Abstract
Purpose: Adolescent and young adults (AYA) with cancer encounter many medical treatment decisions but may have variable desires for involvement in decision-making. This study describes the degree of decisional control AYA patients preferred in complex medical decisions.
Methods: A cross-sectional descriptive correlational design evaluated experienced AYA patients' decision-making role preferences using the Control Preference Scale and explored relationships in a proposed model of decision control.
Results: Overall, most patients preferred an “active collaborative” role (39%), where the patient prefers to make the final decision with input from the provider, or a “shared decision-making” role (34%), wherein the decision is jointly made between patient and provider. Oncology AYA patients tended to prefer a more passive role than nononcology AYA patients. Time since diagnosis also positively correlated with control preference, with patients preferring a more active level of decisional control as the number of days from diagnosis increased. While no other statistically significant relationships were found between factors put forth in the exploratory model and decision control, there were strong associations between the factors themselves that warrant future study.
Conclusion: The findings advance the knowledge of AYA preferences for decision-making involvement, enhancing our ability to identify patients at risk for low health care engagement and explore the consequences of limited or impaired decisional capacity. Future research might examine interventions to promote self-management skills and patient decisional role preferences, fulfilling the need to respect both the desire for decision-making involvement of some patients and the preference to defer to the expertise of providers for others.
Keywords: adolescent, young adult, decision-making, self-efficacy, oncology
Introduction
Shared medical decision-making has emerged as a prominent focus of patient-centered health care.1 While decision-making is admittedly different in pediatrics, further research is needed to explore factors that influence the implementation of shared decision-making (SDM) in children and adolescents.2 For adolescents and young adults (AYAs), participation in medical decisions presents unique challenges to patients, their family members, and health care providers. The adolescent and early adult development stages are marked by a period during which AYA patients are expected to become increasingly independent and engaged in their own self-management. Yet given the complexity of their illnesses, AYAs may be vulnerable to the burden of treatment decisions.
While many providers include AYAs in treatment discussions, our understanding about decision-making preferences in the AYA oncology population is limited.3 Previous research in adolescents with attention-deficit hyperactivity disorders showed that teens assume a more prominent role in medication management as they age, which suggests that adolescents and emerging adults are increasingly capable of self-management.4 Additionally, a recent systematic review of decision-making preferences for oncology fertility preservation showed that adolescents wish to participate in decisions about cancer treatment.5 There are notably barriers to pediatric participation in SDM, however.2,6
Still, pediatric SDM has been shown to reduce decisional conflict and improve knowledge, and thus, it is important to better understand decisional control preferences of AYA patients.6 Evidence is emerging that children with cancer do wish to be involved in decision-making as much as possible, and there is support from health care providers to allow for SDM in pediatric oncology.7 In this study, we aimed to identify AYA decision-making preferences in the context of cancer and other complex diagnoses.
SDM emerged as the hallmark of patient-centered health care.8 Unlike previous models, SDM involves a partnership between patient and provider to reach treatment decisions that best fit the individual patient. This requires a balance between paternalism and consumerism.9 Although evidence suggests improved outcomes using SDM, such as increased adherence and patient satisfaction, SDM is not consistently practiced.9–11 Perhaps this discrepancy can be explained by wide-ranging preferences: some individuals preferred to have active roles in medical decision-making, whereas others preferred to defer to health care providers.10,12 The dearth of empirical evidence in AYA decisional control preference may contribute to the varied degrees of engagement of AYA patients in SDM in clinical practice.
There are also ethical considerations at play for adolescents younger than 18 years who do not have the legal authority to consent for medical care.12,13 Teens are still developing a sense of decisional autonomy that relies on the interplay between factors like the cognitive and psychosocial abilities of the adolescent and the readiness of parents to allow their child to assume a more active role in decision-making and self-care.14 As health care autonomy develops, a shift occurs from parent-focused discussions to a collaborative approach with parent and child, with increasing decisional authority given to the child when developmentally and situationally appropriate.14–16
There is a growing interest in AYA decisional preferences, but additional research is needed to further evaluate issues of decisional autonomy, independence, and supportive needs in AYA patients.17–19 The purpose of this study was to describe decisional control preferences of a previously understudied population and explore factors that may impact desired levels of decisional control for AYAs with cancer and other complex medical conditions.
Methods
This study used an observational cross-sectional design.
Setting and participants
A convenience sample was recruited of AYA patients who received treatment in pediatric hematology/oncology clinics at an academic children's hospital in the Midwestern United States. The study sample included patients between 14 and 25 years with varying oncology and nononcology diagnoses. Patients who were cognitively intact, postinduction chemotherapy or within 12 months of treatment completion in the case of oncology patients, and clinically stable at the time of enrollment were eligible. Oncology diagnoses included leukemia, lymphoma, sarcoma, and other solid organ tumors. Nononcology diagnoses were sickle cell anemia and other chronic anemias, idiopathic thrombocytopenic purpura, and inflammatory bowel diseases (e.g., Crohn's disease and ulcerative colitis). Only English-speaking patients were recruited for participation because measurement instruments were not available in other languages. Written informed consent was obtained from a parent or guardian of participants younger than 18 years; minors provided written informed assent to participate. Participants 18 years or older at the time of enrollment provided written informed consent independently. The study was approved by our center's institutional review board.
Data collection
Data were collected with several previously validated survey instruments and chart extraction. Participants were compensated for their time at the completion of the study. Data were collected with a tablet computer and securely stored electronically in an encrypted database. The following demographic data were collected: age, gender, race, education level, and annual household income. In addition, medical data included diagnosis type, dichotomized to oncology or non-oncology, and time since initial diagnosis in days.
Decisional control preference was measured using the Control Preference Scale (CPS), which has been used in multiple populations, including adolescents, with high validity and reliability.20 We used the CPS ordinal scale to identify a decision role preference from five options: (1) “active role”—I prefer to make the treatment decisions on my own; (2) “active collaboration”—I prefer to make the treatment decision after hearing the physician's opinion; (3) “shared decision-making”—I prefer to make the treatment decision together with the physician; (4) “passive collaboration”—I prefer the physician to make the treatment decision after talking to me; (5) “passive role”—I prefer the physician to make the decision on his/her own.3,21,22
Patient experience with SDM was measured using the three-item CollaboRATE tool. This instrument was developed in response to a need for a quick method to measure SDM in a clinical setting.23 CollaboRATE captures three dimensions of SDM in both patient- and parent-report versions: (1) adequate explanation of health issue, (2) eliciting preferences for decisions, and (3) integrating these preferences in the decision-making process.23 The tool was recently developed using a representative adult sample in the United States and demonstrated excellent concurrent validity with established measurement tools.23
Self-efficacy for decision-making was measured using the Decision Self-Efficacy Scale, which is an 11-item tool with a 5-point scale, ranging from “not at all confident” (0) to “very confident” (4) to measure respondent's confidence in his/her decision-making abilities.24 To produce a score that is more readily interpreted, the individual item scores were summed, divided by 11, and multiplied by 25.25 Total scores range from 0 to 100, with higher scores indicating greater self-efficacy for decision-making.25 The Decision Self-Efficacy Scale has shown adequate internal reliability, Cronbach's alpha ranging from 0.78 to 0.92 in previous studies26 but has not yet been used in an adolescent population.
The Adolescent Self-Regulatory Inventory (ASRI) was used to measure patient self-regulatory skills, an attribute that is an important marker of cognitive development in adolescence and a component of developing decision-making skills. It is a 36-item instrument with 5-point Likert patient-reported responses ranging from “Not at all true for me” to “Really true for me” that was developed to capture both short- and long-term domains of self-regulation.27 Confirmatory factor analysis demonstrated satisfactory internal validity of a two-factor solution.27 Previous psychometric analysis was conducted in healthy adolescents (aged 11 to 17 years) and their parents and revealed a high degree of internal consistency for the scale (Cronbach's α = 0.70–0.82).27 Construct validity was previously evaluated by the concurrent administration of an established measurement tool (Self Regulation Questionnaire, see Novak and Clayton, 2001).28 Based on these sound psychometric properties, we elected to use the ASRI alone to measure self-regulatory skills.
Perceived autonomy was measured by the Health Care Climate Questionnaire (HCCQ), a tool that measures the extent to which a health care provider supports patient autonomy.29 A short form is available in a 6-item, 7-point Likert response scale and was used here to reduce subject response burden. The HCCQ has been used in an adolescent population to evaluate the effects of autonomy support on smoking behaviors.30 More recently, the short form HCCQ was used to assess perceived autonomy support in women with breast cancer (age range: 20–79 years) and found to have high internal consistency in that population (Cronbach α = 0.93).31
Social support was operationalized with the Multidimensional Scale of Perceived Social Support (MSPSS).32 The MSPSS is a 12-item scale that measures perceived social support from family, peers, and significant others. Originally developed in a population of college undergraduates, the MSPSS has since been widely used in various populations, including adolescents, with strong internal consistency for both the total score (Cronbach α = 0.93) and the three subscales (Cronbach α = 0.89–0.91).33 This tool was selected to measure the multiple dimensions of support an AYA with cancer may experience during and after therapy, which may influence the decision-making confidence and role preference.
Data analysis
Descriptive statistics were used to assess frequencies, variability, and percentages, and central tendencies of the data were evaluated by means, and standard deviations (SDs). Bivariate relationships were examined using Pearson correlations, Kendall's tau-b correlation coefficients, and Fisher's exact test of independence. Differences between groups were examined using independent samples t-tests, Mann–Whitney U tests, one-way ANOVA, and Kruskal–Wallis tests. After evaluating bivariate relationships of the variables within an exploratory conceptual model, linear regression was used to explore the predictive associations between self-efficacy for decision-making, self-regulatory skills, perceived autonomy support, social support, and medical factors in a revised conceptual model for shared decisions.
Results
Over a period from October 2016 to December 2017, 47 subjects enrolled in the study (consent rate of 77%), but 1 subject dropped out after completing the informed consent and enrollment procedure due to a time conflict and was lost to follow-up. Fourteen AYA patients declined participation in the study, but there were no statistically significant differences noted in demographic or medical characteristics between these individuals and those who participated. On average, the total amount of time to complete the study (including enrollment and all survey items) was 25 minutes. Response burden and fatigue were minimized by instructing participants to skip any questions and/or stop at any time.
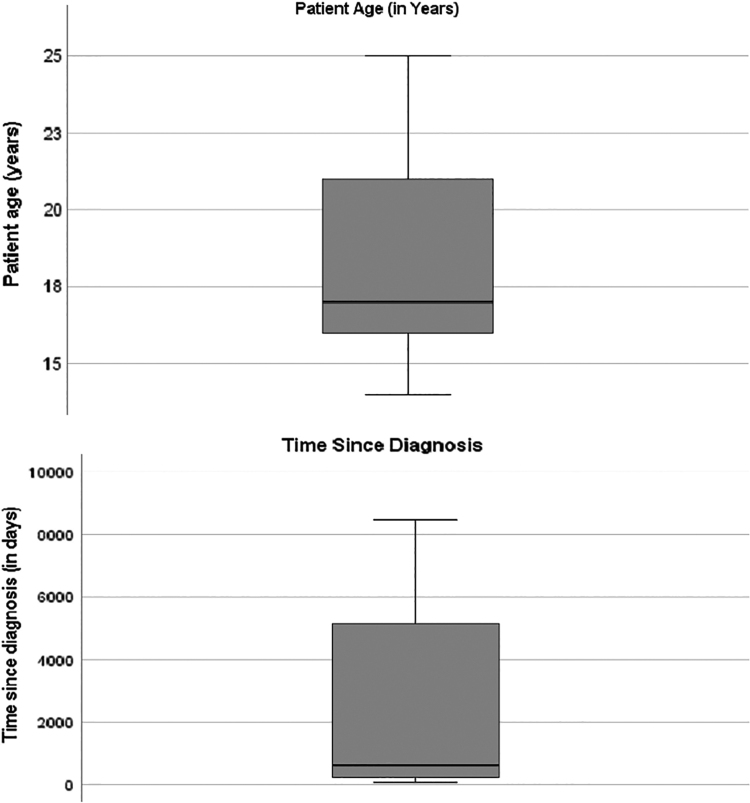
Table 1 describes the demographic and medical characteristics of the sample. The average age of participants was 18.2 years (±3.2 years), and the majority were white (56.5%) and female (60.9%). Notably, the mean time since diagnosis was around 2 years (646 days), but there is a wide range (68–8462 days) since individuals with nononcology diagnoses were included in the sample. Overall, this sample is made up of AYA patients who are experienced with their diagnosis. (Fig. 1) illustrates the distribution of age and time since diagnosis based on nononcology versus oncology patients. For further analyses, medical diagnosis was dichotomized to oncological conditions (n = 22) and nononcology diseases (n = 24). The range, mean, SDs, and reliability of study variables are described in Table 2. Overall, instrument reliability was adequate in the sample (Cronbach's α >0.80)
Table 1.
Sample Characteristics (N = 46)
| Variable | n (%) |
|---|---|
| Age, years | |
| Mean (SD) | 18.2 (3.2) |
| Median (range) | 17.0 (14–25) |
| Gender | |
| Female | 28 (60.9) |
| Male | 18 (39.1) |
| Race | |
| Asian | 2 (4.3) |
| Black or African American | 16 (34.8) |
| White | 26 (56.5) |
| Biracial | 2 (4.3) |
| Level of education | |
| Less than high school | 29 (63.0) |
| High school or equivalent | 16 (34.8) |
| College degree or higher | 1 (2.2) |
| Primary living arrangement (n = 45)a | |
| With parent(s) | 39 (86.7)a |
| Other | 6 (13.3)a |
| Currently employed | |
| Yes | 13 (28.3) |
| No | 33 (71.7) |
| Annual household income (n = 12)a | |
| <$20,000 | 9 (75.0)a |
| $20,000–$50,000 | 1 (8.3)a |
| >$50,000 | 2 (16.7)a |
| Medical diagnosis | |
| Oncology | 22 (47.8) |
| Nononcology | 24 (52.2) |
| Time since diagnosis, days | |
| Median (range) | 646 (68–8462) |
Indicates missing data for variable, sample size provided in parentheses.
SD, standard deviation.
FIG. 1.
Patient age (in years) and time since diagnosis (in days) displayed in quartiles.
Table 2.
Summary of Participants Survey Outcomes (N = 46)
| Possible range | Actual range | Mean | SD | No. of items | Cronbach's α | |
|---|---|---|---|---|---|---|
| DSES | 0–100 | 0–100 | 82.4 | 19.4 | 11 | 0.93 |
| ASRI | 1–5 | 2.25–4.34 | 3.42 | 0.42 | 36 | 0.82 |
| HCCQa | 1–7 | 4.33–7.00 | 6.27 | 1.13 | 6 | 0.93 |
| MSPSS | 1–7 | 3.17–7.00 | 5.78 | 0.98 | 12 | 0.93 |
| RATE | 0–9 | 4.67–9.00 | 7.85 | 1.29 | 3 | 0.86 |
Indicates n = 45 for this variable due to missing data.
ASRI, Adolescent Self-Regulatory Inventory; DSES, Decision Self-Efficacy Scale; HCCQ, Health Care Climate Questionnaire; MSPSS, Multidimensional Scale of Perceived Social Support; RATE, CollaboRATE.
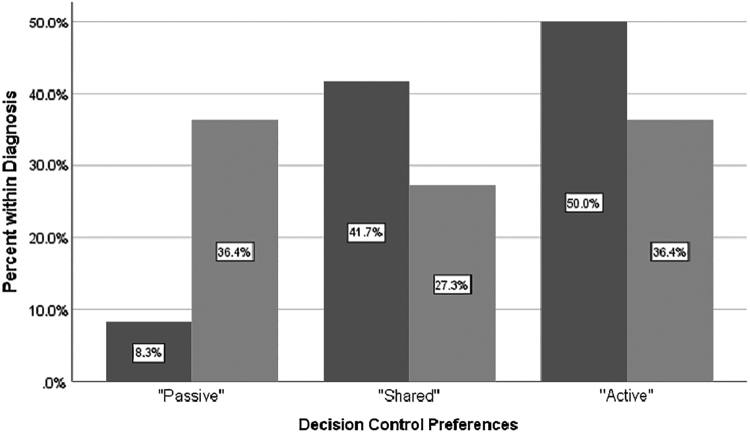
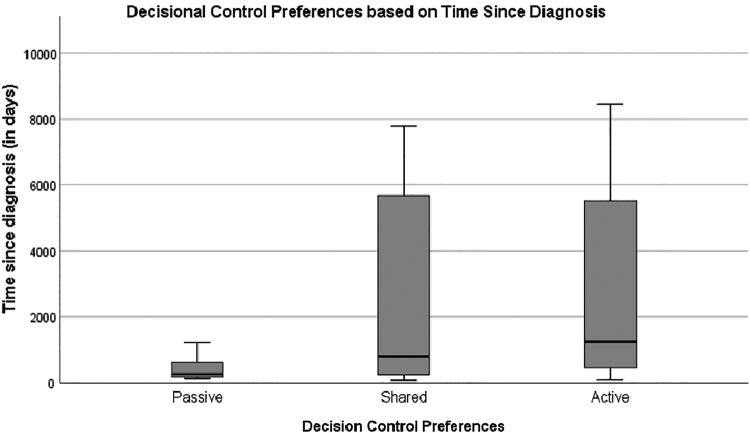
Overall, the majority of AYA patients preferred active collaborative (39%) or shared roles (34%) for decision-making compared with other roles. No statistically significant differences were noted between decision control preferences and age, gender, race, or education level in this sample. Participants with oncology diagnoses tended to prefer to desire less decisional control (“passive”), but the difference was not statistically significant, Fisher's exact = 5.17, p = 0.08 (Fig. 2). Importantly, nononcology AYAs have a greater time since diagnosis on average compared with oncology AYAs, which may influence their decisional control preferences. A one-way ANOVA was conducted to determine if time since diagnosis differed for groups with different decision control role preferences. Those who preferred less decisional control were diagnosed more recently than those who reported a shared or active role preference, Welch's F (2, 23.4) = 10.01, p = 0.001 (Fig. 3). There were no significant associations between decision control preferences and any other variable put forth in the exploratory model.
FIG. 2.
Decision control preferences by medical diagnosis (oncology vs. nononcology).  nononcology diagnosis;
nononcology diagnosis;  oncology diagnosis.
oncology diagnosis.
FIG. 3.
Decisional control preferences based on time since diagnosis (in days), illustrating that those preferred a “passive” role were diagnosed more recently than those who reported a “shared” or “active” role.
However, AYA patients reported high levels of SDM in the most recent medical encounter. The mean CollaboRATE score for the sample was 7.85 (SD = 1.29) (Table 2); though, the responses were highly skewed with 82.6% participants reporting scores 7 or greater. SDM mean scores were significantly higher in the oncology group than in the nononcology group [oncology: M = 8.36, SD = 0.78; nononcology: M = 7.38, SD = 1.49; t (35.6) = 2.85, p = 0.007]. Bivariate relationships between other demographic factors and CollaboRATE were not statistically significant.
When controlling for level of education, self-efficacy was significantly related to reported SDM experience (r = 0.38, p = 0.01). However, this was not true of self-efficacy based on age (r = 0.037, p = 0.81). When controlling for medical diagnosis, the greater the time from diagnosis, the greater the reported self-efficacy (r = 0.42, p = 0.004). Higher degrees of social support were related to higher levels of education (U = 339.5, z = 2.120, p = 0.34). Social support also varied based on medical diagnosis, with oncology groups reporting higher degrees of support (Mdn = 28.11) than nononcology groups (Mdn = 19.27; U = 349.5, z = 2.353, p = 0.02). AYA participants closer to the time of diagnosis tended to report higher degrees of social support, evidenced by an inverse correlation with social support scores that approached statistical significance (rτ = −0.201, p = 0.053). Not surprisingly, self-regulatory skills were positively associated with age (r = 0.379, p = 0.009) and level of education (<high school: Mdn = 19.55; ≥high school: Mdn = 30.24; U = 361.0, z = 2.608, p = 0.009). No other statistically significant relationships were noted.
Exploration of bivariate relationships between study variables
Although we sought to examine the relationships between decision control and several factors, we uncovered no significant relationships. However, Kendall's tau-b correlations were calculated to determine the relationships between other exploratory study variables, which are reported in Table 3. There was a positive relationship between decision self-efficacy and self-regulatory skills, (rτ = 0.266, p = 0.011) (Table 3). Similarly, a positive relationship between decision self-efficacy and perceived autonomy (rτ = 0.311, p = 0.006), self-efficacy and social support (rτ = 0.277, p = 0.009), and self-efficacy and perceived shared decisions (rτ = 0.407, p = 0.000) was discovered in this sample. Perceived autonomy support was also positively associated with perceived social support in general (rτ = 0.222, p = 0.034). Participants who reported higher degrees of SDM were statistically significantly more likely to report high degrees of social support (rτ = 0.518, p = 0.000).
Table 3.
Kendall's Tau Correlations (Rτ) Between Key Variables in Exploratory Model
| Decisional control | Self-efficacy for decisions | Self-regulatory skills | Perceived autonomy | Social support | |
|---|---|---|---|---|---|
| CPS | — | — | — | — | — |
| DSES | 0.09 | — | — | — | — |
| ASRI | −0.11 | 0.27a | — | — | — |
| HCCQ | −0.01 | 0.31b | 0.19c | — | — |
| MSPSS | −0.09 | 0.28b | 0.22a | 0.33b | — |
| RATE | 0.06 | 0.41b | 0.20c | 0.52b | 0.77b |
Indicates p < 0.05.
Indicates p < 0.01.
Indicates p < 0.10.
CPS, Control Preferences Scale.
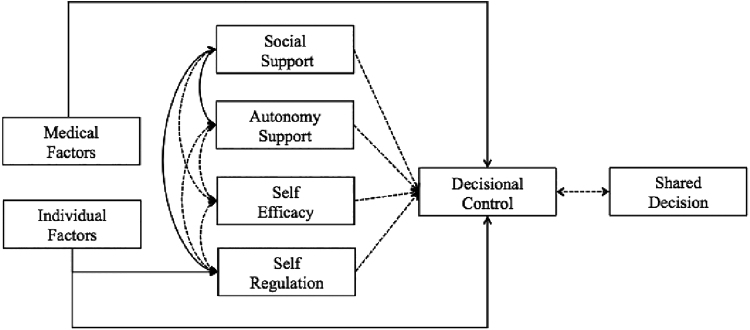
In addition to the associations with demographic characteristics described above, SDM was also significantly related to self-efficacy for decision-making (rτ = 0.41, p < 0.0005) and perceived autonomy support (rτ = 0.52, p < 0.0005). Thus, multivariate analyses were conducted based on a revised conceptual model for shared decisions (Fig. 4).
FIG. 4.
Conceptual model demonstrating relationships between variables.  , statistically significant relationship;
, statistically significant relationship;  , nonsignificant relationship.
, nonsignificant relationship.
Analysis of predictors of SDM
A multiple linear regression examined SDM from patient age, medical diagnosis, time since diagnosis, self-efficacy, self-regulation, autonomy support, and social support. The assumptions of linear regression were met. The multiple regression model was significant, F(7, 37) = 7.131, p < 0.0005, adj. R2 = 0.494. However, only medical diagnosis and autonomy support added significantly to the prediction.
Discussion
Consistent with previous research, the current study confirmed that decisional control role preferences are variable. Although this sample was underpowered to detect true statistical significance, the finding that oncology AYA patients tended to prefer more passive roles is consistent with previous literature in adult oncology populations. This might be explained by a desire to defer to the expertise of a pediatric oncologist when faced with treatment decisions about cancer. Alternatively, a preference for less decisional control for AYA oncology patients may be due to a perception that the decision is limited to receiving cancer treatment or electing to not treat a life-limiting condition. Further research is needed to expand our understanding and more accurately explain the differences for AYA cancer patients compared with nononcology patients. It is also important to note the significance of time since initial diagnosis and a varied degree of desire for decision control. As with other studies, the more recent a diagnosis, the less the desired decisional control. Being newly diagnosed with a complex medical condition requires time for education, acceptance, and finding a new normal. Thus, a desire to defer decision control to the expertise of the provider may shift over time as knowledge, self-management skill, and comfort increase. Future research should explore decision control preferences over time to examine if the role preference changes over time as individuals move further away from the time of diagnosis.
Unlike in adult populations, where younger age is related to higher degrees of decision control, age was not significantly related to role preference in this AYA sample. It is possible that this finding is due to a shift in health care culture that lends itself to consumer-driven participatory decision-making. However, it is important to note that while we did not find a difference in role preferences based on age, this may be due to a small heterogeneous sample underpowered to detect to statistically significant differences. Further research is warranted to determine if these findings hold up in a large sample of AYA oncology patients alone.
The current study revealed strong correlations between self-efficacy for decision-making and other factors related to AYA decision-making. Higher degrees of self-efficacy for decision-making were significantly related to higher degrees of self-regulatory skills, perceived autonomy, social support, and share decisions. Since previous studies have shown that shared decisions are associated with improved patient outcomes, nursing interventions targeting self-efficacy for decision-making to promote its relationship with SDM may impact overall outcomes for our AYA patients. Similarly, perceived social support from one's family and friends, and perceived autonomy support from one's health care providers, were positively related to each other and to SDM scores. This may be another target for nursing intervention to promote shared decisions through a supportive environment. Indeed, the importance of autonomy support as a predictor of shared decisions in AYA patients was reinforced by the findings of the regression analysis.
This study advanced the knowledge of AYA decision-making in hematology/oncology care and described patient preferences for decisional control. This will enhance our ability to identify AYAs at risk for low engagement in their treatment planning in the future and demonstrates a need for interventions to increase AYA perceived capacity and efficacy for decision-making. There is also an opportunity to explore the consequences of limited or impaired decisional capacity and low control preferences with future studies. In the case of too much involvement, there is potential for increased anxiety, pressure, and uncertainty, leading to poor decisions or decisional regret. However, with too little involvement in decision-making, there is risk for a violation of rights (e.g., autonomy), increased distrust of providers or surrogate decision-makers (e.g., parents), and lower treatment adherence. By establishing the decisional control preferences in this AYA population, this study enhances our ability to meet the individual needs of those who prefer more active or shared roles in decision-making compared with those who prefer to delegate decisions to providers. This study provides a basis for future intervention research to improve our ability to meet the needs of AYA decision-makers. The findings suggest that there may be opportunities to increase self-efficacy for decision-making or self-regulatory skills in AYAs, leading to improved adherence to treatment plans and SDM experience.
This study has several limitations. First, the generalizability of the findings is limited to similar tertiary pediatric hospitals. Although the sample was fairly representative of pediatric hematology/oncology patient populations, the sample was drawn from a Midwestern region of the United States and may not be generalized to other regions within the country, or other global populations. Second, the instruments used in this study were self-report tools. Although participants were informed that responses were anonymous and did not impact care received at the institution, it is possible response bias factored into the reported scores. Third, as discussed above, the sample was heterogenous (including oncology and nononcology diagnoses for comparison) and the study had many variables, potentially leading to an inability to find statistically significant differences that may exist in the population. Of note, this sample consisted of experienced AYA patients, that is, they were undergoing treatment or off-treatment, not newly diagnosed; inferences from these findings cannot be applied to newly diagnosed individuals, where the decision-making role preferences may be very different. Finally, this exploratory study was underpowered to detect any significant associations or group differences with respect to decision control preferences. Based on a linear regression analysis with a small effect size and alpha of 0.05, a post hoc power analysis in G*Power3.1.9.2 revealed that the observed power in this sample (N = 46) was 64.6%. Further analyses in a larger sample are advised.
Our findings support the need for assessment of individual role preference for decision-making involvement in AYAs, due to the varying degree of control preferences influenced by medical diagnosis and time since learning of the disease. The results serve as a foundation for future research on interventions aimed to increase SDM by supporting patient autonomy and taking into consideration differences between oncology and nononcology AYA patient populations.
Acknowledgments
The authors wish to acknowledge the patients, families, and staff of Angie Fowler's Cancer Institute at University Hospitals Cleveland Medical Center Rainbow Babies' and Children's Hospital for their participation in this research study. Additionally, the authors gratefully acknowledge Heather Hardin, PhD, RN, who provided editorial support to this work.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The research received financial support from the Alumni Association of Frances Payne Bolton School of Nursing at Case Western Reserve University. Additional financial support was obtained from the National Institute of Nursing Research, Palliative Care and Symptom Management in Adults with Advanced Disease, 5T32NR014213-02.
References
- 1. Barry MJ, Edgman-levitan S, Billingham V. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–1 [DOI] [PubMed] [Google Scholar]
- 2. Boland L, Graham ID, Légaré F, et al. Barriers and facilitators of pediatric shared decision-making: a systematic review. Implement Sci. 2019;14(1). DOI: 10.1186/s13012-018-0851-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knopf JM, Hornung RW, Slap GB, et al. Views of treatment decision making from adolescents with chronic illnesses and their parents: a pilot study. Health Expect. 2008;11(4):343–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinkman WB, Sherman SN, Zmitrovich AR, et al. In their own words: adolescent views on ADHD and their evolving role managing medication. Acad Pediatr. 2012;12(1):53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quinn GP, Murphy D, Knapp C, et al. Who decides? Decisiong making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health. 2011;49(4):337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wyatt KD, List B, Brinkman WB, et al. Shared decision making in pediatrics: a systematic review and meta-analysis. Acad Pediatr. 2015;15(6):573–583 [DOI] [PubMed] [Google Scholar]
- 7. Coyne I, O'Mathuna DP, Gibson F, et al. Interventions for promoting participation in shared decision-making for children with cancer. Cochrane Database Syst Rev. 2013;(6):CD008970. [DOI] [PubMed] [Google Scholar]
- 8. Godolphin W. Shared decision-making. Healthc Q. 2009;12:e186–90 [DOI] [PubMed] [Google Scholar]
- 9. Braddock CH, Edwards KA, Hasenberg NM, et al. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–20 [DOI] [PubMed] [Google Scholar]
- 10. Edbrooke-childs J, Jacob J, Argent R, et al. The relationship between child- and parent-reported shared decision making and child-, parent-, and clinician-reported treatment outcome in routinely collected child mental health services data. 2016. DOI: 10.1177/1359104515591226 [DOI] [PubMed] [Google Scholar]
- 11. Elwyn G, Edwards A, Wensing M, et al. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care. 2003;12(2):93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Degner LF, Sloan JA. Decision making during serious illness: what role do physicians really want to play? J Clin Ethics. 1992;45(9):941. [DOI] [PubMed] [Google Scholar]
- 13. Noone J. Concept analysis of decision making. Nurs Forum. 2002;37(3):21–32 [DOI] [PubMed] [Google Scholar]
- 14. Grootens-Wiegers P, Hein IM, van den Broek JM, de Vries MC. Medical decision-making in children and adolescents: developmental and neuroscientific aspects. BMC Pediatr 2017:1–10. DOI: 10.1186/s12887-017-0869-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sisk BA, Johnson L, Canavera K, et al. Ethical issues in the care of adolescent and young adult oncology patients. Pediatr Blood Cancer. 2019;66(5):e27608. [DOI] [PubMed] [Google Scholar]
- 16. Beacham BL, Deatrick JA. Health care autonomy in children with chronic conditions. Implications for self-care and family management. Nurs Clin North Am. 2013;48(2):305–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gormley-Fleming L, Campbell A. Factors involved in young people's decisions about their health care. Nurs Child Young People. 2011;23(9):19–22 [DOI] [PubMed] [Google Scholar]
- 18. Coyne I, Amory A, Kiernan G, Gibson F. Children's participation in shared decision-making: children, adolescents, parents and healthcare professionals' perspectives and experiences. Eur J Oncol Nurs. 2014;18(3):273–80 [DOI] [PubMed] [Google Scholar]
- 19. Day E, Jones L, Langner R, Bluebond-langner M. Current understanding of decision-making in adolescents with cancer: a narrative systematic review. 2016. DOI: 10.1177/0269216316648072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29(3):21–43 [PubMed] [Google Scholar]
- 21. Bruera D, Sweeney C, Calder K, et al. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol. 2001;11(1): 2883–5 [DOI] [PubMed] [Google Scholar]
- 22. Lipstein EA, Dodds CM, Lovell DJ, et al. Making decisions about chronic disease treatment: a comparison of parents and their adolescent children. Health Expect. 2016;19(3):716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barr PJ, Thompson R, Walsh T, et al. The psychometric properties of collaborate: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16(1). DOI: 10.2196/jmir.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bunn H, O'Connor AM. Validation of client decision-making instruments in the context of psychiatry. Canadian Journal of Nursing Research. 1996;28(3):13–27 [PubMed] [Google Scholar]
- 25. O'Connor AM. Decision self efficacy scale – user manual. Accessed January5, 2018 from: www.decisionaid.ohri.edu
- 26. Cranney A, O'Connor AM, Jacobsen MJ, et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Education and Counseling. 2002:47(3):245–55 [DOI] [PubMed] [Google Scholar]
- 27. Moilanen KL. The adolescent Self-Regulatory inventory: the development and validation of a questionnaire of short-term and long-term self-regulation. J Youth Adolesc. 2007;36(6):835–48 [Google Scholar]
- 28. Novak SP, Clayton RR. The influence of school environment and self-regulation on transitions between stages of cigarette smoking: a multilevel analysis. Health Psychology. 2001;20(3):196–207 [PubMed] [Google Scholar]
- 29. Williams GC, Deci E. Perceived Autonomy Support: the Climate Questionnaires The Learning Climate Questionnaire (LCQ). 1996:7–10. Accessed March31, 2018 from: www.selfdeterminationtheory.org/pas-learning-climate
- 30. Williams GC, Cox EM, Kouides R, Deci EL. Presenting the facts about smoking to adolescents: effects of an autonomy-supportive style. Arch Pediatr Adolesc Med. 1999;153(9):959–64 [DOI] [PubMed] [Google Scholar]
- 31. Shumway D, Griffith KA, Jagsi R, et al. Psychometric properties of a brief measure of autonomy support in breast cancer patients. BMC Med Inf Decis Mak. 2015;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. Journal of Personality Assessment. 1988;52(1):30–41 [DOI] [PubMed] [Google Scholar]
- 33. Canty-Mitchell J, Zimet GD. Psychometric properties of the multidimensional scale of perceived social support in urban adolescents. Am J Community Psychol. 2000;28(3):391–400 [DOI] [PubMed] [Google Scholar]