Abstract
Background: A female survival advantage in cutaneous melanoma has been long recognized. However, whether this extends across all age groups, with risk stratification using the latest prognostic staging system or in the current era of efficacious systemic therapies is unknown. Therefore, we evaluated whether sex-based differences in melanoma survival persisted within a recent population-based patient cohort with consideration of these factors.
Materials and Methods: We identified stage II−IV cutaneous melanoma patients from 2010 to 2014 Surveillance, Epidemiology, and End Results cancer registries data. We recalculated stage per American Joint Committee on Cancer 8th edition guidelines. Cancer-specific survival (CSS) was estimated by using the Kaplan–Meier method and multivariable Cox proportional hazards regression.
Results: Of 16,807 patients (39.8% female), 8,990 were stage II, 4,826 stage III, and 2,991 stage IV at diagnosis. Unadjusted 3-/5-year CSS estimates for females versus males were 64.2% versus 59.7%, and 53.5% versus 49.9%, respectively, p ≤ 0.0001. Five-year CSS varied within each stage and across age strata of <45, 45 − 59, and ≥60 years. Within each stage, females <45 had better CSS than all other sex/age groups (p < 0.0001). In multivariable analysis of stage II/III patients, female sex, younger age, and lower mitotic index retained favorable CSS prognostic significance (p < 0.001).
Conclusions: Sex-based differences in melanoma survival persist in a contemporary patient cohort staged with the latest prognostic system. These data may guide decision marking regarding adjuvant therapy, highlight the importance of including sex as a pre-specified clinical trial variable, and suggest that investigation of underlying biologic mechanisms may drive discovery of biomarkers and therapeutic targets to improve patient care.
Keywords: melanoma, sex, females, survival, outcomes, AJCC staging
Introduction
A female survival superiority for cutaneous melanoma has been recognized for more than half a century.1 In 2018, an estimated 91,270 new cases of invasive melanoma were diagnosed in the United States, 55,150 (60.4%) in males and 36,120 (39.6%) in females.2 Further, among the 9,320 deaths attributable annually to melanoma, a disproportionately higher number (5,990 or 64.3%) will occur in males.
Prior studies have either not addressed3 or reported conflicting findings regarding the influence of patient age and stage of disease on sex differences in outcomes. Although some investigators have concluded that a survival advantage exists only for younger females,4−6 others have demonstrated persistence of this female survival advantage across all ages.7,8 Similarly, with respect to stage of disease, some have reported that the female survival advantage extends to all stages of disease9−11 whereas others have observed this only in earlier stage disease,8,10,11 or have not found a female survival advantage among those with stage IV disease.12
More recently, sex has been shown to remain an independent prognostic factor even with consideration of other known prognostic variables, such as primary tumor mitotic rate.7 Although prior studies confirm sex as an independent prognostic factor for melanoma-related survival, the effect of patient age at diagnosis, as a possible surrogate for a hormonal or immunologic influence on outcomes, is unclear. In addition, sex differences in outcomes may vary with cancer stage at diagnosis irrespective of patient age. For example, it has been suggested that among patients enrolled in clinical trials, sex differences diminish with more advanced disease stage.13
Thus, it remains unclear whether the female survival superiority in melanoma persists in a contemporary patient population, staged using the most current American Joint Committee on Cancer (AJCC) prognostic staging iteration that went into use on January 1, 2018, and treated in the current era of microstaging with sentinel lymph node surgery and efficacious systemic therapy with immune checkpoint inhibition and targeted agents. Therefore, the aim of this study was to evaluate the effect of female sex on survival within a modern patient cohort classified with the most current prognostic staging system and with consideration of other potentially relevant prognostic variables.
Materials and Methods
We evaluated the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) program set of cancer registries data from 2010 to 2014. This time period was selected as mitotic rate, a relevant prognostic marker,14−16 was included in the SEER data set beginning in 2010. We identified all cutaneous melanoma patients (n = 223,501). We then excluded patients with stage I and in situ disease (n = 119,682) given their relatively high rate of survival, as our aim was to assess differences in survival outcomes in a recent patient cohort. We also excluded those with a prior cancer or a new cancer diagnosis subsequent to the index melanoma diagnosis (n = 75,569), patients diagnosed at autopsy (n = 286), and those with missing variables of interest (thickness or ulceration or AJCC T stage, n = 11,126; age, n = 31) to arrive at our study cohort of 16,807 stage II−IV cutaneous melanoma patients. Stage was recalculated by using the AJCC 8th edition staging system.17
Cancer-specific survival (CSS) was estimated by using the Kaplan–Meier method. Demographic and tumor variables were compared by sex using chi-squared and Wilcoxon rank-sum tests. Multivariable Cox proportional hazards regression was used to analyze the effect of critical variables on outcomes. We tested for interactions between our exposure variables (age, stage, and sex) on survival. Analyses were performed by using SAS software, version 9.4 (SAS, Inc., Cary, NC).
Results
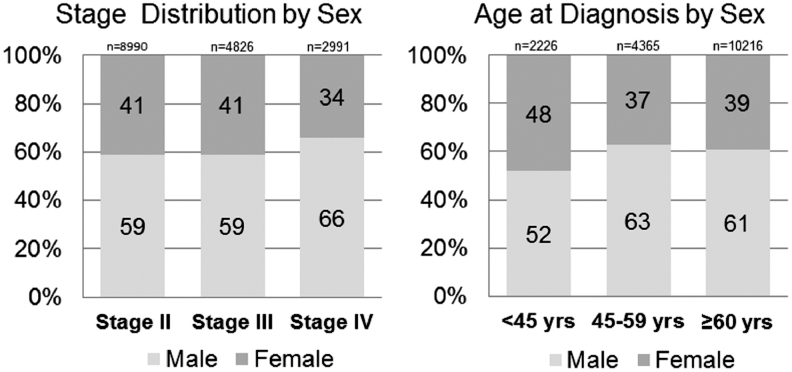
Among 16,807 patients, the majority, 10,111 (60.2%), were male. Median patient age was 64 years for both males and females. We constructed age strata to approximate premenopause, perimenopause, and postmenopause as age <45, age 45 − 59, and age 60 and older, respectively. Distribution of sex by stage and age strata is shown in Figure 1. The distribution of demographic, clinical, and tumor features by sex is summarized in Table 1. Statistically significant differences between male and females were observed with respect to race, anatomic location of the primary tumor, tumor thickness, T classification, mitotic rate, and ulceration. The proportion of male patients was substantially greater than the proportion of female patients with thin (T1) primary tumors. The proportion of female patients presenting with N3 disease was actually higher than for male patients, whereas the proportion classified as node-negative (N0 disease) was similar.
FIG. 1.
Proportion of patients stratified by sex within stage and age strata. Among the 16,807 Surveillance, Epidemiology and End Results (SEER) melanoma patients analyzed, the p-value was <0.0001 for both comparisons.
Table 1.
Demographic and Tumor Variables Stratified by Sex for 16,807 Cutaneous Melanoma Patients
| Male (n = 10,111) | Female (n = 6,696) | pa | |
|---|---|---|---|
| Age, median (interquartile range) | 64 (54 − 74) | 64 (51 − 78) | 0.0706 |
| Race, n (%) | <0.0001 | ||
| Non-Hispanic White | 9,607 (95.0) | 6,214 (92.8) | |
| Hispanic | 162 (1.6) | 219 (3.3) | |
| Non-Hispanic Black | 30 (0.3) | 29 (0.4) | |
| Non-Hispanic Asian or Pacific Islander | 161 (1.6) | 127 (1.9) | |
| Other or unknown | 151 (1.5) | 107 (1.6) | |
| Anatomic location (primary tumor), n (%) | <0.0001 | ||
| Head/neck | 2,413 (23.9) | 860 (12.8) | |
| Trunk | 3,247 (32.1) | 2,242 (33.5) | |
| Upper extremity | 1,933 (19.1) | 1,388 (20.7) | |
| Lower extremity | 1,109 (11.0) | 1,566 (23.4) | |
| Other or not specified | 1,409 (13.9) | 640 (9.6) | |
| Tumor thickness (Breslow depth), n (%) | <0.0001 | ||
| ≤1.0 mm | 1,171 (11.6) | 567 (8.5) | |
| 1.01 − 2.00 mm | 1,441 (14.3) | 1,032 (15.4) | |
| 2.01 − 4.00 mm | 3,297 (32.6) | 2,010 (30.0) | |
| ≥4.00 mm | 2,506 (24.8) | 1,508 (22.5) | |
| Missing | 1,696 (16.8) | 1,579 (23.6) | |
| T classification, n (%) | <0.0001 | ||
| T0 (unknown primary) | 937 (9.3) | 392 (5.9) | |
| T1 | 596 (5.9) | 317 (4.7) | |
| T2 | 1,627 (16.1) | 1,427 (21.3) | |
| T3 | 3,644 (36.0) | 2,524 (37.7) | |
| T4 | 2,686 (26.6) | 1,705 (25.5) | |
| Tx (T stage missing) | 621 (6.1) | 331 (4.9) | |
| Ulceration (primary tumor), n (%) | <0.0001 | ||
| Present | 4,189 (41.4) | 2,607 (38.9) | |
| Absent | 3,649 (36.1) | 2,268 (33.9) | |
| Unknown | 2,273 (22.5) | 1,821 (27.2) | |
| Mitotic rate, mitoses/mm2 (primary tumor), n (%) | <0.0001 | ||
| 0, <1 | 563 (5.6) | 392 (5.9) | |
| 1 | 902 (8.9) | 621 (9.3) | |
| ≥2 | 4,997 (49.4) | 3,058 (45.7) | |
| Unknown | 3,649 (36.1) | 2,625 (39.2) | |
| Pathologic lymph node status, n (%) | 0.0050 | ||
| Node negative | 5,885 (58.2) | 3,915 (58.5) | |
| Node positive | 3,412 (33.8) | 2,330 (34.8) | |
| No pathologic nodal staging | 814 (8.1) | 451 (6.7) | |
| N classification, n (%) | <0.0001 | ||
| N0 | 5,885 (58.2) | 3.915 (58.5) | |
| N1 | 1,574 (15.6) | 1,022 (15.3) | |
| N2 | 982 (9.7) | 547 (8.2) | |
| N3 | 856 (8.5) | 761 (11.4) | |
| NX (not staged) | 814 (8.1) | 451 (6.7) | |
p Value for comparisons between male and female patients for each variable.
Unadjusted 3- and 5-year CSS estimates for females versus males were 64.2% versus 59.7%, and 53.5% versus 49.9%, respectively (p ≤ 0.0001). Unadjusted 5-year CSS estimates within overall stage alone for females versus males were 76.2% versus 74.5% for stage II patients (p = 0.11), 61.0% versus 56.8% for stage III patients (p = 0.64) and 24.6% versus 17.9% for stage IV patients (p = 0.04).
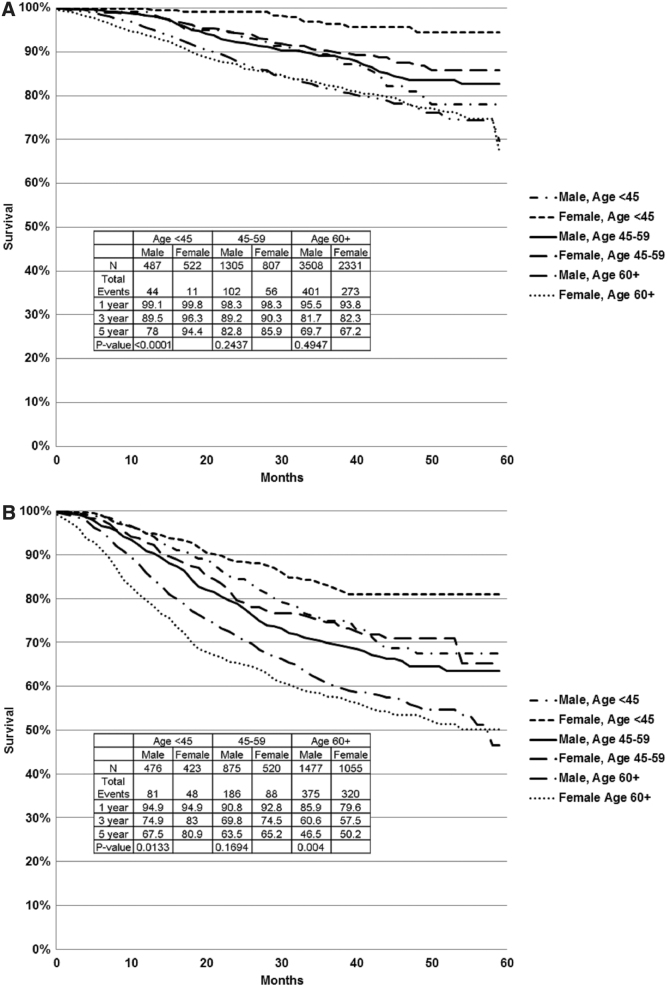
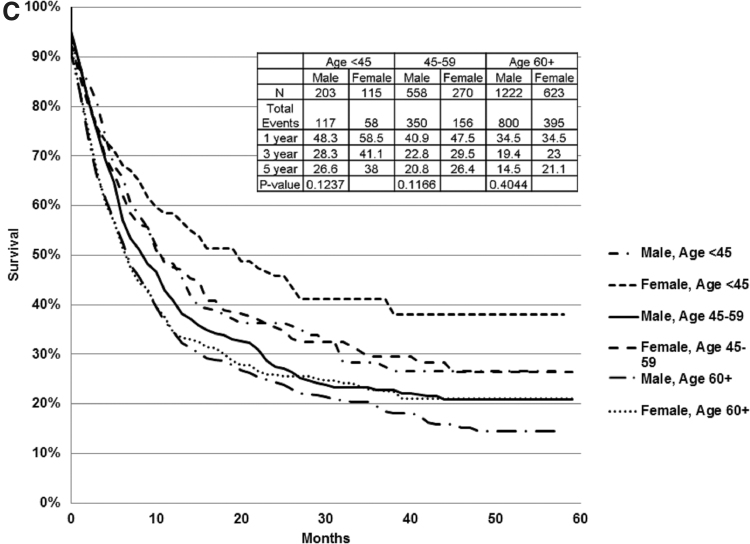
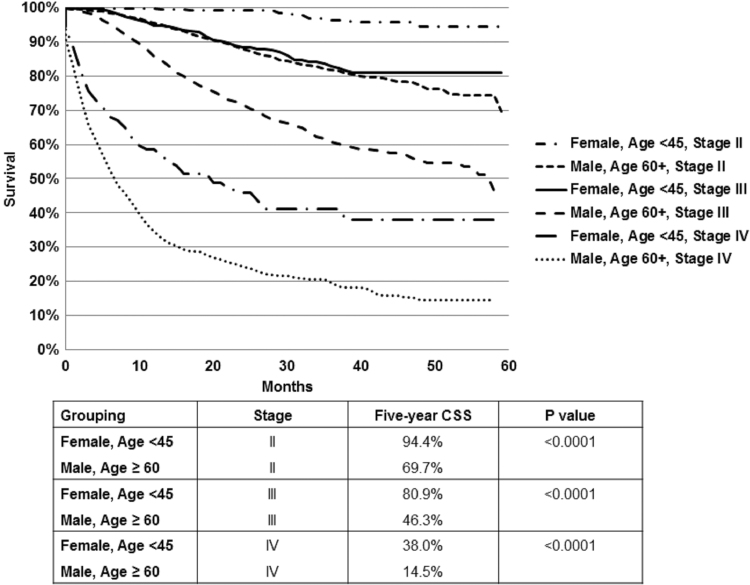
Further evaluation of CSS estimates within age strata of <45, 45 − 59, and ≥60 years within each stage grouping identified statistically significant differences in several of these subgroups. Specifically, females age <45 years had superior 5-year CSS compared with same age males for stage II (Fig. 2A) and III (Fig. 2B) disease, as well as for females and males in other age strata, whereas females age 45 − 59 had no statistically significant CSS advantage over same age males, and females age ≥60 had better CSS than same age males only for stage III disease (Fig. 2B). Within age strata comparisons for stage IV, patients did not reach statistical significance (Fig. 2C). Within stage III substages, females <45 with stage IIIa disease and females <45 and ≥60 with stage IIIc/d disease had statistically significantly better CSS than same age males, 5-year estimates 95.4% versus 80.4% (p = 0.05), 74.5% versus 60.1% (p = 0.05) and 41.2% versus 21.4% (p = 0.001), respectively. Further, it was clear that within each stage females age <45 had far superior survival compared with any other patient population, as depicted in Figure 3.
FIG. 2.
CSS within each stage, stratified by sex and age. Within each stage, females age <45 had superior CSS to same age group males and both females and males of other (older) age strata. (A) Stage II: Within age strata, female CSS was superior to males, but it reached statistical significance only for patients age <45 (p < 0.0001). (B) Stage III: Within age strata, the female CSS superiority was statistically significant for patients age <45 (p = 0.01) and ≥60 years (p = 0.004). (C) Stage IV: Within age strata, female CSS was superior to males, but it did not achieve statistical significance in subgroup analysis within age strata. CSS, cancer-specific survival.
FIG. 3.
CSS estimates by stage for females age <45 versus males age ≥60. Within each stage grouping, females age <45 had the best outcomes, which was significantly better than the oldest males diagnosed at an earlier stage (p < 0.0001).
Multivariable analysis with consideration of the primary tumor mitotic rate for stage II and III patients in the subset for whom this variable was recorded (71.4%) was performed with the covariates of age, sex, and mitotic rate. This showed a persistent effect of female sex, mitotic rate, and oldest versus youngest age strata on CSS (Table 2). On testing for interactions, we found significant interactions between age strata and sex for stage II (p value for interaction terms <0.01), but not for stage III patients (p value for interaction term = 0.38). Therefore, we stratified our models by stage and age to investigate the impact of female sex on CSS for each group (Tables 3 and 4).
Table 2.
Multivariable Analysis of Cancer-Specific Survival in Stage II and III Melanoma Patients
| Variable | HR (CIs) | p |
|---|---|---|
| Age 45–59 vs. <45 years | 1.16 (0.94 − 1.43) | 0.1582 |
| Age ≥60 vs. <45 years | 1.66 (1.38 − 1.99) | <0.0001 |
| Female sex | 0.76 (0.67 − 0.86) | <0.0001 |
| Mitotic rate >1 vs. 0 or ≤1 | 1.58 (1.25 − 1.98) | <0.0001 |
CI, confidence interval; HR, hazard ratio.
Table 3.
Stage II: Effect of Patient Sex on Cancer-Specific Survival, Stratified by Agea
| Sex | HR for female vs. male sex (CIs) | p |
|---|---|---|
| In patients <45 years | 0.18 (0.08 − 0.42) | <0.0001 |
| In patients age 45–59 | 0.72 (0.48 − 1.10) | 0.1317 |
| In patients age ≥60 | 0.96 (0.75 − 1.17) | 0.6755 |
p Value for interaction <0.01; models adjusted for anatomic site and mitotic rate category.
Table 4.
Stage III: Effect of Patient Sex on Cancer-Specific Survival, Stratified by Agea
| Sex | HR for female vs. male sex (CIs) | p |
|---|---|---|
| In patients <45 years | 0.67 (0.43 − 1.03) | 0.0647 |
| In patients age 45–59 | 0.98 (0.69 − 1.38) | 0.8929 |
| In patients age ≥60 | 0.83 (0.65 − 1.06) | 0.1376 |
p Value for interaction = 0.38; models adjusted for anatomic site and mitotic rate category.
Discussion
Using a modern patient cohort and the latest AJCC prognostic staging system for risk stratification, we found an overall significant effect of female sex and age on CSS for stage II, III, and IV melanoma patients. Within stages, sex differences existed, but they were influenced by age, largely driven by women age <45. The key findings of our study are (1) that there are female sex-specific survival advantages for melanoma patients across all stages of disease and (2) this effect is influenced by age. In fact, the most striking finding, that women <45 across all stages of disease had far superior survival to any other patient population, supports further investigation of the hypotheses that hormonal and/or age and sex-based immunologic differences influence melanoma outcomes.
Our findings with respect to patient age are supported by data from several previous studies. An early study evaluating National Cancer Data Base (NCDB) 1985–1988 data found superior overall survival for females age 45 and younger versus females age 55 and older and all ages of males across all stages of melanoma.4 A large single-institution retrospective review of stage I–III melanoma patients diagnosed between 1976 and 2001 found patient sex as a significant predictor of CSS, and that age, evaluated as age 65 and younger versus age >65, exerted a greater influence on CSS for females than for males.6 In fact, in subgroup analysis, sex had a significant effect on CSS only within the younger (≤65) age group. From this, the authors concluded that the survival superiority of females vanished after age 65. An expanded study from the same group, including patients diagnosed through 2008, analyzed sex differences in survival by age groups of 43 years and younger, 44–60, and age >60 and found that the female CSS survival advantage persisted to age 60.18 Further, analysis of 73,720 melanoma patients from the EUROCARE-4 database found the relative excess risk of death for females versus males to be the lowest for melanoma (vs. 25 other cancer types analyzed) and more significant for younger females age 15–54 (hazard ratio [HR]: 0.4) than older females age 55–99 (HR: 0.6).5 A prior SEER study, evaluating 1992–2011 data, also showed a female survival advantage across all age groups among melanoma patients with stage I–III disease, but it found a more marked effect of sex on cancer outcomes for younger patients (age 18–54).8
On the other hand, analysis of patients enrolled in nine European Organization for Research and Treatment of Cancer (EORTC) clinical trials showed a female CSS advantage overall and across all age groups.7,13 Also in contrast to our findings, two other reports from cancer registry data skewed toward early stage disease (<10% of patients presented with stage III or IV disease) found no significant interaction between sex and age evaluated as a continuous variable nor within age strata.9,19 Another retrospective single-institutional study of stage III melanoma patients treated between 1970 and 2013 found that the female CSS advantage persisted only for the first 3 years after diagnosis whereas the favorable influence of younger age persisted throughout the conditional survival time points evaluated.20
Although the influence of disease stage on the female survival advantage in melanoma has not been specifically addressed in many studies, a few investigations have also observed that the female survival advantage extends to all stages of disease. Registry studies from both the Netherlands and Australia found a similar protective effect of female sex on melanoma outcomes among patients with local, regional, and metastatic melanoma.9,21 Pooled analysis of more than 1,300 patients enrolled in eight ECOG trials also showed a female survival advantage for patients with stage IV disease.22 In contrast, a SEER study found a female survival advantage across earlier disease stages but not for patients with stage IV disease.8 Additional studies have noted a lack of or diminishing survival benefit for female patients with stage IV disease. Among patients enrolled in 15 Southwest Oncology Group (SWOG) trials between 1982 and 1996, there was no difference in outcomes for female versus male patients whereas a post hoc analysis of patients enrolled in two EORTC clinical trials reported that the female sex advantage decreased with higher metastatic stage.12,13
Possible explanations for these prior contradictory reports include differences in study design, study year (influencing both staging and treatment), and selection of prognostic variables. Prior studies have included analyses performed on differing patient populations such as those enrolled in clinical trials, regional and national registries, as well as single-institution tertiary referral centers, each with inherent patient selection biases. We suggest that this study may overcome some of these biases and provide accurate and clinically relevant information based on analysis of a contemporary patient cohort re-staged using the current AJCC prognostic system and consideration of tumor mitotic rate. Key differences highlighted in this newest staging iteration include classification changes across all stages of disease to refine risk stratification and improve estimates of prognosis.16
Past reasons for sex-based outcome disparities for melanoma patients have included differences in health care behaviors regarding timeliness of seeking medical care, pursuit of routine medical care, and different attitudes toward sun-seeking activities and prevention with males who are less likely to engage in preventive behaviors.23,24 Differences in the mode of detection of melanoma are also reported, with females performing skin surveillance and self-detecting their primary tumors more frequently than males.25
Other possible reasons for the reported variability in association of sex and melanoma outcomes across age and stage groups include exclusion of what we now recognize as relevant prognostic factors such as tumor thickness, mitotic rate, ulceration, and current prognostic stage, as well as patient and treatment factors. Further, the patient populations studied also have been variable, many retrospective and single institution, whereas the prospective study has been limited to post hoc analyses of patients enrolled in clinical trials designed to address other questions. Despite these differences, there is general agreement across multiple studies that the relatively favorable outcomes for female melanoma patients are not fully explained by sex differences in preventive and health care behavior.19,24 Taken together, these data suggest a biological basis for this phenomenon.
More recently, it has become clear that the female survival benefit remains even with consideration of established and relatively recently identified prognostic indicators such as mitotic rate, ulceration, and sentinel lymph node status.9,10,19 These observations give further support to the theory that intrinsic biological differences may exist between melanomas in females and males. In fact, whole exome sequencing of melanoma metastases has shown a greater frequency of missense mutations in samples from males without a significant sex difference in UV hotspot mutated tumors, suggesting genetic differences unexplained by UV light exposure.26 Other lines of data support that melanoma is a hormone-related cancer. Unlike estrogen receptor α, which stimulates cell proliferation, estrogen receptor β (ERβ) is postulated to act as a tumor suppressor gene across several tissue types, and it is expressed in skin. ERβ expression diminishes from benign to pre-cancerous to malignant disease and with increasing tumor aggressiveness.27−29 At least one study has shown diminished ERβ expression in melanomas from male versus female patients.30
Sex differences in the immune system have been recognized for many years and, more recently, immune checkpoint pathway dysregulation has been found to play a critical role in immune suppression in melanoma patients. This has led to the recent and rapid uptake of immune checkpoint inhibition for the efficacious treatment of advanced melanoma.31,32 It has been postulated that differences in tumor-specific T-helper cells may exist between the sexes, which, in turn, might influence clinical response to immunotherapy and thus melanoma-specific patient outcomes. However, to date, these data exist largely in animal models and await further investigation in humans.33,34 A better understanding of sex differences in response to immune checkpoint inhibition, if they do indeed exist, is likely to drive further advances in the care of melanoma patients. Further, understanding the biological basis for sex differences in melanoma may lead to identification of critical predictive, prognostic, and therapeutic targets with the potential to benefit all melanoma patients. Taken as a whole, these observations highlight the importance of including sex as a pre-specified variable in clinical trial design and observational studies. These data might also be incorporated into shared decision making with patients regarding the relative benefit of adjuvant therapy.
Limitations of our study include that patient comorbidities and data regarding treatment preferences, surgeon specialty training and other factors that may influence the treatment of melanoma, granular data on the details of treatment, as well as data on health-related behaviors were unavailable. Evaluation of CSS rather than overall survival as an endpoint mitigates some of these potential shortcomings. There also exists the potential for bias, as data on mitotic rate were not complete for all patients. Although missing data are a well-recognized challenge for researchers using SEER data, it is generally considered reasonable for statistical comparison as long as the deficiency is in less than 50% of cases.35 Although we used the 8th Edition AJCC Classification that has been developed to improve prognostic stratification, especially among patients with node-positive disease, we recognize that clinical practice is based on earlier staging systems. However, treatment recommendations are unlikely to have changed during this recent 5-year study period. Further, the SEER data set has many strengths, including its longitudinal nature, provision of detailed tumor-specific information, an absence of referral bias, and the substantial size of the patient cohort that facilitates sub-group analysis with adequate statistical power.
Conclusions
Analyzing a contemporary population-based patient cohort staged with the latest 8th edition AJCC prognostic system, we found an overall significant effect of female sex and age on CSS for stage II, III, and IV melanoma patients driven by a marked difference within stage II patients younger than age 45. Within stages, sex differences existed, and were further influenced by patient age, with women age <45 having far superior survival to any other patient population. Our findings support hypotheses for a hormonal or age and sex-based immune influence on melanoma outcomes. These data suggest that even with optimal risk stratification and effective targeted and immune therapies, women retain a survival advantage over men that is unaccounted for by potential health behavior variance.
Our observations also highlight the importance of including sex as a variable in clinical trial design and observational studies. These data may also be used to counsel patients regarding prognosis and may influence decision making regarding relative benefits of adjuvant therapies. Understanding sex differences in melanoma, and changes across the lifespan, may identify critical host and tumor mechanisms that influence both biologic aggressiveness and treatment response. Investigation of the biological basis underlying these observations may drive discovery of new biomarkers and therapeutic targets to improve melanoma patient care.
Acknowledgments
The authors would like to acknowledge the support of the Mayo Clinic Department of Surgery (Rochester, MN) and the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery (Rochester) as substantial contributors of resources to the project.
Author Disclosure Statement
T.J.H. reports research funding from Genentech unrelated to this work.
M.S.B. reports research grants from Marker Therapeutics, Immune Design, Merck, Bristol Myers Squibb, and Genentech, all outside the scope of the submitted work.
The remaining authors have no relevant disclosures or conflicts of interest and declare that no competing financial interests exist.
Funding Information
E.A.L.E. is supported by a Career Development Award from the National Institute of Child and Human Development (NICHD) through the National Institute of Health (NIH) K12 HD065987.
References
- 1. White LP. Studies on melanoma. II. Sex and survival in human melanoma. N Engl J Med 1959;260:789–797 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 3. Bristow BN, Casil J, Sorvillo F, Basurto-Davila R, Kuo T. Melanoma-related mortality and productivity losses in the USA, 1990–2008. Melanoma Res 2013;23:331–335 [DOI] [PubMed] [Google Scholar]
- 4. Kemeny MM, Busch E, Stewart AK, Menck HR. Superior survival of young women with malignant melanoma. Am J Surg 1998;175:437–444; discussion 444–445. [DOI] [PubMed] [Google Scholar]
- 5. Micheli A, Ciampichini R, Oberaigner W, et al. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur J Cancer 2009;45:1017–1027 [DOI] [PubMed] [Google Scholar]
- 6. Lasithiotakis K, Leiter U, Meier F, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008;112:1795–1804 [DOI] [PubMed] [Google Scholar]
- 7. Joosse A, Collette S, Suciu S, et al. Superior outcome of women with stage I/II cutaneous melanoma: Pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol 2012;30:2240–2247 [DOI] [PubMed] [Google Scholar]
- 8. Enninga EAL, Moser JC, Weaver AL, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med 2017;6:2203–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Vries E, Nijsten TE, Visser O, et al. Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol 2008;19:583–589 [DOI] [PubMed] [Google Scholar]
- 10. Scoggins CR, Ross MI, Reintgen DS, et al. Gender-related differences in outcome for melanoma patients. Ann Surg 2006;243:693–698; discussion 698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–3634 [DOI] [PubMed] [Google Scholar]
- 12. Unger JM, Flaherty LE, Liu PY, Albain KS, Sondak VK. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer 2001;91:1148–1155 [DOI] [PubMed] [Google Scholar]
- 13. Joosse A, Collette S, Suciu S, et al. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: A pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol 2013;31:2337–2346 [DOI] [PubMed] [Google Scholar]
- 14. Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: An analysis of 3661 patients from a single center. Cancer 2003;97:1488–1498 [DOI] [PubMed] [Google Scholar]
- 15. Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: An analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 2011;29:2199–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin MB, et al. (eds.). AJCC cancer staging manual, 8th ed. New York: Springer International Publishing, 2017 [Google Scholar]
- 18. Mervic L, Leiter U, Meier F, et al. Sex differences in survival of cutaneous melanoma are age dependent: An analysis of 7338 patients. Melanoma Res 2011;21:244–252 [DOI] [PubMed] [Google Scholar]
- 19. Crocetti E, Fancelli L, Manneschi G, et al. Melanoma survival: Sex does matter, but we do not know how. Eur J Cancer Prev 2016;25:404–409 [DOI] [PubMed] [Google Scholar]
- 20. Haydu LE, Scolyer RA, Lo S, et al. Conditional survival: An assessment of the prognosis of patients at time points after initial diagnosis and treatment of locoregional melanoma metastasis. J Clin Oncol 2017;35:1721–1729 [DOI] [PubMed] [Google Scholar]
- 21. Khosrotehrani K, Dasgupta P, Byrom L, Youlden DR, Baade PD, Green AC. Melanoma survival is superior in females across all tumour stages but is influenced by age. Arch Dermatol Res 2015;307:731–740 [DOI] [PubMed] [Google Scholar]
- 22. Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 2000;18:3782–3793 [DOI] [PubMed] [Google Scholar]
- 23. Courtenay WH. Constructions of masculinity and their influence on men's well-being: A theory of gender and health. Soc Sci Med 2000;50:1385–1401 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Freemantle N, Nazareth I, Hunt K. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: An analysis of routinely collected UK general practice data. PLoS One 2014;9:e101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandian TK, Krishnamurthy V, Hieken TJ. The impact of patient, physician and tumor factors on awareness and early diagnosis of cutaneous melanoma. J Dermatol Res Ther 2017;3:049 [Google Scholar]
- 26. Gupta S, Artomov M, Goggins W, Daly M, Tsao H. Gender disparity and mutation burden in metastatic melanoma. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marzagalli M, Montagnani Marelli M, Casati L, Fontana F, Moretti RM, Limonta P. Estrogen receptor beta in melanoma: From molecular insights to potential clinical utility. Front Endocrinol (Lausanne) 2016;7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hieken TJ, Carter JM, Hawse JR, et al. ERbeta expression and breast cancer risk prediction for women with atypias. Cancer Prev Res (Phila) 2015;8:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reese JM, Bruinsma ES, Monroe DG, et al. ERbeta inhibits cyclin dependent kinases 1 and 7 in triple negative breast cancer. Oncotarget 2017;8:96506–96521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Giorgi V, Gori A, Gandini S, et al. Oestrogen receptor beta and melanoma: A comparative study. Br J Dermatol 2013;168:513–519 [DOI] [PubMed] [Google Scholar]
- 31. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–482 [DOI] [PubMed] [Google Scholar]
- 32. Sadozai H, Gruber T, Hunger RE, Schenk M. Recent successes and future directions in immunotherapy of cutaneous melanoma. Front Immunol 2017;8:1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dronca RS, Dong H. A gender factor in shaping T-cell immunity to melanoma. Front Oncol 2015;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wesa AK, Mandic M, Taylor JL, et al. Circulating type-1 anti-tumor CD4(+) T cells are preferentially pro-apoptotic in cancer patients. Front Oncol 2014;4:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim HM, Goodman M, Kim BI, Ward KC. Frequency and determinants of missing data in clinical and prognostic variables recently added to SEER. J Registry Manag 2011;38:120–131 [PubMed] [Google Scholar]