Abstract
Rationale: Obesity is associated with an increased risk of pulmonary hypertension (PH); however, regional adipose tissue deposition is heterogeneous with distinct cardiovascular phenotypes.
Objectives: To determine the association of body mass index (BMI) and thoracic visceral and subcutaneous adipose tissue areas (VAT and SAT, respectively) with PH in patients with advanced lung disease referred for lung transplantation.
Methods: We studied patients undergoing evaluation for lung transplantation at three centers from the Lung Transplant Body Composition Study. PH was defined as mean pulmonary artery pressure >20 mm Hg and pulmonary vascular resistance ≥3 Wood units. VAT and SAT were measured on chest computed tomography and normalized to height squared.
Results: One hundred thirty-seven (34%) of 399 patients included in our study had PH. Doubling of thoracic VAT was associated with significantly lower pulmonary vascular resistance (β, −0.24; 95% confidence interval [95% CI], −0.46 to −0.02; P = 0.04), higher pulmonary arterial wedge pressure (β, 0.79; 95% CI, 0.32 to 1.26; P = 0.001), and decreased risk of PH (relative risk, 0.86; 95% CI, 0.74 to 0.99; P = 0.04) after multivariate adjustment. Vaspin levels were higher in patients without PH (median, 101.8 vs. 92.0 pg/ml; P < 0.001) but did not mediate the association between VAT and the risk of PH. SAT and BMI were not independently associated with risk of PH.
Conclusions: Lower thoracic VAT was associated with a higher risk of PH in patients with advanced lung disease undergoing evaluation for lung transplantation. The role of adipokines in the pulmonary vascular disease remains to be evaluated.
Keywords: pulmonary hypertension, adipose tissue, vaspin, advanced lung disease
Obesity has been implicated as a risk factor for several types of pulmonary hypertension (PH) (1), and almost two-thirds of patients with PH are overweight or obese at the time of diagnosis (2, 3). PH occurs in approximately 20% of patients with parenchymal lung disease (4–6) and increases the risk of death in these patients (7–9). The link between obesity and the development of PH is not well understood. Obesity is a heterogeneous disease with multiple fat deposition phenotypes, which have distinct cardiovascular effects (10–13). For example, abdominal visceral adipose tissue area (VAT) is particularly metabolically active and is associated with greater risk of cardiovascular disease compared with subcutaneous adipose tissue area (SAT) (14, 15). VAT secretes various adipokines leading to endothelial dysfunction (16–18).
In the thorax, epicardial and pericardial fat make up the main source of VAT, but the two fat depots are anatomically and embryologically distinct with unique characteristics (19). Epicardial fat was traditionally considered to be white adipose tissue (similar to abdominal VAT), although more recent evidence suggests that epicardial fat more closely resembles brown adipose tissue with the highest rates of lipid-consuming and fatty acid metabolism (19–21). Epicardial fat (unlike other fat depots) is contiguous with the myocardium with no fascia separation and strong evidence for paracrine effects on the coronary microcirculation (19, 22, 23). Pericardial fat, on the other hand, is located anteriorly to the epicardial fat between the visceral and parietal pericardium and is a source of proinflammatory cytokines (24, 25). Pericardial fat has been associated with adverse cardiovascular outcomes and coronary artery calcifications (26, 27). Thoracic adipose tissue is of particular interest in pulmonary disease, especially PH, because its lymphatics drain directly into the pulmonary circulation and it can exert local and systemic effects (28).
Although most adipokines promote inflammatory and atherogenic processes, there are some adipokines that have a more favorable cardiovascular profile, such as vaspin (29). Vaspin inhibits endothelial cell apoptosis caused by increased free fatty acids and promotes endothelial nitric oxide synthase with resulting vasodilation (30, 31).
Collectively, these data highlight the complex association of obesity with PH. Although obesity may be associated with PH, intrathoracic VAT may actually be protective via production of adipokines such as vaspin, which has vasodilatory properties and could impact the nitric oxide pathway. We sought to determine the association of body mass index (BMI) and intrathoracic VAT and SAT with PH in patients with advanced lung disease being evaluated for lung transplantation. We hypothesized that higher BMI and lower thoracic VAT would be associated with a higher risk of PH.
Methods
Study Population
We performed a cross-sectional analysis of LTBC (the Lung Transplant Body Composition study) (32), a prospective multicenter cohort study investigating the mechanistic links between adiposity and primary graft dysfunction after lung transplantation. The LTBC study enrolled adult patients age greater than or equal to 18 years with advanced lung disease evaluated for lung transplantation at Duke University, Columbia University Medical Center, and the University of Pennsylvania between 2011 and 2013 (33). For this study, we included patients from LTBC who had a diagnosis of interstitial lung disease (ILD), chronic obstructive pulmonary diseases (COPD), sarcoidosis, or pulmonary arterial hypertension (PAH) and who underwent chest computed tomography (CT) examination as part of the LTBC protocol. We excluded patients with cystic fibrosis (CF), non-CF bronchiectasis, or a prior lung transplantation as PH in the setting of chronic inflammation from infection or long-standing calcineurin inhibitors and corticosteroid use may have a different etiology from other forms.
PH was defined according to the sixth World Symposium on Pulmonary Hypertension recommendations as mean pulmonary artery pressure (mPAP) >20 mm Hg and a pulmonary vascular resistance (PVR) ≥3 Wood units as determined by right heart catheterization at the time of lung transplant evaluation (34). We performed a secondary analysis after excluding patients with elevated pulmonary arterial wedge pressure (PAWP) (>15 mm Hg). Participants provided written informed consent for participation in the LTBC study. This study was approved by the institutional review board of the University of Pennsylvania.
CT Measurements of Fat Depots
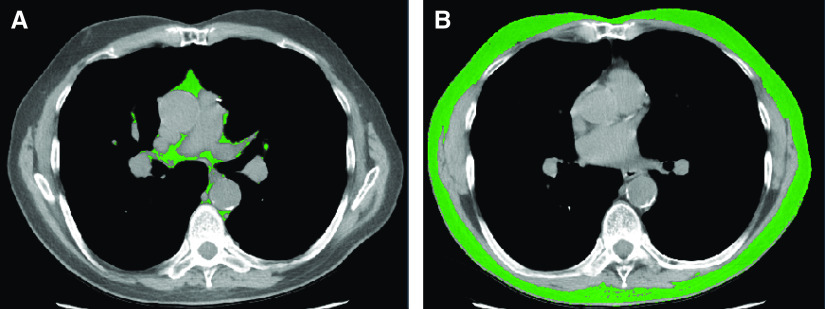
LTBC study participants underwent chest imaging during full inspiration performed with multidetector row CT scanners at the time of evaluation for lung transplantation. Details of chest fat quantification have been previously described (35–37). The thoracic SAT–VAT interface was defined as the interior surface of the rib cage; fat within this surface was considered VAT and that external to this surface was considered SAT for all slices that are superior to the diaphragm. Using a standardized anatomical space approach that was originally developed and validated in a cohort of lung transplant candidates, the thoracic VAT was measured at the mid-T7 vertebral level, as this has been previously shown to have maximum correlation with total volume of thoracic VAT (r = 0.86) (Figure 1A) (35). The thoracic SAT was measured at the mid-T8 vertebral level, which has shown maximum correlation with total volume of thoracic SAT (r = 0.97) (Figure 1B) (35). The primary exposure variables were BMI and thoracic VAT and SAT. We indexed each of the VAT and SAT areas to height squared (m2) (37, 38).
Figure 1.
Thoracic adipose tissue delineation at the level of mid-thorax. (A) Thoracic visceral adipose tissue (green area) (B). Thoracic subcutaneous adipose tissue (green area).
Covariates
Demographic variables and clinical parameters were collected prospectively including age, sex, race/ethnicity, spirometric volumes, and 6-minute walk distance. Weight was measured wearing light indoor clothing and no shoes to the nearest 0.1 kg. Height was measured with a wall-mounted stadiometer to the nearest 0.1 cm.
Vaspin Measurement
Phlebotomy was performed at the lung transplant evaluation visit and blood was collected into citrate tubes. We measured plasma vaspin in duplicate in citrated samples stored at −80°C using a commercially available enzyme-linked immunosorbent assay (R&D Systems). The intraassay coefficient of variation was 2.17%. As our analysis was ancillary to the LTBC study, we only had available samples on a subset of patients (n = 78), which was independent of their PH status.
Statistical Analysis
Baseline characteristics of study participants were compared using two-tailed Student’s t test, Wilcoxon rank sum test, and Fisher exact test, as appropriate. Spearman correlation was used to test the association between adipose measures and weight and BMI. These associations were then tested for nonlinear effects by using quadratic terms for weight and BMI using polynomial regression. Logistic regression models were used to assess the crude association between VAT and SAT and the odds of PH. Models were adjusted for a priori confounders including age, sex, race/ethnicity, primary lung diagnosis, forced vital capacity (FVC), and PAWP. We then transformed odds ratios to relative risks (RRs) using the orsk package (39). We performed a sensitivity analysis of generating RRs using a modified Poisson regression (40).
Generalized additive models with loess smoothing functions were used to demonstrate the nonlinear association between adipose tissue measures and risk of PH. Similarly, linear regression models were used to assess the association between hemodynamic measures (dependent variables) and the adipose measures (independent variable) in the entire cohort (with and without PH) and adjusted these models for the same confounders. We generated five data sets using multiple imputation by chained equations to handle missing data using the mice package in R (41).
We performed sensitivity analysis after restricting the study sample to those with normal PAWP, after restricting the sample to those without PAH, and checked for effect modification by underlying lung disease diagnosis. We also performed mediation analyses to explore whether vaspin mediated the association of adipose tissue with PH using nonparametric bootstrapping estimation methods using the mediation package in R (42). All analyses were performed using R 2018 version 3.6.1.
Results
Patient Demographics
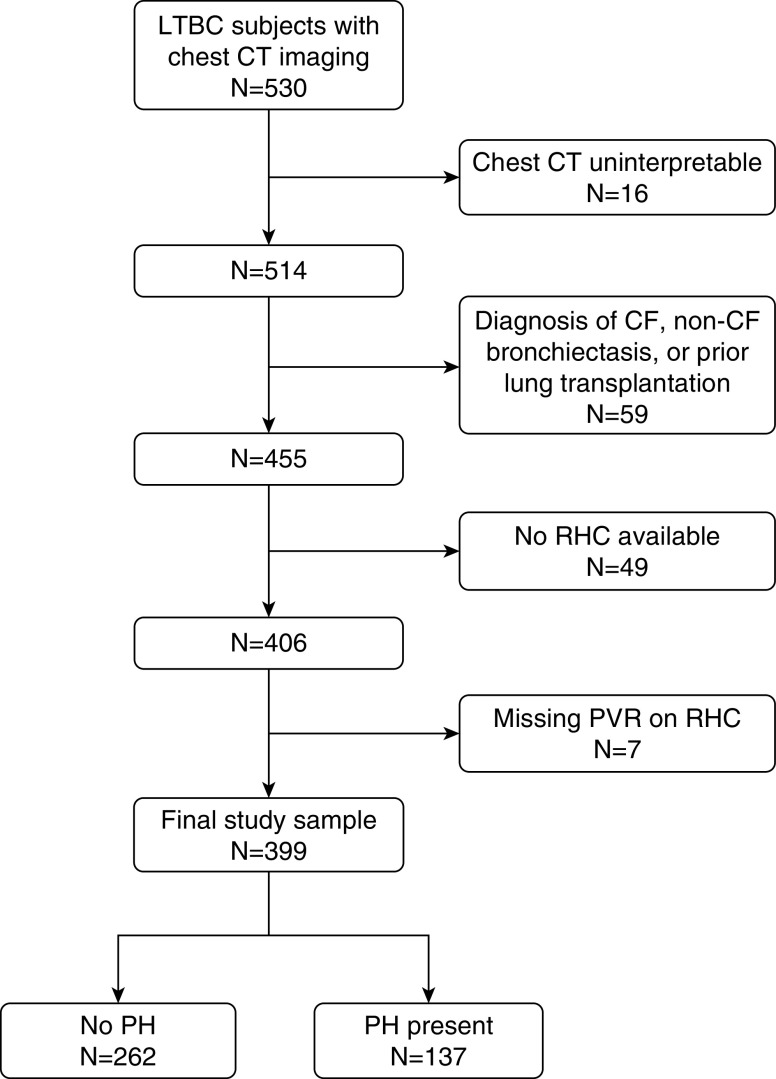
There were 530 patients in the LTBC study, 514 of whom had interpretable chest CT scans (Figure 2). After excluding patients with a diagnosis of CF or non-CF bronchiectasis and those who had previously received lung transplantation as well as those who either did not have a right heart catheterization or were missing hemodynamic measurements, 399 patients were included in our final study sample. Of these, 137 subjects (34%; 95% confidence interval [95% CI], 29–39%) had PH (Table 1). There were no differences in age or sex between patients with and without PH. There was also no difference in BMI between patients with and without PH (25.7 ± 4.5 vs. 25.8 ± 4.1 kg/m2; P = 0.82). Patients with PH were more likely to be non-Hispanic Black or Asian than those patients without PH. Patients with PH were more likely to have higher forced expiratory volume in 1 second and more commonly identified as needing some assistance rather than full assistance. Not surprisingly, patients with PH had lower 6-minute walk distance, higher PVR, and lower cardiac index.
Figure 2.
Flowchart of study inclusion. CF = cystic fibrosis; CT = computed tomography; LTBC = Lung Transplant Body Composition; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; RHC = right heart catheterization.
Table 1.
Baseline characteristics of patients by pulmonary hypertension status
| No PH (n = 262) | PH (n = 137) | |
|---|---|---|
| Age, yr | 61 ± 9 | 59 ± 11 |
| Sex, F, % | 38 | 46 |
| Race/ethnicity, % | ||
| Non-Hispanic white | 84 | 66 |
| Non-Hispanic Black | 9 | 20 |
| Hispanic | 4 | 5 |
| Asian | 2 | 7 |
| Other/multiracial | 1 | 2 |
| Body mass index, kg/m2 | 25.8 ± 4.1 | 25.7 ± 4.5 |
| Primary lung diagnosis, % | ||
| Interstitial lung disease | 59 | 63 |
| Chronic obstructive pulmonary disease | 35 | 15 |
| Pulmonary arterial hypertension | — | 12 |
| Sarcoidosis | 4 | 7 |
| Other | 2 | 3 |
| Forced expiratory volume in 1 s, % pred (n = 371) | 42 ± 21 | 47 ± 21 |
| Forced vital capacity, % pred (n = 371) | 53 ± 18 | 53 ± 21 |
| 6-min walk distance, m (n = 397) | 351 (252–427) | 287 (201–382) |
| Functional status, % (n = 246) | ||
| Needs no assistance | — | 1 |
| Needs some assistance | 32 | 48 |
| Needs full assistance | 68 | 51 |
| Hemodynamics | ||
| Mean pulmonary artery pressure, mm Hg | 20 (17–24) | 33 (27–40) |
| Pulmonary artery wedge pressure, mm Hg | 10 (7–14) | 9 (6–13) |
| Pulmonary vascular resistance, Wood units | 1.9 (1.3–2.4) | 4.7 (3.8–6.9) |
| Cardiac output, L/min | 5.29 (4.60–6.16) | 4.51 (3.92–5.30) |
| Cardiac index, L/min/m2 | 2.80 (2.44–3.19) | 2.49 (2.14–2.85) |
Definition of abbreviations: PH = pulmonary hypertension; pred = predicted.
Mean ± SD or median (interquartile range).
Association of Thoracic Visceral and Subcutaneous Adipose Tissues with Body Mass Index
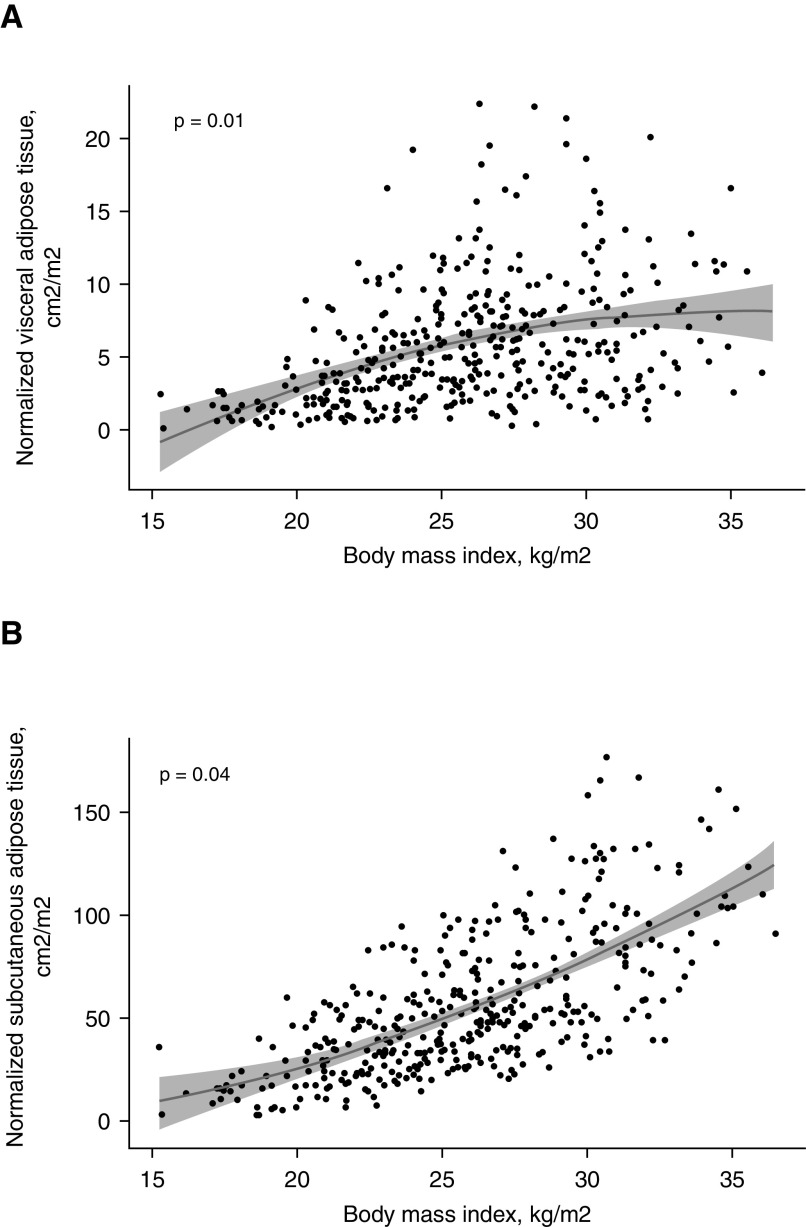
Thoracic VAT was nonlinearly associated with BMI (Figure 3A; P for quadratic trend 0.01). Similarly, thoracic SAT was nonlinearly associated with BMI (Figure 3B; P for quadratic trend 0.04).
Figure 3.
(A) Nonlinear association between thoracic visceral adipose tissue and body mass index. The 95% confidence intervals are depicted in gray shade. (B) Nonlinear association between thoracic subcutaneous adipose tissue and body mass index. The 95% confidence intervals are depicted in gray shade.
Association between Thoracic Visceral and Subcutaneous Adipose Tissue and Hemodynamic Variables
Doubling of the thoracic VAT area was associated with significantly lower PVR (β, −0.24; 95% CI, −0.46 to −0.02; P = 0.04) and higher PAWP (β, 0.79; 95% CI, 0.32 to 1.26; P = 0.001) after adjustment for age, sex, race/ethnicity, underlying lung disease, and FVC in the entire study cohort (Table 2). A doubling of thoracic SAT area was also independently associated with lower PVR and higher PAWP.
Table 2.
Association between thoracic visceral and subcutaneous adipose tissue measures and hemodynamics
| mPAP, mm Hg |
PVR, Wood units |
PAWP, mm Hg |
CI, L/min/m2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | β | 95% CI | P Value | β | 95% CI | P Value | |
| Doubling of VAT cm2/m2 | 0.08 | −0.79 to 0.95 | 0.86 | −0.24 | −0.46 to −0.02 | 0.04 | 0.79 | 0.32 to 1.26 | 0.001 | −0.02 | −0.08 to 0.04 | 0.48 |
| Doubling of SAT cm2/m2 | 0.70 | −0.03 to 1.71 | 0.17 | −0.32 | −0.57 to −0.06 | 0.02 | 1.11 | 0.56 to 1.65 | <0.001 | −0.03 | −0.10 to 0.04 | 0.38 |
| Body mass index per 1 kg/m2 increase | 0.33 | 0.11 to 0.56 | 0.003 | −0.04 | −0.09 to 0.02 | 0.02 | 0.33 | 0.21 to 0.45 | <0.001 | −0.03 | −0.04 to −0.01 | <0.001 |
Definition of abbreviations: β = standardized regression coefficient; 95% CI = 95% confidence interval; CI = cardiac index; mPAP = mean pulmonary artery pressure; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
Adjusted for age, sex, race/ethnicity, lung disease diagnosis, and forced vital capacity.
A 1-kg/m2 increase in BMI was independently associated with higher mPAP, lower PVR, higher PAWP, and lower cardiac index.
Association between Thoracic Visceral and Subcutaneous Adipose Tissue and Risk of Pulmonary Hypertension
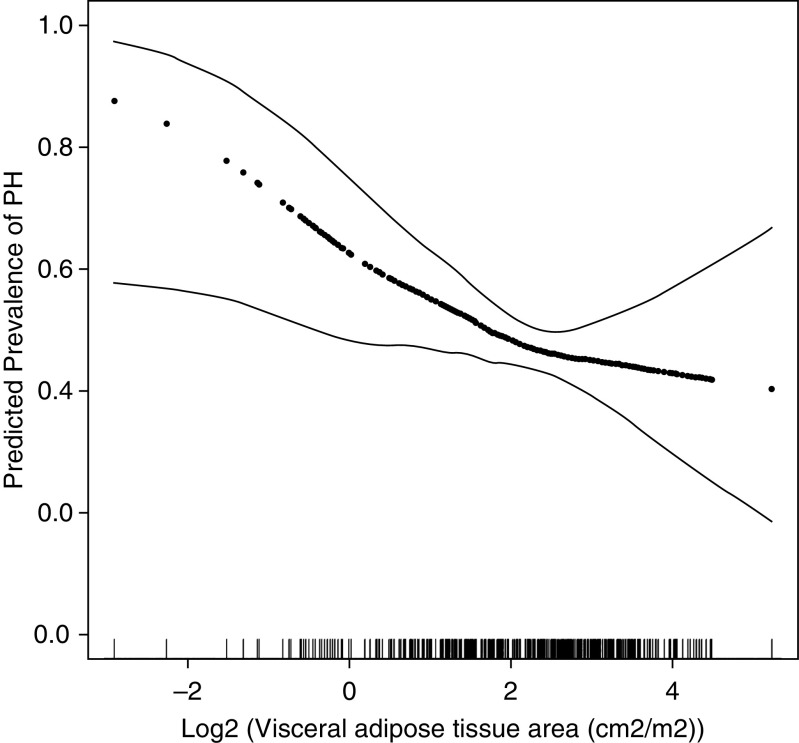
Higher thoracic VAT area was associated with decreased risk of PH (RR per doubling of VAT area = 0.88; 95% CI, 0.78–0.99; P = 0.03) (Table 3), which persisted after adjustment for age, sex, race/ethnicity, FVC, lung disease diagnosis, and PAWP (Table 3 and Figure 4). The association of thoracic VAT and PH held even after additional adjustment for BMI. Thoracic SAT and BMI were not associated with risk of PH in this cohort (RR per doubling of thoracic SAT = 0.92; 95% CI, 0.78-1.09 and RR per 1 kg/m2 increase in BMI = 1.00; 95% CI 0.97-1.04), Table 3). The associations observed in the full cohort were maintained in a sensitivity analysis restricted to patients with normal PAWP and restricted to those without PAH after adjustment for age, sex, race/ethnicity, FVC, and lung disease diagnosis (RR per doubling of thoracic VAT area = 0.86; 95% CI, 0.74–0.99; P = 0.05 and RR per doubling of thoracic VAT area = 0.84; 95% CI, 0.72–0.98; P = 0.03, respectively). The use of binomial modified Poisson regression resulted in RRs that were identical to those in our main analysis.
Table 3.
Unadjusted and adjusted relative risks for the presence of pulmonary hypertension
| Model 1* |
Model 2† |
Model 3‡ |
Model 4§ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | ||||
| Doubling of VAT cm2/m2 | 0.88 (0.78–0.99) | 0.03 | 0.84 (0.72–0.97) | 0.02 | 0.86 (0.74–0.99) | 0.04 | 0.83 (0.70–0.97) | 0.02 | |||
| Doubling of SAT cm2/m2 | 0.96 (0.84–1.09) | 0.56 | 0.90 (0.76–1.06) | 0.21 | 0.92 (0.78–1.09) | 0.36 | 0.84 (0.63–1.08) | 0.17 | |||
| 1 kg/m2 increase in BMI | 0.99 (0.97–1.03) | 0.81 | 0.99 (0.96–1.03) | 0.63 | 1.00 (0.97–1.04) | 0.98 | — | — | |||
Definition of abbreviations: 95% CI = 95% confidence interval; BMI = body mass index; RR = relative risk; SAT = subcutaneous adipose tissue area; VAT = visceral adipose tissue area.
Unadjusted.
Adjusted for age, sex, race/ethnicity, lung disease diagnosis, and forced vital capacity.
Adjusted for age, sex, race/ethnicity, lung disease diagnosis, forced vital capacity, and pulmonary artery wedge pressure.
Adjusted for age, sex, race/ethnicity, lung disease diagnosis, forced vital capacity, pulmonary artery wedge pressure, and body mass index.
Figure 4.
Continuous associations between visceral adipose tissue area and the prevalence of pulmonary hypertension using a generalized additive model. Thick dotted lines: smoothed regression lines adjusted to age, sex, race/ethnicity, lung disease diagnosis, forced vital capacity, and pulmonary artery wedge pressure. Thin solid lines: 95% confidence intervals. PH = pulmonary hypertension.
There was no significant effect modification by underlying lung disease of the association between thoracic VAT and PH risk (P for interaction = 0.16). After limiting the study population to subjects with ILD (n = 239) or COPD (n = 113), we observed comparable results (but with wider 95% CI due to smaller sample size, as expected) (data not shown).
Association between Thoracic Visceral Adipose Tissues, Vaspin, and Risk of Pulmonary Hypertension
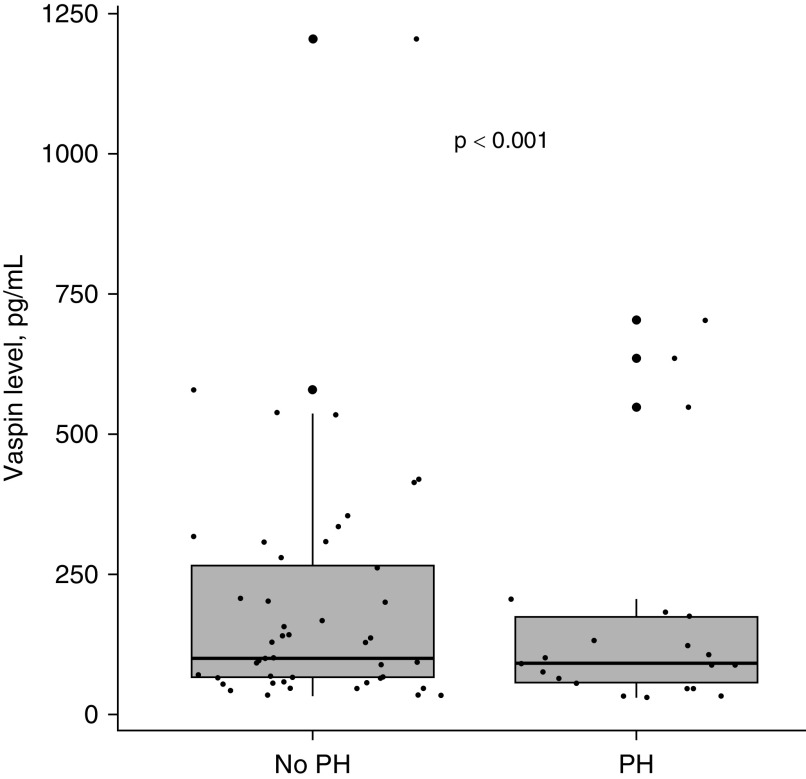
Blood samples were available for 78 subjects. Circulating vaspin levels were positively correlated with thoracic VAT (r = 0.38; P = 0.001) but not with thoracic SAT (r = 0.02; P = 0.86) or with BMI (r = 0.08; P = 0.47). Vaspin levels were higher in the patients without PH as compared with those with PH (median [interquartile range], 101.8 [66.5–266.5] vs. 92.0 [57.2–175.8] pg/ml; P < 0.001; Figure 5). However, circulating vaspin levels did not appear to mediate the association between thoracic VAT and the risk of PH (indirect effect 1.0%).
Figure 5.
Median vaspin levels and PH status (P < 0.001). Median and interquartile range are shown. PH (n = 24) and Non-PH (n = 54). PH = pulmonary hypertension.
Discussion
We found that higher thoracic VAT area was independently associated with decreased risk of PH in patients with COPD, ILD, sarcoidosis and PAH undergoing evaluation for lung transplantation. This association persisted in the subset of patients with normal PAWP and was not modified by the underlying lung diagnosis. Higher thoracic VAT was also independently associated with lower PVR and higher PAWP. Even though thoracic SAT was also associated with PVR and PAWP, it was not independently associated with the risk of PH. Thoracic VAT and SAT were nonlinearly associated with BMI, which was not associated with risk of PH.
Although VAT is most commonly associated with increased cardiovascular risk and endothelial dysfunction, higher thoracic VAT was associated with a decreased risk of PH in our study. Thoracic VAT, particularly epicardial adipose tissue, has unique properties that distinguishes it from other VAT depots because of a delicate equilibrium between protective and harmful cardiovascular effects. Epicardial fat has been postulated to function similarly to brown adipose tissue, with cardioprotection in the setting of hypothermia and hypoxia (19). Epicardial adipose tissue expresses genes associated with brown adipose tissue including UCP1 (uncoupling protein-1), which is absent in other fat depots such as SAT (20). Epicardial fat secretes cardioprotective adipokines, such as adiponectin, adrenomedullin, and vaspin (19, 43–46), which could be contributing to the decrease in PVR associated with higher thoracic VAT. Vaspin is a serine protease inhibitor that has protective effects on the vascular endothelium by inhibiting free fatty acid–induced apoptosis of endothelial cells and increasing the effect of nitric oxide and endothelial nitric oxide synthase mediated by human signal transducer and activator of transcription 3 (30, 47). The nitric oxide signaling pathway is thought to play a major role in the pathogenesis of pulmonary hypertension (48–51).
The association between increased levels of thoracic VAT and decreased risk of PH in our cohort could be potentially explained by decreased pulmonary vasoconstriction or vascular remodeling. In our cohort, we found that circulating vaspin levels were positively correlated with thoracic VAT and were lower in patients with PH. The serum vaspin levels measured in our cohort were on the lower spectrum of reported serum vaspin ranges (52). Serum vaspin levels are impacted by obesity and impaired glucose tolerance. We were not able to show that the association between thoracic VAT and PH is mediated by circulating vaspin levels. A single sample of the peripheral circulation may not reflect local vaspin levels over time, and we only had samples on a small subset of our study sample, so this adipokine (or others) could still play a role in this relationship.
Higher BMI has been shown to be positively correlated with increased PAWP likely as a result of increased intrathoracic pressure in the obese (53). In our study, BMI as well as VAT and SAT were associated with higher PAWP. However, this study demonstrates that BMI itself does not sufficiently account for the heterogeneity of fat depots and deposition (54). In fact, thoracic VAT and SAT were not linearly associated with BMI. Even though we found an association between higher BMI and higher mPAP, higher PAWP, and lower PVR, BMI was not independently associated with the risk of pulmonary hypertension whereas thoracic VAT was, even after adjustment for BMI. One possible explanation is in the requirement of PVR ≥3 Wood units in the sixth World Symposium on Pulmonary Hypertension definition of PH, which many patients in our cohort did not meet as they had mild elevations in mPAP but low or normal PVR. Another possible explanation is a truncated BMI distribution excluding the morbidly obese as patients were referred for a lung transplant evaluation. Our work supports the need for improved phenotyping of obesity and fat distribution in patients with PH to allow for improved personalization of treatments, a priority in pulmonary vascular disease research (55).
Our study has limitations. Our study measured intrathoracic VAT but did not differentiate between epicardial fat and pericardial fat. Although our hypothesis is based on the role of epicardial fat, which is known to have paracrine and endocrine effects on the heart, our results refer to overall intrathoracic VAT. The cross-sectional design does not permit inferences regarding the temporal sequence/causality between adipose measures and development of PH. We included a heterogeneous population of patients with advanced lung disease presenting for lung transplant evaluation at multiple centers, which increases our generalizability; however, our findings may not apply to patients with less severe lung disease or who are not suitable candidates for lung transplant evaluation. Obesity is a relative contraindication to lung transplantation, potentially leading to referral bias. Additionally, many of the subjects included in our cohort could have been on corticosteroid therapy, which could impact glucose tolerance and vaspin levels. Thus, it is unclear if our findings hold true for patients with higher BMI. Finally, we adjusted our models for several variables that could affect the relationship between adipose tissue and PH, although the possibility of residual or unmeasured confounding effects remains.
In conclusion, we found that lower thoracic VAT area was associated with a higher risk of PH in patients with advanced lung disease undergoing evaluation for lung transplantation, an association that was not observed with thoracic SAT. Our results support the need for deep phenotyping of obesity to better understand its association with PH and explore novel therapeutic avenues.
Supplementary Material
Footnotes
Supported by an ENTELLIGENCE Young Investigator Award and by U.S. National Institutes of Health grants K23 HL141584, R01 HL114626, R01 HL087115, K24 HL103844, K24 HL115354, and K24 HL131937.
Author Contributions: N.A.-N., J.D.C., D.J.L., and S.M.K. contributed to the study design. N.A.-N., H.-M.P., M.R.A., D.A.T., Y.T., J.K.U., and S.M.K. analyzed the data. N.A.-N., H.-M.P., M.R.A., D.A.T., Y.T., M.O., M.K.P., S.P., S.M.A., J.M.D., J.K.U., J.D.C., D.J.L., and S.M.K. contributed to the interpretations and the drafting of the manuscript. All authors collected data, provided critical revisions to the study manuscript, and approved the final version submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, Simon R Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev. 2014;23:450–457. doi: 10.1183/09059180.00007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 4.Barberà JA, Blanco I. Gaining insights into pulmonary hypertension in respiratory diseases. Eur Respir J. 2015;46:1247–1250. doi: 10.1183/13993003.01288-2015. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 6.Heresi GA, Platt DM, Wang W, Divers CH, Joish VN, Teal SA, et al. Healthcare burden of pulmonary hypertension owing to lung disease and/or hypoxia. BMC Pulm Med. 2017;17:58. doi: 10.1186/s12890-017-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a REferral centre. Eur Respir J. 2012;39:945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 9.Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(Suppl):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Arsenault BJ, Lachance D, Lemieux I, Alméras N, Tremblay A, Bouchard C, et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med. 2007;167:1518–1525. doi: 10.1001/archinte.167.14.1518. [DOI] [PubMed] [Google Scholar]
- 11.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 12.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 13.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 14.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Schatzkin E, Omoluabi E, Fajemisin M, Onuoha C, Erinfolami T, et al. Introducing the subcutaneous depot medroxyprogesterone acetate injectable contraceptive via social marketing: lessons learned from Nigeria’s private sector. Contraception. 2018;98:438–448. doi: 10.1016/j.contraception.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, Gabrielsson BG, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 17.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 18.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 20.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 21.Bambace C, Telesca M, Zoico E, Sepe A, Olioso D, Rossi A, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol. 2011;20:e153–e156. doi: 10.1016/j.carpath.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 24.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 25.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 29.Sawicka M, Janowska J, Chudek J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol. 2016;222:581–589. doi: 10.1016/j.ijcard.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 30.Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, et al. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun. 2011;413:264–269. doi: 10.1016/j.bbrc.2011.08.083. [DOI] [PubMed] [Google Scholar]
- 31.Sun N, Wang H, Wang L. Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol. 2015;8:482–489. [PMC free article] [PubMed] [Google Scholar]
- 32.Singer JP, Peterson ER, Snyder ME, Katz PP, Golden JA, D’Ovidio F, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190:1012–1021. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond JM, Arcasoy S, McDonnough JA, Sonett JR, Bacchetta M, D’Ovidio F, et al. Lung Transplant Body Composition Study. Adipose gene expression profile changes with lung allograft reperfusion. Am J Transplant. 2017;17:239–245. doi: 10.1111/ajt.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong Y, Udupa JK, Torigian DA, Odhner D, Wu C, Pednekar G, et al. Chest fat quantification via CT based on standardized anatomy space in adult lung transplant candidates. PLoS One. 2017;12:e0168932. doi: 10.1371/journal.pone.0168932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y, Udupa JK, Torigian DA. Optimization of abdominal fat quantification on CT imaging through use of standardized anatomic space: a novel approach. Med Phys. 2014;41:063501. doi: 10.1118/1.4876275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson MR, Udupa JK, Edwin E, Diamond JM, Singer JP, Kukreja J, et al. Adipose tissue quantification and primary graft dysfunction after lung transplantation: the Lung Transplant Body Composition study. J Heart Lung Transplant. 2019;38:1246–1256. doi: 10.1016/j.healun.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. J Stat Softw. 2013;55:1–11. [Google Scholar]
- 40.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 41.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 42.Tingley DYT, Hirose K, Keele L, Imai K.Mediation: R package for causal mediation analysis J Stat Softw 2014591–38.26917999 [Google Scholar]
- 43.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- 45.Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58:248–255. doi: 10.1016/j.jacc.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 46.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung CH, Lee WJ, Hwang JY, Lee MJ, Seol SM, Kim YM, et al. Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. PLoS One. 2012;7:e52346. doi: 10.1371/journal.pone.0052346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue C, Johns RA. Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:1642–1644. doi: 10.1056/NEJM199512143332416. [DOI] [PubMed] [Google Scholar]
- 49.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension Circulation 20091192894–2903.[Published erratum appears in Circulation 124:e279.] [DOI] [PubMed] [Google Scholar]
- 50.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [Published erratum appears in N Engl J Med 354:2400–2401.] [DOI] [PubMed] [Google Scholar]
- 51.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, et al. PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 52.Youn BS, Klöting N, Kratzsch J, Lee N, Park JW, Song ES, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. doi: 10.2337/db07-1045. [DOI] [PubMed] [Google Scholar]
- 53.LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J. 2014;44:425–434. doi: 10.1183/09031936.00209313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlecht I, Fischer B, Behrens G, Leitzmann MF. Relations of visceral and abdominal subcutaneous adipose tissue, body mass index, and waist circumference to serum concentrations of parameters of chronic inflammation. Obes Facts. 2016;9:144–157. doi: 10.1159/000443691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman JH, Rich S, Abman SH, Alexander JH, Barnard J, Beck GJ, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach: a joint NHLBI-cardiovascular medical research and education fund workshop report. Am J Respir Crit Care Med. 2017;195:1661–1670. doi: 10.1164/rccm.201701-0150WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.