Abstract
The explosion of the opioid epidemic in the United States and across the world has been met with advances in pharmacologic therapy for the treatment of opioid use disorder. Long-acting naltrexone is a promising strategy, but its use has important implications for critical care, as it may interfere with or complicate sedation and analgesia. Currently, there are two available formulations of long-acting naltrexone, which are distinguished by different administration routes and distinct pharmacokinetics. The use of long-acting naltrexone may be identified through a variety of strategies (such as physical examination, laboratory testing, and medical record review), and is key to the safe provision of sedation and analgesia during critical illness. Perioperative experience caring for patients receiving long-acting naltrexone informs management in the intensive care unit. Important lessons include the use of multimodal analgesia strategies and anticipating patients’ demonstrating variable sensitivity to opioids. For the critically ill patient, however, there are important distinctions to emphasize, including changes in drug metabolism and medication interactions. By compiling and incorporating the currently available literature, we provide critical care physicians with recommendations for the sedation and analgesia for critically ill patients receiving long-acting naltrexone therapy.
Keywords: critical care, opioid-related disorders, narcotic antagonists, hypnotics and sedatives
As of 2016, there were approximately 2 million people in the United States suffering from opioid use disorder (1). To combat this epidemic, there has been a renewed focus on medication-assisted treatment. Despite improvements in creating and expanding access to treatment for these patients, critical care practitioners may not be informed about how different therapies for opioid use disorder may alter inpatient management. Specifically, long-acting naltrexone for treatment of opioid use disorder poses a challenge to intensive care unit (ICU) providers when caring for a critically ill patient who requires sedation or analgesia. Given the increasing burden of substance use disorders in this country (1) and the availability of long-acting naltrexone as treatment, it is inevitable that critical care practitioners will care for, and provide sedation and analgesia to, patients with opioid use disorder receiving long-acting naltrexone. In this review, we discuss the current available formulations of long-acting naltrexone and strategies to identify patients receiving this therapy, summarize available literature on sedation and analgesia for these patients, and provide recommendations for the ICU provider caring for these patients.
Long-Acting Naltrexone as a Strategy to Manage Opioid Use Disorder
By antagonizing the opioid receptor, naltrexone attenuates and may abolish the addictive effects of opioid exposure. There is also evidence to support the use of long-acting naltrexone for the treatment of opioid use disorder. In clinical trials, long-acting naltrexone was more effective at maintaining opioid abstinence, preventing relapse, reducing cravings, and improving treatment retention than placebo, usual care, or oral daily naltrexone (2–4). Naltrexone is also effective as alcohol deterrence therapy (5).
There are two available forms of long-acting naltrexone—a long-acting injectable (LAI) formulation and a long-acting subcutaneous implant (SI) formulation. The LAI formulation, marketed with the brand name Vivitrol, is dosed at 380 mg injected intramuscularly on a monthly basis (Alkeremes). This medication is currently approved by the U.S. Food and Drug Administration (FDA) to prevent relapse after opioid detoxification and to treat alcohol dependence. The SI formulation is composed of pellets, which are implanted surgically into the subcutaneous tissue of the abdomen, allowing for slow release over time. This formulation is not currently FDA-approved in the United States.
However, the SI strategy has become popular in other countries, particularly in Russia, where it has been studied extensively by researchers at Pavlov Medical University in St. Petersburg, Russia (6–8). In Russia, this formulation is known as Prodetoxone (GP Healthcare SIA). In 2016, BioCorRx Pharmaceuticals reportedly acquired the North American rights to Prodetoxone implant formulations. BioCorRx is currently in the preclinical stages of developing and testing this formulation, referred to as BICX102. In Australia, the SI formulation is marketed as the O’Neill Long-Acting Naltrexone Implant and is manufactured by Go Medical Industries. American practitioners should be aware of the SI formulation, as some private clinics may offer this treatment, and ongoing clinical trials in the United States (for example, ClinicalTrials.gov identifier NCT 03810495) may lead to wider adoption of the SI route of administration as treatment for opioid use disorder.
Beyond different delivery mechanisms, the LAI and SI formulations have different pharmacokinetics. LAI naltrexone achieves a therapeutic concentrations rapidly, within 1–2 hours after injection. The naltrexone concentration remains above 1 ng/ml (the concentration considered effective for blocking street heroin) for at least 35 days (9–11). The SI, however, has a variable duration of effect depending on the manufacturer. The O’Neill Long-Acting Naltrexone Implant advertises a 6-month duration, whereas Prodetoxone is marketed as having a duration of 3 months. However, at least one case report describes a patient whose naltrexone implant was advertised to last 6 weeks (12). Therefore, there may be other products offered to patients with varying durations of effect based on what has been published in the medical literature. For reference, Table 1 lists the advertised durations for different long-acting naltrexone formulations.
Table 1.
Duration of effect of different long-acting naltrexone formulations
| Formulation | Duration of Effect |
|---|---|
| Long-acting injection | |
| Depot injection | ∼35 d |
| Subcutaneous implant | |
| Go Medical Industries | 180 d |
| BioCorRx | 90 d |
| GP Healthcare SIA | 60–90 d |
Identification of Patients on Long-Acting Naltrexone Therapy
When caring for a critically ill patient requiring sedation and/or analgesia, determining whether the patient is receiving long-acting naltrexone is necessary to be able to provide optimized management. Different methods of identifying whether patients are receiving long-acting naltrexone therapy are summarized in Table 2.
Table 2.
Methods to identify patients who may be receiving long-acting naltrexone therapy
| Method | Details |
|---|---|
| Communication | Contact continuity providers, family, and friends who may have knowledge of treatments for opioid abuse disorder |
| Medical records | May be listed under “procedures” or “medication” records |
| Examination | If an implant has been placed, inspect and palpate the abdomen for a firm, subcutaneous object |
| Laboratory tests | Available commercially from Quest Diagnostics (urine) and NMS Labs (serum) |
Depending on the context, different clinical strategies may be useful. If the patient is unable to provide a history, the medical record can be interrogated for information about prior long-acting naltrexone therapy. Specifically, this information may be recorded under “medications” or in a “procedure note,” particularly for patients who have an SI mode of delivery. Connecting with continuity providers may be a useful resource to determine whether a patient has received or is receiving long-acting naltrexone treatment. We recommend querying family and friends who may have knowledge of treatments the patient has received for opioid use disorder.
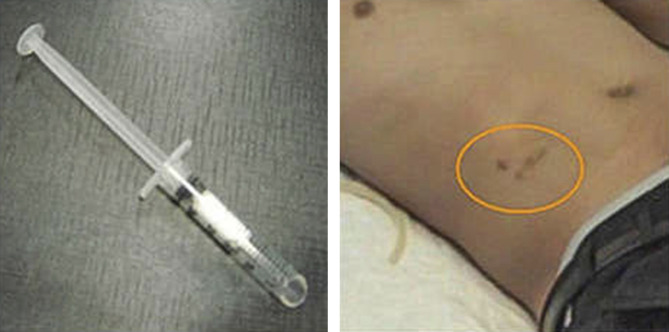
The physical examination may provide insights on whether the patient has had an SI placed. As pictured in Figure 1, the implant is typically inserted in the lower abdomen, will leave overlying skin changes, and can be palpated on examination (12).
Figure 1.
Long-acting naltrexone implant and post-procedure appearance in the right lower quadrant (as indicated by the orange circle). Image courtesy of Dr. George Woody and Dr. Evgeny Krupitsky.
Finally, there may be a role for laboratory testing. Importantly, naltrexone is not captured on common toxicology panels and will not cause a false-positive result on opiate assays. There are, however, commercially available laboratory tests for naltrexone and its major metabolite, 6-beta-naltrexol, which is produced in the liver and excreted in the urine (13). In the United States, Quest Diagnostics offers urine testing, and NMS Labs performs serum testing. At our institution, a Quest Diagnostics test for naltrexone is estimated to take approximately 4 days to return (R. Haspel, M.D., written communication, January 2020). Therefore, the clinical utility of testing for naltrexone concentrations is limited by the time it takes to receive the test result.
Important Pharmacologic Considerations for the Patient on Long-Acting Naltrexone
An important consequence of opioid use disorder is a reduced pain threshold and abnormal pain sensitivity (14). For patients receiving opioid antagonist therapy for opioid use disorder, it is unknown whether (and when) the pain threshold returns to normal. To achieve analgesia, opioid antagonism can be overcome by high doses of opioids in an acute setting. In rats, the dose of opioids needed to accomplish this may be 10–20 times the standard clinical doses (15). Importantly, however, long-term opioid receptor antagonism also leads to upregulation of opioid receptors (16). Therefore, if a patient ceases naltrexone exposure (or if the formulation is no longer active) and opioids are provided, the patient may be at risk of having an exaggerated response to opioid exposure. These pharmacologic consequences of long-term opioid antagonist therapy have important implications for the clinical care of critically ill patients.
Current Knowledge: Perioperative Analgesia for Patients on Long-Acting Naltrexone
The majority of the available literature about managing patients on long-acting naltrexone is limited and primarily involves patients in the perioperative environment. Specifically, there are three case reports describing the clinical details of four patients receiving long-acting naltrexone undergoing surgical procedures and three clinical reviews on this topic (12, 14, 17–20).
The case reports stress the importance of determining the timing of the most recent long-acting naltrexone dose, specifically the date of last intramuscular injection or the date that the implant was placed. If there has been sufficient duration between implant or injection and subsequent opioid exposure, standard doses of opioids will likely be effective. This is highlighted in a case report from O’Brien and colleagues in which a 28-year-old man suffering from multiple traumatic injuries required analgesia (12). Family informed staff of an existing naltrexone implant, which was palpable on examination. Despite the presence of the implant, standard doses of analgesic medications appeared effective. Later, it became apparent that the implant had been placed 3 months before presentation, with an expected duration of efficacy of 6 weeks. Providers noted that his pain was easily controlled, suggesting that opioid receptors had been upregulated in the presence of long-term blockade. However, because of upregulated opioid receptors, there is increased potential for unintentional overdose, so close clinical monitoring is necessary. Some suggest using ultra–short-acting opioids, such as remifentanil, in this setting (18), although others have argued against this approach because of the risk of acute tolerance and hyperalgesia (14).
A second report by the same authors details the case of a patient with an active naltrexone implant who required a surgical procedure (12). In this case, analgesia and sedation were challenging. The authors report using a combination of multiple analgesic agents, including nonopioid medications such as acetaminophen and ketamine.
All case reports and reviews in the perioperative setting emphasize the need for a multimodal approach to analgesia that incorporates adjunctive therapies whenever possible. This includes prescribing nonopioid analgesics, anxiolysis, and local anesthesia (e.g., local infiltration, nerve block, and epidural anesthesia) if appropriate. Nonopioid analgesics to consider include nonsteroidal antiinflammatory drugs and acetaminophen, which can be intravenous and/or scheduled if necessary (14). Ketamine can be considered via low-dose infusion or bolus. Providing gabapentin or pregabalin preoperatively has been recommended (20, 21). Glucocorticoids for antiemetic and antiinflammatory properties may be useful (14). Anxiolysis may be achieved with low-dose benzodiazepines or clonidine (12, 22). Of note, tramadol, a weak opioid agonist, may still retain some analgesic properties in the face of naltrexone, though other effects such as nausea and vomiting may be enhanced (23).
Considerations for the Critically Ill Patient
Although some components of perioperative care can be translated into the ICU setting, the literature about caring for critically ill patients receiving long-acting naltrexone therapy is sparse. There are no national guidelines regarding the optimal management of these patients, including no specific guidance from the Society of Critical Care Medicine, whose sedation guidelines were last updated in 2018 (24). There is a single review article that considers pain management in critically ill patients in light of the opioid epidemic, but this does not incorporate the impact of therapies for opioid use disorder (25). Furthermore, because there are limited data for the use of sedative and/or analgesic medications in critically ill patients with opioid use disorder, much of this is derived from accepted practice patterns. Despite the lack of evidence or clinical practice guidelines for the optimal management of patients receiving long-acting naltrexone therapy, there are important considerations for managing these critically ill patients, which we highlight in the remaining sections.
First, in contrast to the perioperative period for an elective surgery, the onset of critical illness cannot be scheduled or predicted, making it impossible for patients to discontinue long-acting therapy in advance. Second, there is a theoretical consideration that pharmacokinetic and pharmacodynamic properties of medications may be altered in the setting of critical illness (26). This is particularly pertinent in patients with renal and/or hepatic dysfunction, as naltrexone is contraindicated in acute hepatitis or liver failure and has not been studied in patients with moderate to severe renal impairment (27, 28). As such, circulating naltrexone concentrations may be increased in the setting of organ dysfunction commonly seen in critical illness. Importantly, because naltrexone is not metabolized through the cytochrome P450 pathway, there is less potential for medication interactions (13). Important drug interactions to consider are those with other opioid-containing medications, including antitussive and antidiarrheal agents.
Recommendations and details regarding specific medications in the critically ill patient are highlighted in Table 3.
Table 3.
Adjunctive medications for critically ill patients receiving long-acting naltrexone requiring analgesia or anxiolysis
| Medication | Indication | Considerations |
|---|---|---|
| Dexmedetomidine | Anxiolysis and analgesia | Dose may be limited by bradycardia and there is risk for withdrawal with prolonged infusion |
| Nonsteroidal antiinflammatory medications | Analgesia | Concern for adverse drug reactions and for acute renal injury |
| Acetaminophen | Analgesia | May need dose reduction with certain comorbidities, such as liver disease |
| Ketamine | Anxiolysis and analgesia | Limited evidence base in critically ill patients, and concern for increased risk of acute delirium in the ICU setting |
| Gabapentin, pregabalin, carbamazepine | Neuropathic pain and anxiolysis | Dose reduction required in renal failure and concern for medication interactions with carbamazepine |
| Dexamethasone | Antiinflammatory | Multiple adverse effects |
| Phenobarbital | Anxiolysis | Prolonged duration of action and potential for medication interactions via cytochrome P450 system |
| Benzodiazepines | Anxiolysis | Risk of delirium, prolonged ventilation, and paradoxical reactions |
| Propofol | Sedation and hypnosis | Commonly associated with hypotension |
Definition of abbreviation: ICU = intensive care unit.
Recommendations for the Care of Critically Ill Patients Receiving Long-Acting Naltrexone Therapy
Based on the available literature, consultation with pharmacists and clinical chemists, and our experience as critical care physicians, we propose the following recommendations, recognizing that as knowledge and experience increases, these may be subject to change.
To begin, early identification of patients receiving long-acting naltrexone, whether by history, physical examination, or medical records, is critical. Understanding the timing of the last naltrexone exposure is key to devising a strategy for analgesia and to predict potential risks of opioid administration. Laboratory testing may be helpful to confirm naltrexone exposure, although it does not provide information about the duration of exposure. Given a turnaround time of several days for naltrexone assays, however, decisions about optimal clinical management will have to be made without this information. When providing analgesia, providers should maximize nonopioid analgesia if possible (Table 3). If severe pain is present or sedation is required, opioids may be used, but they should be provided with substantial caution and diligent monitoring. We favor short-acting opioids initially to assess the patient’s response, given that patients who have received long-acting naltrexone may have heightened sensitivity and upregulation of opioid receptors. Because naltrexone competitively binds with the mu receptor, opioids with higher binding affinity, such as fentanyl and hydromorphone, may be preferable (29). If the patient is within 4 weeks of his or her last naltrexone injection, we recommend close monitoring with low-ratio nursing, waveform capnography, and consideration of placing a nasal trumpet to maintain airway patency for patients who are not already intubated. For patients with a secure airway in place who are receiving mechanical ventilation, we recommend using a controlled mode of ventilation to ensure adequate minute ventilation. For all patients receiving long-acting naltrexone therapy, we recommend frequent assessment of pain because of concerns about hyperalgesia. Providers should consider the use of objective methods for assessing pain, such as the Critical Care Pain Observation Tool (30).
If opioids are used for analgesia, withdrawing these medications can present challenges. Patients may require a taper of opioids to attenuate pain and withdrawal symptoms. We recommend partnering with pharmacists, addiction medicine specialists, and/or pain specialists when possible. Providers should maintain vigilance for withdrawal symptoms when reducing opiate medications. Tools such as the Clinical Opioid Withdrawal Scale, Clinical Institute Narcotic Assessment, and others can be used to monitor patients (31, 32).
Finally, providers must also consider the ramifications of opioid exposure in patients with opioid use disorder. Available data suggest that patients on medication-assisted therapy for opioid use disorder who are exposed to opioids for treatment of acute pain are not at an increased risk of relapse (33). However, critical illness and its sequelae, such as post-ICU syndrome, may place a patient at greater risk for opioid relapse, given the associated stress, anxiety, and possible prolonged recovery with resultant disability. Furthermore, the development of chronic pain after an ICU stay and opioid prescriptions upon discharge from the ICU are not uncommon (34–36), and uncontrolled pain has been cited as a major factor inciting relapse (37–39). With these potential triggers in mind, providers should ensure appropriate follow-up care and potential reinitiation of long-acting naltrexone therapy. Importantly, communication, coordination, and planning with continuity providers are key for the long-term health and safety of these patients.
Conclusions
As the opioid epidemic evolves and available therapies expand, ICU providers will undoubtedly care for patients receiving long-acting naltrexone therapy who require analgesia and sedation. In this review, we have highlighted the multiple long-acting naltrexone options available for patients at this time, with the goal of increasing the awareness of naltrexone implants, which may become more popular. Because long-acting naltrexone therapy impacts medications for sedation and analgesia frequently used in the ICU, updated clinical practice guidelines incorporating available data and expert consensus are needed. In the meantime, we encourage case reporting of the management of patients on long-acting naltrexone given the limited evidence regarding the optimal management of these patients in the critical care setting. Future studies of different analgesia strategies, data regarding actual risk of hypoventilation when opioids are reintroduced, and more complete understanding of the risk of relapse for patients treated with opioids while receiving long-acting naltrexone therapy should be considered.
Supplementary Material
Footnotes
Author Contributions: C.R.P. and J.B.R. contributed to the content and writing of this manuscript.
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Committee on Medication-Assisted Treatment for Opioid Use Disorder. Medications for opioid use disorder save lives. In: Leshner AI, Mancher M, editors. Washington, D.C.: National Academies Press; 2019. p. 174. [PubMed] [Google Scholar]
- 2.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan MA, Bisaga A, Pavlicova M, Carpenter KM, Choi CJ, Mishlen K, et al. A randomized trial comparing extended-release injectable suspension and oral naltrexone, both combined with behavioral therapy, for the treatment of opioid use disorder. Am J Psychiatry. 2019;176:129–137. doi: 10.1176/appi.ajp.2018.17070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- 6.Krupitsky E, Blokhina E, Zvartau E, Verbitskaya E, Lioznov D, Yaroslavtseva T, et al. Slow-release naltrexone implant versus oral naltrexone for improving treatment outcomes in people with HIV who are addicted to opioids: a double-blind, placebo-controlled, randomised trial. Lancet HIV. 2019;6:e221–e229. doi: 10.1016/S2352-3018(18)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin M, et al. Anhedonia, depression, anxiety, and craving in opiate dependent patients stabilized on oral naltrexone or an extended release naltrexone implant. Am J Drug Alcohol Abuse. 2016;42:614–620. doi: 10.1080/00952990.2016.1197231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin M, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973–981. doi: 10.1001/archgenpsychiatry.2012.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunøe N, Lobmaier P, Ngo H, Hulse G. Injectable and implantable sustained release naltrexone in the treatment of opioid addiction. Br J Clin Pharmacol. 2014;77:264–271. doi: 10.1111/bcp.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res. 2006;30:480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsen L, Christophersen AS, Frogopsahl G, Waal H, Mørland J. Plasma concentrations during naltrexone implant treatment of opiate-dependent patients. Br J Clin Pharmacol. 2004;58:219–222. doi: 10.1111/j.1365-2125.2004.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien B, Cody C. Analgesia and sedation in the presence of a naltrexone implant: a novel pharmacological challenge. Eur J Emerg Med. 2006;13:315–316. doi: 10.1097/00063110-200610000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Wall ME, Perez-Reyes M, Brine DR, Cook CE. Naltrexone disposition in man after subcutaneous administration. Drug Metab Dispos. 1984;12:677–682. [PubMed] [Google Scholar]
- 14.Coluzzi F, Bifulco F, Cuomo A, Dauri M, Leonardi C, Melotti RM, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017;13:1163–1173. doi: 10.2147/TCRM.S141332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean RL, Todtenkopf MS, Deaver DR, Arastu MF, Dong N, Reitano K, et al. Overriding the blockade of antinociceptive actions of opioids in rats treated with extended-release naltrexone. Pharmacol Biochem Behav. 2008;89:515–522. doi: 10.1016/j.pbb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Yoburn BC, Sierra V, Lutfy K. Chronic opioid antagonist treatment: assessment of receptor upregulation. Eur J Pharmacol. 1989;170:193–200. doi: 10.1016/0014-2999(89)90539-6. [DOI] [PubMed] [Google Scholar]
- 17.Israel JS, Poore SO. The clinical conundrum of perioperative pain management in patients with opioid dependence: lessons from two cases. Plast Reconstr Surg. 2013;131:657e–658e. doi: 10.1097/PRS.0b013e31828277bd. [DOI] [PubMed] [Google Scholar]
- 18.Curatolo C, Trinh M. Challenges in the perioperative management of the patient receiving extended-release naltrexone. A A Case Rep. 2014;3:142–144. doi: 10.1213/XAA.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 19.Bryson EO. The perioperative management of patients maintained on medications used to manage opioid addiction. Curr Opin Anaesthesiol. 2014;27:359–364. doi: 10.1097/ACO.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 20.Ward EN, Quaye AN-A, Wilens TE. Opioid use disorders: perioperative management of a special population. Anesth Analg. 2018;127:539–547. doi: 10.1213/ANE.0000000000003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stromer W, Michaeli K, Sandner-Kiesling A. Perioperative pain therapy in opioid abuse. Eur J Anaesthesiol. 2013;30:55–64. doi: 10.1097/EJA.0b013e32835b822b. [DOI] [PubMed] [Google Scholar]
- 22.Vickers AP, Jolly A. Naltrexone and problems in pain management. BMJ. 2006;332:132–133. doi: 10.1136/bmj.332.7534.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoops WW, Lofwall MR, Nuzzo PA, Craig LB, Siegel AJ, Walsh SL. Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology (Berl) 2012;223:427–438. doi: 10.1007/s00213-012-2739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 25.Erstad BL. Implications of the opioid epidemic for critical care practice. J Am Coll Clin Pharm. 2019;2:161–166. [Google Scholar]
- 26.Tse AHW, Ling L, Lee A, Joynt GM. Altered pharmacokinetics in prolonged infusions of sedatives and analgesics among adult critically ill patients: a systematic review. Clin Ther. 2018;40:1598–1615. doi: 10.1016/j.clinthera.2018.07.021. e2. [DOI] [PubMed] [Google Scholar]
- 27.Reus VI, Fochtmann LJ, Bukstein O, Eyler AE, Hilty DM, Horvitz-Lennon M, et al. The American psychiatric association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175:86–90. doi: 10.1176/appi.ajp.2017.1750101. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. Naltrexone prescribing information. Waltham, MA: Alkeremes; 2010 [updated 2020 May; accessed 2020 Jun 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021897s049lbl.pdf.
- 29.Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59:385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Gélinas C, Johnston C. Pain assessment in the critically ill ventilated adult: validation of the Critical-Care Pain Observation Tool and physiologic indicators. Clin J Pain. 2007;23:497–505. doi: 10.1097/AJP.0b013e31806a23fb. [DOI] [PubMed] [Google Scholar]
- 31.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 32.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 33.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battle CE, Lovett S, Hutchings H. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 2013;17:R101. doi: 10.1186/cc12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karamchandani K, Pyati S, Bryan W, Pepin M, Lehman EB, Krishnamoorthy V, et al. New persistent opioid use after postoperative intensive care in US veterans. JAMA Surg. 2019;154:778–780. doi: 10.1001/jamasurg.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;32:429–435. doi: 10.1177/0885066615618189. [DOI] [PubMed] [Google Scholar]
- 37.Karasz A, Zallman L, Berg K, Gourevitch M, Selwyn P, Arnsten JH. The experience of chronic severe pain in patients undergoing methadone maintenance treatment. J Pain Symptom Manage. 2004;28:517–525. doi: 10.1016/j.jpainsymman.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsui JI, Lira MC, Cheng DM, Winter MR, Alford DP, Liebschutz JM, et al. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend. 2016;166:26–31. doi: 10.1016/j.drugalcdep.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.