID-19 emergency if resources are available). Mail delivery of devices to and from the patient’s home is encouraged. Use of disposable HSAT equipment, if available, can also be considered. Upon receipt by mail, the bagged device should be isolated for 72 hours and then cleaned and disinfected with the U.S. Environmental Protection Agency (EPA)-registered product recommended by the manufacturer (12, 13). Concurrent initiation of in-laboratory PSG overnight testing might be considered by some laboratories where the prevalence of COVID-19 remains low and staff are appropriately trained and comfortable with local infection-control policies.
The next phase is the initiation of in-laboratory diagnostic PSG without positive-airway-pressure (PAP) titration. Testing should begin at 50% capacity to facilitate physical distancing between technologists in the control room and allow for acclimation to the new workflows and increased use of PPE. This service could be ramped up as these concerns are adequately overcome.
Patients who are at high risk for adverse outcomes should be prioritized. This includes patients with significant sleepiness (e.g., due to narcolepsy), safety concerns (e.g., rapid-eye-movement behavior disorder), or comorbid conditions that raise concerns for central sleep apnea and/or hypoventilation syndromes (e.g., congestive heart failure, stroke, chronic obstructive pulmonary disease, asthma, morbid obesity), which should be assessed by attended PSG. For each patient, the benefits of in-laboratory testing should be weighed against the potential risk of developing COVID-19–related illness (e.g., for older adults, pregnant women, and those with serious medical conditions) before deciding on in-laboratory PSG (14). The multiple-sleep-latency test/maintenance-of-wakefulness test should follow guidelines similar to those for in-laboratory PSG. Mattresses should have plastic covers that can be cleaned and disinfected with an EPA-registered disinfectant (12, 13). All room surfaces should also be cleaned and disinfected between patients, and the same disinfectants should be used (15).
Children are more likely to have asymptomatic cases; therefore, testing for SARS-CoV-2 48 hours before the sleep study should be considered in high-prevalence areas. Use of PPE by staff conducting pediatric testing should be strongly considered. Because most pediatric and even some adult sleep studies require a family member to stay overnight, any family member that comes to the sleep center should undergo similar testing for COVID-19 before being allowed to stay at the sleep center, and if they test negative, they may stay in the room with the patient.
The final phase of restoring sleep services consists of the strategic introduction of PAP titrations, which are associated with an increased aerosolization risk. During PAP titration, the technologist should wear appropriate PPE equipment (N95 mask, face shield, gown, and gloves) and should be assigned one patient per night to mitigate risk of transmission. Portable HEPA filter units in the rooms should also be considered. If available, use of nonvented masks with a disposable expiratory port and attached HEPA filter may further reduce the risk of aerosolization (16). If PAP titrations cannot be performed, patients can be initiated on automatic continuous PAP (auto-CPAP) units on the basis of HSAT or PSG results and followed closely with remote monitoring of PAP data. Alternatively, during the COVID-19 public emergency, patients can be initiated on auto-PAP without a diagnostic sleep study, on the basis of clinician judgment and payer coverage policies.
Pulmonary Rehabilitation
PR is standard care for patients with chronic respiratory disease and continued symptoms and/or functional limitations despite otherwise maximal medical therapy. It is typically delivered as a group program with shared exercise equipment and group learning. This format has not been feasible during the COVID-19 pandemic because of infection-control policies. Home PR alternatives to center-based PR have not been extensively studied, but several remotely delivered PR models are available with some published evidence of their efficacy (17–19). If remote PR is used, it should deliver the essential components of PR, which include exercise training, education, and behavior change.
There appears to be a great need for rehabilitative services for those with post–COVID-19 cardiopulmonary impairment and musculoskeletal compromise after critical illness. In this population, consideration should be given to acute rehabilitation during hospitalization once patients are hemodynamically stable and within the context of infectious-control policies and protocols. Routine rehabilitative services in COVID-19 survivors should be considered when patients are no longer contagious to address any unmet rehabilitation needs.
Assessment should include measurements of physiological limitations (e.g., lung function, exercise and functional capacity, muscle function, balance) as well as patient-centered outcomes (e.g., symptoms and health-related quality of life). Collection of data regarding the outcomes of rehabilitative interventions will be necessary to inform future decision-making regarding rehabilitative interventions for survivors of COVID-19.
From a logistical standpoint, before each PR session, patients should undergo the same screening and SARS-CoV-2 testing strategy described above for elective in-person services. Throughout each session, healthcare workers and patients should maintain social distancing. A surgical mask should be worn during the educational and behavioral-change portions of the session, and healthcare workers should change to an N95 mask, gloves, and gown during the exercise-training portion because of the risk of aerosolization during exercise. Only necessary equipment with adequate spacing should be kept in the training area, which should be thoroughly decontaminated after each session.
Conclusions
Elective medical services were cancelled in response to the COVID-19 pandemic and mitigation strategies, such as physical distancing, were undertaken to reduce the number of cases and deaths and to prevent healthcare resources from being overwhelmed. The suspension of elective clinical services cannot be indefinite because patients’ health needs must be addressed. The CDC and CMS both indicate the resumption of clinical services can be considered once there is a 14-day downward trajectory of new cases, assuming that institutions have an operational strategy for the mitigation of viral transmission within the healthcare facility. Such a plan should consist of patient prioritization, screening, diagnostic testing, physical distancing, infection control, and follow-up surveillance. Importantly, a static plan is unlikely to remain maximally effective in the context of a dynamic process like SARS-CoV-2 transmission; therefore, the operational strategy should be frequently reassessed and modified as needed to emphasize strengths and correct faults.
Acknowledgments
Acknowledgment
Members of the task force are as follows:
Coordination
Kevin C. Wilson, M.D., Boston University School of Medicine, Boston, Massachusetts
General Framework and Outpatient Clinics
Charles A. Powell (group leader), M.D., M.B.A., Icahn School of Medicine at Mount Sinai, New York, New York
Geoffrey, L. Chupp, M.D., Yale University, New Haven, Connecticut
Anne E. Dixon, M.D., University of Vermont, Burlington, Vermont
James H. Finigan, M.D., National Jewish Health, Denver, Colorado
Renda Soylemez-Wiener, M.D., M.P.H., VA Boston Healthcare System, Boston University School of Medicine, Boston, Massachusetts
Pulmonary Function Labs
David A. Kaminsky (group leader), M.D., University of Vermont, Burlington, Vermont
Igor Barjaktarevic, M.D., University of California, Los Angeles, California
Nirav R. Bhakta, M.D., Ph.D., University of California, San Francisco, San Francisco, California
Adam Cole, M.D., University of Kentucky, Lexington, Kentucky
Rodney J. Folz, M.D., Ph.D., Case Western Reserve University, Cleveland, Ohio
Brian Graham, Ph.D., University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Teal S. Hallstrand, M.D., M.P.H., University of Washington, Seattle, Washington
Jeffrey Haynes, R.P.F.T., St. Joseph Hospital, Nashua, New Hampshire
John Hankinson, Ph.D., Hankinson Consulting, Inc., Athens, Georgia
Neil MacIntyre, M.D., Duke University, Durham, North Carolina
Kevin McCarthy, R.P.F.T., R.R.T., ERT, Inc., Matthews, North Carolina
Meredith McCormack, M.D., M.H.S., Johns Hopkins University, Baltimore, Maryland
Margaret Rosenfeld, M.D., Seattle Children’s Hospital, University of Washington, Seattle, Washington
Erik R. Swenson, M.D., University of Washington, Seattle, Washington
Sanja Stanojevic, Ph.D., Sick Kids, University of Toronto, Toronto, Ontario, Canada
Daniel J. Weiner, M.D., Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, Pennsylvania
Bronchoscopy and Procedure Suite
Gaetane Michaud (group leader), M.D., New York University, New York, New York
George Cheng, M.D., University of California San Diego, San Diego, California
Jess Mandel, M.D., University of California San Diego, San Diego, California
Sonali Sethi, M.D., Cleveland Clinic, Cleveland, Ohio
Michal Senitko, M.D., University of Mississippi Medical Center, University of Mississippi, Jackson, Mississippi
Polysomnography Laboratory
Sunil Sharma (group leader), M.D., West Virginia University, Morgantown, West Virginia
Susheel Patil, M.D., Johns Hopkins University, Baltimore, Maryland
Mihaela Teodorescu, M.D., M.S., University of Wisconsin, Madison, Wisconsin
Pulmonary Rehabilitation
Linda Nici, M.D., Brown University, Providence, Rhode Island
Footnotes
This work does not necessarily represent the position of the Veterans Health Administration or the U.S. government.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.U.S. Centers for Medicare and Medicaid Services. Baltimore, MD: U.S. Centers for Medicare and Medicaid Services; 2020. Centers for Medicare & Medicaid Services (CMS) recommendations re-opening facilities to provide non-emergent non-COVID-19 healthcare. [updated 2020 Jun 8; accessed 2020 Jun 15]. Available from: https://www.cms.gov/files/document/covid-recommendations-reopening-facilities-provide-non-emergent-care.pdf. [Google Scholar]
- 2.White House; U.S. Centers for Disease Control and Prevention. Washington, DC: WhiteHouse.gov; 2020. Guidelines: opening up America again. [accessed 2020 May 9]. Available from: https://www.whitehouse.gov/openingamerica/ [Google Scholar]
- 3.U.S. Centers for Medicare and Medicaid Services. Baltimore, MD: U.S. Centers for Medicare and Medicaid Services; 2020. Non-emergent, elective medical services, and treatment recommendations. [updated 2020 Apr 7; accessed 2020 May 11]. Available from: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf. [Google Scholar]
- 4.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. Symptoms of coronavirus. [accessed 2020 May 15]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. [Google Scholar]
- 5.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. Criteria for return to work for healthcare personnel with suspected or confirmed COVID-19 (interim guidance) [accessed 2020 Aug 27]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. [Google Scholar]
- 6.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanger J. ATS pulmonary function laboratory management and procedure manual. 3rd ed. New York, NY: American Thoracic Society; 2016. [Google Scholar]
- 8.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2003. Appendix B: air. Guidelines for environmental infection control in health-care facilities. [accessed 2020 May 15]. Available from: https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html#b1. [Google Scholar]
- 9.Prachand VN, Milner R, Angelos P, Posner MC, Fung JJ, Agrawal N, et al. Medically necessary, time-sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020;231:281–288. doi: 10.1016/j.jamcollsurg.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett MA, Oberg CL, Belanger A, De Cardenas J, Cheng G, Nacheli GC, et al. Society for Advanced Bronchoscopy consensus statement and guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis. 2020;12:1781–1798. doi: 10.21037/jtd.2020.04.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahidi MW, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et al. American Association for Bronchology and Interventional Pulmonology. St. Paul, MN: American Association for Bronchology and Interventional Pulmonology; 2020. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. [accessed 2020 Apr 24]. Available from: https://aabronchology.org/wp-content/uploads/2020/03/2020-AABIP-Statement-on-Bronchoscopy-COVID.GAE-updated-Version.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Center for Biocide Chemistries; American Chemistry Council. Washington, DC: American Chemistry Council; 2020. Novel coronavirus (COVID-19)–fighting products. [accessed 2020 May 8]. Available from: https://www.americanchemistry.com/Novel-Coronavirus-Fighting-Products-List.pdf. [Google Scholar]
- 14.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. People with certain medical conditions. [accessed 2020 May 8]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html. [Google Scholar]
- 15.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. Cleaning and disinfecting your facility: everyday steps, steps when someone is sick, and considerations for employers. [accessed 2020 May 8]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html. [Google Scholar]
- 16.Kryger M, Thomas R. Home PAP devices in COVID-19 infected patients. J Clin Sleep Med. 2020;16:1217–1219. doi: 10.5664/jcsm.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Chronic Obstructive Pulmonary Disease Axis of Respiratory Health Network, Fonds de Recherche en Santé du Québec. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149:869–878. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 18.Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Cox NS, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017;72:57–65. doi: 10.1136/thoraxjnl-2016-208514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton EJ, Mitchell KE, Johnson-Warrington V, Apps LD, Sewell L, Morgan M, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2018;73:29–36. doi: 10.1136/thoraxjnl-2016-208506. [DOI] [PubMed] [Google Scholar]


