Abstract
Background: The basis for the treatment of hypothyroidism with levothyroxine (LT4) is that humans activate T4 to triiodothyronine (T3). Thus, while normalizing serum thyrotropin (TSH), LT4 doses should also restore the body's reservoir of T3. However, there is evidence that T3 is not fully restored in LT4-treated patients.
Summary: For patients who remain symptomatic on LT4 therapy, clinical guidelines recommend, on a trial basis, therapy with LT4+LT3. Reducing the LT4 dose by 25 mcg/day and adding 2.5–7.5 mcg liothyronine (LT3) once or twice a day is an appropriate starting point. Transient episodes of hypertriiodothyroninemia with these doses of LT4 and LT3 are unlikely to go above the reference range and have not been associated with adverse drug reactions. Trials following almost a 1000 patients for almost 1 year indicate that similar to LT4, therapy with LT4+LT3 can restore euthyroidism while maintaining a normal serum TSH. An observational study of 400 patients with a mean follow-up of ∼9 years did not indicate increased mortality or morbidity risk due to cardiovascular disease, atrial fibrillation, or fractures after adjusting for age when compared with patients taking only LT4. Desiccated thyroid extract (DTE) is a form of combination therapy in which the LT4/LT3 ratio is ∼4:1; the mean daily dose of DTE needed to normalize serum TSH contains ∼11 mcg T3, but some patients may require higher doses. The DTE remains outside formal FDA oversight, and consistency of T4 and T3 contents is monitored by the manufacturers only.
Conclusions: Newly diagnosed hypothyroid patients should be treated with LT4. A trial of combination therapy with LT4+LT3 can be considered for those patients who have unambiguously not benefited from LT4.
Keywords: hypothyroidism, levothyroxine, triiodothyronine, desiccated thyroid extract, combination therapy
Background and Statement of a Problem
Hypothyroidism is caused by a failure to produce sufficient amounts of thyroid hormone (TH), as a result of genetic defects, autoimmune disease, and surgical or actinic thyroidectomy (1). It is considered one of the most common chronic diseases worldwide, with a prevalence of 3–7% in the United States. Desiccated thyroid extract (DTE) dosed according to clinical assessment was the preferred form of treatment until the 1970s. However, early clinical studies demonstrated the advantages of using synthetic levothyroxine (LT4) alone as replacement therapy (2,3) and that serum thyrotropin (TSH) measurements were an ideal tool for monitoring LT4 dosage (4–6). In addition, two recent large population studies have shown that, provided hypothyroid patients achieve sustained normal serum TSH levels, mortality is comparable to the background population (7,8). Indeed, all current clinical guidelines recommend daily tablets of LT4 at doses that normalize serum TSH levels, usually between 1.5 and 1.8 μg/kg (9–11).
The rationale for LT4 therapy resides on the fact that multiple tissues are capable of T4 deiodination to triiodothyronine (T3), the active form of TH, “restoring” the body's T3 reservoir. This dogma has been challenged by findings that, in LT4-treated hypothyroid patients with normal serum TSH, serum T3 levels are ∼10% lower whereas serum T4 levels are ∼12% higher (12–16). When compared with normal individuals with similar TSH levels, LT4-treated patients weigh ∼10 pounds more (14), exhibit a slightly lower basal metabolic rate (BMR) (15,17,18), and have higher serum cholesterol and low density lipoprotein levels (19–21), even as they are more likely to be on statin medications (14). This suggests that T3 signaling and homeostasis in LT4-treated patients may not be fully restored in all tissues using LT4 alone (22).
At face value, these data call for the next logical step to be the attempted normalization of serum T3 in LT4-treated patients, including restoring its normal diurnal rhythmicity, provided a suitable slow-release or long-acting preparation and dosing schedule can be achieved. However, several clinical trials that focused on comparing therapy with LT4 versus LT4+liothyronine (LT3) failed to identify rock-solid differences between these two forms of treatment (10). These trials focused on mood, cognition, and quality of life, and despite lack of data supporting explicit superiority of the LT4+LT3 therapy over LT4, and that serum T3 was not related to psychological well-being (23), many seem to still indicate a patient preference for combination therapy (24). This was also identified in an online survey of about 12,000 hypothyroid patients in whom preference was the highest for treatment with DTE, followed by LT4+LT3 and LT4 alone (25). Selection bias and lack of initial focus on symptomatic patients may be important complicating factors in the design and interpretation of these trials (26). For example, other causes of residual symptoms such as suboptimal LT4 treatment, ascertainment bias, somatoform disorders, and the co-existence of chronic diseases that may present with similar symptoms were not consistently assessed in these trials (27,28).
While considering all of these factors, the most recent clinical guidelines prepared by the British Thyroid Association (BTA) (29) and the European Thyroid Association (ETA) (9) explicitly recognize that a trial with LT4+LT3 can be attempted in patients who have unambiguously not benefited from LT4 (Table 1); the American Thyroid Association (ATA) (10) and the U.K. National Institute for Health and Care Excellence (NICE) guidelines (30) tolerate therapy with LT4+LT3, provided that it is not used as a routine approach to hypothyroid patients (Table 1). In addition, many patients come to see providers while already on stable therapy that contains LT3 or on DTE, and they are unwilling to switch to monotherapy with LT4. In 2017, DTE was the 130th most prescribed medication in the United States with ∼5.5 million prescriptions per year (31).
Table 1.
Statements in Clinical Guidelines on the Use of LT3, Desiccated Thyroid Extract, and LT3+LT4 on Treatment of Hypothyroidism
| Sponsor | DTE | LT3 | LT3+LT4 |
|---|---|---|---|
| AACE | Not be used | Not to be used | Does not support |
| AGS | Potentially inappropriate use in older adults | N/A | N/A |
| ATA | Against the routine use | Against the routine use | Against the routine use |
| BTA | No routine use | No routine use | No routine use; can be use as a trial in patients who have unambiguously not benefited from LT4 |
| ETA | Not to be used | N/A | Might be considered as an experimental approach in compliant LT4-treated hypothyroid patients who have persistent complaints |
| LATS | N/A | N/A | Not recommended |
| NICE | Do not offer | Do not routinely offer | Do not routinely offer |
AACE, American Association of Clinical Endocrinologist; AGS, American Geriatrics Society; ATA, American Thyroid Association; BTA, British Thyroid Association; DTE, desiccated thyroid extract; ETA, European Thyroid Association; LT3, liothyronine; LT4, levothyroxine; NICE, U.K. National Institute for Health and Care Excellence; N/A, not available.
For 50 years now, providers have been steered away from using anything other than LT4 in the treatment of hypothyroidism. This has resulted in two generations of physicians with limited clinical experience prescribing LT3 or DTE, and with the taught perception that these drugs were unsafe. This constitutes a major challenge for those willing to follow the guidelines and try combination therapy for specific situations. Even as we follow guidelines and initiate trials with LT3, how should this best be done? What should we monitor to ensure proper/safe TH replacement?
Thus, the goal of this article is to review the past and current use of LT3 and DTE in hypothyroidism, focusing on their pharmacokinetics and pharmacodynamics, clinical T3-dependent parameters, and undesirable effects reported as adverse drug reactions (ADRs). We searched PubMed, Google Scholar, and ClinicaTrials.Gov for clinical trials by using the keywords “liothyronine” or “desiccated thyroid extract”; the search was completed on December 2019. We also reviewed the topic “treatment of hypothyroidism” in 115 textbooks, published between 1883 and 1980, on medical and surgical aspects of goiter and/or the thyroid gland. This search strategy led us to 832 publications, of which 55 contained pharmacokinetic or pharmacodynamic data on LT3 or DTE, and/or their use in the treatment of hypothyroidism, as well as psychiatric or metabolic disorders.
Pharmacokinetics of LT3
The daily T3 production in a 70-kg healthy adult is ∼30 mcg, with the thyroid producing only ∼5 mcg T3; an additional ∼25 mcg T3 is produced daily outside of the thyroid parenchyma via T4 deiodination (32). The deiodination pathway is predominantly via the type 2 deiodinase (D2), which tends to play a homeostatic role, that is it is accelerated during hypothyroidism and is suppressed during hyperthyroidism (33,34). Nevertheless, the thyroidal contribution to daily T3 production can increase according to the TSH levels. For example, the minimal circadian plasma T3 rhythmicity is secondary to a circadian TSH rhythmicity (35). Further, during iodine deficiency or primary hyperthyroidism due to Graves' disease, the enhanced stimulation of TSH receptors accelerates relatively more T3 production (36).
The hypothalamus-pituitary-thyroid axis seems to be hardwired to preserve plasma T3 levels within a narrow range (37). For example, plasma T3 levels are normal in mice with inactivation of all T3-producing deiodinases thanks to a compensatory increase in T3 secretion by the thyroid gland (38,39). In fact, there are only a few known situations in which plasma T3 is below normal in individuals with a healthy thyroid gland, that is, caloric restriction or fasting, and nonthyroidal illness (40). In these situations, the hypothalamus-pituitary-thyroid axis deliberately allows for a drop of plasma T3 levels, without responding with increased TSH secretion, up until caloric intake is restored or illness is resolved (41).
The physiological stability of plasma T3 levels contrasts with the rapid fluctuation of serum T3 after oral administration of LT3. A number of promising LT3 formulations and strategies are being developed to minimize/avoid this, but for the time being tablets containing the sodium salt of LT3 (Cytomel™, Triostat™), or liotrix, a 4:1 mixture of LT4:LT3 (Thyrolar™), are the only Food and Drug Administration (FDA) approved therapy containing LT3 (42). Alternatively, capsules containing LT3 are frequently prepared by compounding pharmacies, but these are not FDA approved (43). Quality data about these preparations are not always available, which is critical given the difficulties in accurately and consistently dispensing a few mcg of any active pharmaceutical ingredient, including LT3.
DTE is an active pharmaceutical ingredient that, on consumption and subsequent digestion, releases T4 and T3 that are absorbed into the bloodstream. It is produced as a powder of porcine thyroid gland tissue containing T4:T3 at ∼4:1 ratio. It predates the passage of the new drug approval requirements in 1938, and to this date prescription drug products containing DTE have continued to be marketed outside of formal FDA oversight. Notably, some manufacturers are planning or actively pursuing a development program to establish the safety and efficacy of their products for purposes of gaining FDA approval (44).
The oral administration of LT3-containing tablets is followed by a spike of T3 in the circulation that peaks around 2–3 hours and rapidly subsides after two distinct phases of linear elimination: a fast distribution phase with a half-life of ∼2 hours and a slow elimination phase with a half-life of ∼23 hours, supporting a two-compartment model (Fig. 1A–C) (45,46). The post-dose spike of serum T3 is the result of a rapid, very efficient intestinal absorption that reaches >95% by 4 hours (47), and it can be seen after either LT3 or DTE administration (48,49). This T3 spike creates practical problems for therapy with LT3-containing tablets (50,51). Given the velocity with which serum T3 fluctuates after oral administration, it is difficult to assess serum T3 levels in patients taking LT3-containing tablets without at least two to three time points.
FIG. 1.
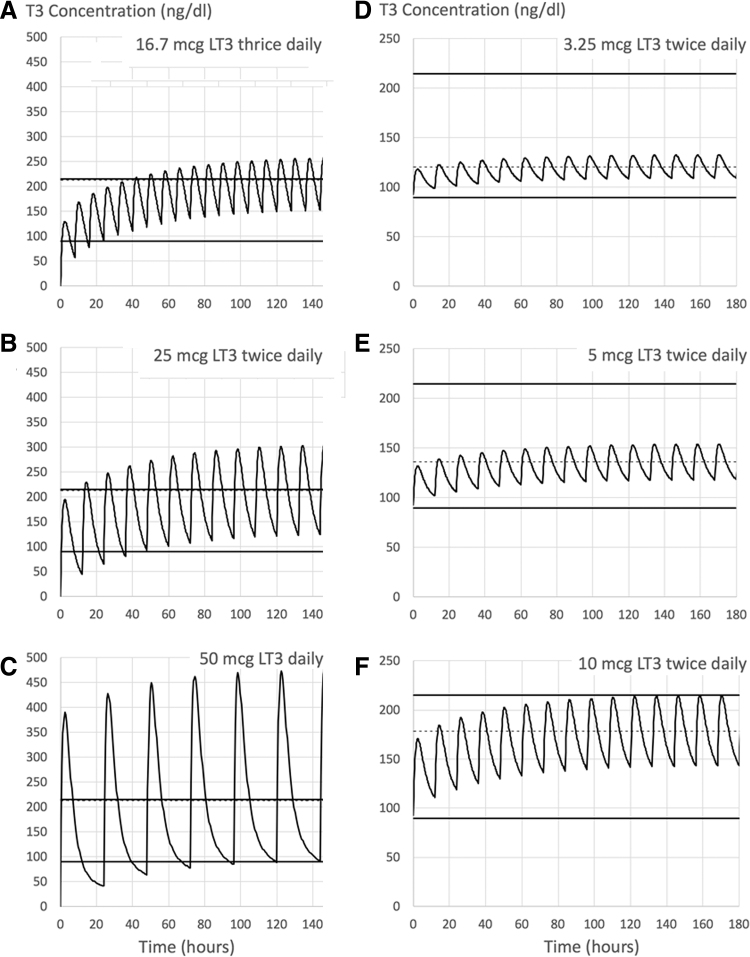
Projected serum T3 levels according to mathematical model: (A–C) Modeling of 50 mcg LT3 administered on a thrice (A), twice (B), or single (C) daily regimen. (D) Hypothetical 72.5 kg hypothyroid patient treated with 112 mcg LT4 alone, being transitioned to LT4/LT3 therapy at a 16:1 weight/weight combination therapy, that is, LT3 dose of 3.25 mcg twice daily and LT4 dose of 92.5 mcg daily; (E) same as (D) except that the LT4 dose was reduced by 25 or 50 mcg and substituting with 5 or 10 mcg LT3 on twice daily (E, F), respectively (i.e., to an LT3 dose of 5 or 10 mcg twice daily and LT4 dose of 88 or 62 mcg daily, respectively); dashed line is the mean concentration of T3. Modified from Van Tassell et al. (45). LT3, liothyronine; LT4, levothyroxine.
These rapid plasma T3 kinetics can be seen in both hypothyroid patients kept on TH replacement therapy and euthyroid healthy volunteers. The study of 16 hypothyroid patients (21–76 years old; 14 females) during chronic treatment with LT3 (25 or 50 mcg/day) shows that, at steady state, the administration of LT3 resulted in an acute elevation of serum T3 ∼ 3 hours after the dose, to above the upper limit of the reference range (from ∼100 to ∼225 ng/dL; and from ∼110 ng/dL vs. ∼350 ng/dL, respectively) (52). Similar numbers were seen in healthy volunteers who received a single dose of LT3 (46,53). Mathematical modeling was used to predict changes in serum T3 concentration after LT3 doses were given in combination with LT4 (Fig. 1D–F). In 14 hypothyroid patients (∼49 years old, 72 kg, and body mass index 26) taking 113 mcg/day LT4, the model predicted that reducing the dose of LT4 by 25 mcg/day and adding 5 mcg LT3 given twice daily (to an LT3 dose of 10 mcg daily) would increase integrated serum T3 levels, without reaching the upper limit of normal (45). At the same time, the substitution of 50 mcg LT4 for 10 mcg LT3 twice daily (to an LT3 dose of 20 mcg daily) would elevate serum T3 levels above the reference range only 14% of the time (45).
Pharmacodynamics of LT3 and ADRs
The T3 acts by interacting with nuclear TH receptors (TRs), changing the rate at which genes are transcribed. This affects the transcriptome and proteome of target tissues, explaining T3-dependent biological effects (54). Studies in mice indicate that TRs can also rapidly activate intracellular second-messenger signaling pathways independently of gene expression (55). The chain of events triggered by T3 involves time, explaining why at least one hour is needed for the first biological effects to become evident (56). However, given that T3 is rapidly metabolized, its effects dissipate within a few hours and are mostly reversible. Even though TH actions are pleiotropic, there is general agreement that the sympathetic, cardiovascular, and musculoskeletal systems, as well as mood and behavior, are particularly sensitive to minimal excess of TH. Thus, these have been the most commonly studied areas when assessing safety and ADRs for TH-containing formulations.
At or shortly after the serum T3 peak that follows oral LT3, and depending on the LT3 dose, patients might complain of palpitations, chest tightness, and/or sweating (57), but this seems to be associated with relatively high doses of LT3 or DTE as not all double-blinded studies reveal cardiovascular symptoms (50).
Two independent studies monitored healthy, relatively young volunteers after a single 10 or 50 mcg dose of LT3 (46,53). Eleven males (21–30 years old) received 10 mcg LT3 and exhibited a ∼45% elevation in free T3 serum levels after 2.5 hours while no changes were observed in serum TSH. Heart rate was not affected, and no ADRs were reported (53). In another study (46), eight males and four females (29.3 ± 8.6 years old) received 50 mcg LT3 and exhibited a ∼300% elevation (114 ± 26 to 346 ± 77 ng/dL) in serum T3 after 2.5 hours. The maximum drop in serum TSH, 65%, occurred at 12 hours (1.34 ± 0.76 to 0.49 ± 0.32 μU/L); earlier time points did not show a reduction in serum TSH (51). Heart rate accelerated from 60 ± 7 to 76 ± 9 bpm at post-dose 10 hours, only returning back to 65 ± 8 bpm post-dose at 48 h. No electrocardiographic changes were observed; changes in blood pressure (systolic and diastolic), body temperature, or body weight were apparent (46). Notably, chronic administration of LT3 (50–62.5 mcg/day) for 5–9 weeks to 15 healthy volunteers (21–38 years old; 12 males) resulted in (i) reduction in blood lipids and body weight, (ii) ∼11% acceleration of energy expenditure, and (iii) transient elevation in heart rate, which returned to normal at the end of the study. None of the subjects exhibited ADRs (58,59). However, because hypothyroid patients are typically older, extrapolation to these patients must be done with caution.
Most studies that addressed TH safety and ADRs looked at patients with stably elevated levels of T4 and/or T3, and/or low/suppressed serum TSH, as in hyperthyroidism or sub-clinical hyperthyroidism, or in those taking excessive doses of LT4. The conclusions of these studies are clear; lower serum TSH levels are associated with increased risk of atrial fibrillation (AF) (60) and osteoporosis (61). However, an issue that has not been resolved is whether the episodes of hypertriiodothyroninemia, which follow administration of LT3 and do not reduce serum TSH outside the reference range, pose short- and/or long-term safety risk. In addition, is having a morning serum T3 peak of 160 ng/dL that drops to 100 ng/dL in the afternoon similar to keeping serum T3 at 130 ng/dL all this time? How about if the T3 levels reach outside the reference range? What if this pattern repeats during a lifetime? Two recent studies on this subject may be used to address these questions indirectly. The first is an analysis of 174,914 patients followed for 7.0 ± 4.9 years. It revealed that the incidence and prevalence of AF was greater with elevated serum fT4 levels, even within the normal reference range, but no relationship was noted for serum total T3 and fT3 within their normal reference ranges (62). The second study is an analysis of 55,114 individuals with AF and 482,295 referents. It revealed that a polymorphism in the type 1 deiodinase gene (DIO1) that genetically predicts an increase in circulating fT3:fT4 ratio was associated with increased risk of AF (63). However, and most importantly, the increase in fT3:fT4 ratio associated with this polymorphism is driven by the strong association with decreased circulating fT4 and not an elevation in fT3 levels; indeed, fT3 levels were not associated with the DIO1 polymorphism (64). Thus, to our knowledge, there is currently no evidence that fluctuations in serum T3 within the normal reference range, with normal serum TSH levels, constitute a risk factor for AF. However, the number of studies is limited and more research is needed to confirm/clarify these points.
Clinical Experience with LT3 Preparations
Historically, the diagnosis of hypothyroidism was clinical, based on well-known symptoms and signs (65–67) that reflected a reduced basal rate of metabolism, that is BMR (67,68); when available, blood count, serum cholesterol, protein bound iodine (PBI) levels (69,70), time response of the ankle jerk (71), and serum T4, T3, and 131I thyroid uptake levels (72) were later used. Initially, however, assessment of the adequacy of treatment was judged, without the benefit of objective parameters. “The object of treatment should be to rid the patient of symptoms with the smallest ration of thyroid that will accomplish this purpose. An arbitrarily selected BMR should not be the mark aimed at… unless we had by pure chance seen the patient and measured his metabolism before his thyroid underwent atrophy.” (68,73). In retrospect, it seems that this strategy invariably led to overtreatment. At some point, during the 1970s, serum TSH became the ever-desired objective parameter that reflects thyroid status, and it subsequently became the gold standard to diagnose and assess treatment of hypothyroidism (4–6).
DTE therapy for hypothyroidism
Different DTE preparations have been used in clinical practice since the 1880s and, from the beginning, physicians were well aware of frequent “untoward symptoms,” nervousness, precordial pain, and/or palpitation, an indication that the DTE dose had to be reduced. Early in its use, DTE was given in gradually increasing amounts, up until clinical evidence of thyrotoxicosis had developed (headache, palpitation, anxiety, and angina). Therapy was then withdrawn and resumed later at a lower dose. Some physicians would automatically prescribe monthly one-week DTE holidays; others would fractionate the DTE doses, to be given two or three times a day (65,67,74,75). The maintenance replacement dose of DTE varied between 100 and 200 mg daily, but many patients did not tolerate starting with a full dose (67). For patients with severe hypothyroidism, physicians would first stabilize any preexisting cardiovascular condition, even include a short period of bed rest before starting DTE. With time, an array of TH-sensitive criteria was used to guide the replacement dose, minimizing the problem of overtreatment. However, more significance was invariably given to “freedom from symptoms with the minimum dose that will accomplish it” rather than aiming at predefined laboratory results (72,76), which commonly led to some degree of overtreatment.
In the 1960s, backed by 80 years of experience and at the height of its use (77), it was believed that “physiologic doses” of DTE reproduced the metabolic effects of natural thyroid secretions and, when properly managed, were without significant toxicity (72,76,78). “A diagnosis of hypothyroidism being made—in an uncomplicated patient—the next logical step is to give thyroid (i.e., DTE). The results that follow such treatment are prompt and dramatic. They are of scientific as well as of practical interest” (76). However, despite being accepted as an effective and safe therapy, DTE preparations had a major disadvantage; they could not be standardized from lot to lot to provide uniform hormonal activity (67,78,79) given that standardization was based on iodine levels and not on T4 and T3 content (80,81). In addition, DTE was occasionally found to be clinically inactive, or of variable potency between batches (81–83).
Synthetic LT4 and LT3 became commercially available in the 1950s and each, consistently, reproduced the desired metabolic actions of endogenous TH (78). Later, the discovery that most circulating T3 is derived from extrathyroidal T4 deiodination provided momentum for the adoption of synthetic TH as the mainstream form of replacement therapy (84,85). The LT4 gained rapid acceptance because of its uniform potency and absorption, predictable bioavailability given its narrow therapeutic range, and relatively low cost (2,3,86–88). In addition, serum T3 levels did not fluctuate in patients treated with LT4 as it did with patients treated with DTE (52,89,90). During the intervening 10 years, DTE was almost completely replaced with LT4 monotherapy for management of hypothyroidism (77,91).
Contemporary manufacturing and clinical use of DTE
Today, the manufacturing of DTE must comply with current Good Manufacturing Practice as enforced by the FDA, the failure of which may result in product recall. In addition, companies that manufacture and commercialize DTE in the United States must follow the procedures and standards described in the United States Pharmacopeia (USP) monography (92). In short, currently thyroid glands are harvested from pigs used in the food supply (USDA-approved slaughter houses), frozen, and finally shipped to manufacturers. The glands are subsequently processed to a fine powder that contains thyroglobulin and very little free T3 and T4: Most TH is covalently bound to the thyroglobulin peptide chain. Thus, USP protocol requires proteolytic enzymatic digestion of the powder followed by high-performance liquid chromatography to measure T3 and T4 contents followed by, and comparison against, the respective USP standards. The thyroid powder is formulated into tablets based on the amount of T4 and the final weight is adjusted to ensure that the potency of the resulting tablet conforms with USP recommendations, that is, a 1 grain (65 mg) tablet contains 38 mcg T4 and 9 mcg T3 with a margin of error of ±10%; the measured amount of T4 and T3 in a batch of tablets must, therefore, be 34.2–41.8 mcg T4 and 8.1–9.9 mcg T3. It is up to the manufacturers to review the data for each DTE lot and ensure that their product meets those standards both at release and throughout its shelf life.
A study in which 40 patients were assessed while taking DTE after being switched to LT4 is often cited as a reason to abandon DTE as a treatment for hypothyroidism. In this study, DTE usage was associated with hypertriiodothyroninemia and thyrotoxic symptoms (93). However, 23 of these patients were not being treated for hypothyroidism; they were on DTE “as medical therapy for gland suppression.” In addition, serum TSH was not reported; it was only used to exclude hypothyroidism and not to adjust the replacement doses (93). Indeed, the availability of sensitive methods to measure serum TSH transformed the approach for defining and adjusting the replacement dose of DTE (94), even as its utilization is now limited to a much smaller number of patients (77). For example, a comparative study of 12 hypothyroid patients receiving progressively larger doses of LT4 and 9 hypothyroid patients receiving progressively larger doses of DTE (monthly increments of 30 mg/day; mean 138 ± 27; range 114–180 mg/day) revealed that both treatments can normalize plasma TSH levels and similarly eliminate signs and symptoms of hypothyroidism, with no ADRs, although resulting in markedly different serum T3/T4 ratios ( × 1000), 16 versus 35, respectively (90).
A major difference and residual point of concern with DTE, particularly in the older adults and/or patients with cardiovascular diseases (57,95), is the occurrence of daily transient episodes of hypertriiodothyroninemia after consumption (10,48,49,57,95). Indeed, patients receiving DTE exhibit an elevation in serum T3 that peaks at about 3 hours after taking the tablet, and this might fluctuate above the upper limit of the reference range depending on the dose taken; however, serum T3 returns to within normal range 12–24 hours after the last dose. Serum levels of T4 and TSH might fluctuate as well, but much less and within the normal range (48,49,57). Notwithstanding, this probably goes unnoticed by patients on doses that normalize serum TSH, as illustrated in the analysis of 2575 individuals who were part of the Framingham Heart Study (examined during 1980–1984) to assess the appropriateness of therapy with TH: 178 older adults (154 women; all patients >60 years old; mean 68.6 years) were receiving treatment with TH for 2–50 years (average 19 years) (96). Notably, 122 were on DTE whereas 51 were taking either LT4 or LT4+LT3. During the follow-up examination 6.9 years later (2–8 years), 91 had remained on DTE. Serum TSH was obtained at baseline and follow-up visits, and although the study was not designed to assess ADRs, no mention was made about thyrotoxic symptoms (96). In addition, two recent studies in which the dose of DTE was also adjusted, using sensitive methods to measure serum TSH, failed to identify reasons for concern.
The first study is a randomized, double-blind, crossover study of 70 hypothyroid patients (age 23–65 years old; 53 females) that compared treatment with DTE (mean 80 ± 30 mg/day; range 43–172 mg/day) versus LT4 for 16 weeks (97). The study used an equivalency conversion formula: 1 mg DTE = 1.667 mcg LT4, and revealed that while serum TSH remained within normal range in all patients, serum T3 was ∼55% higher and fT4 was ∼40% lower in patients taking DTE. Blood samples were collected early in the morning, before patients took the treatment tablets, with no information about episodes of hypertriiodothyroninemic-related symptoms during the day. Nevertheless, there were no differences in heart rate, blood pressure, serum lipids, or symptoms and neurocognitive parameter measurements between the two therapies, although patients on DTE lost ∼3 lbs. of weight; no ADRs were reported (97).
The second study is a 6-year observational retrospective chart review that identified 100 patients (20–81 years old; 95 females) receiving combination therapy and compared them with 2400 patients receiving LT4 monotherapy (98). The analysis of 60 patients on DTE indicated that ∼97% patients had therapeutic TSH levels, with <5% exhibiting supra-therapeutic levels of fT3. The mean follow-up duration was 27 months (median 22; range 1–111 months) (98). The starting dose for DTE was 15 mg/day, which was titrated to obtain physiologic and therapeutic TSH, fT4, and fT3 levels along with symptom relief. The average maintenance dose of DTE was 30 mg/day, but no information was provided about episodes of hypertriiodothyroninemic-related symptoms during the day. None of the patients who had abnormally low TSH or elevated fT3 or fT4 levels was hospitalized for arrhythmias or thyrotoxicosis, and DTE use did not result in additional risk of AF, cardiovascular disease, or mortality in patients of any ages (98).
Summary
The DTE is manufactured in the United States according to USP recommendations and standards with limited FDA oversight; at the time of this writing, there is no independent surveillance regarding the contents of T3 and T4 in DTE. Typically, DTE at mean doses of 80 mg/day (containing 11 mcg T3/day) restores clinical and biochemical euthyroidism with no reports of ADRs, as long as fasting serum TSH levels are used to adjust the replacement dose. The main differences between therapies that normalize serum TSH with DTE versus LT4 alone are that DTE treatment results in a much higher serum T3/T4 ratio and DTE patients exhibit a daily peak in serum T3 levels two to three hours after taking a DTE tablet. The peak depends on the dose taken, but it could reach 40–80% above the baseline values, and last for several hours; only at the higher DTE doses could serum T3 rise outside the reference range. Further, the clinical euthyroidism and absence of cardiovascular ADRs in all reported patients indicate that, at the doses used and during a mean observational period of 27 months, a daily transient elevation in serum T3 levels was not associated with cardiovascular ADRs; however, long-term assessment of bone density or turnover markers is not available. Patients on DTE can be switched to LT4 and vice versa by using an accepted conversion formula. This formula is a good starting point but fine tuning the DTE or LT4 doses should ultimately be guided by fasting serum TSH levels. The American Association of Clinical Endocrinologist (AACE), ATA, BTA, ETA, and NICE guidelines do not recommend the use of DTE (Table 1) (9,10,11,29,30). The Centers for Medicare and Medicaid Services (CMS) includes DTE in the “high-risk medication list in the elderly” based on “concerns about cardiac effects” stated in The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (99).
Liothyronine therapy for hypothyroidism
The LT3 has been available since the 1956 as a therapy to treat hypothyroidism (70). In one of the first studies, the daily oral administration of 70–105 mcg LT3 to patients with myxedema resulted in a return to clinical euthyroidism within 10–14 days (100). There was rapid and complete disappearance of all signs and symptoms of hypothyroidism without any distressing or toxic effects being noted. Patients lost weight during the first two weeks of treatment and remained euthyroid for the duration of the therapy (100). While oral preparations of LT3 were well absorbed, LT3 could also be given subcutaneously or intravenously with similar effects (70,101). As expected, the treatment of hypothyroidism with LT3 alone resulted in pronounced T3 peak in the circulation, the magnitude of which is proportionally less if LT3 is given twice or trice daily (Fig. 1A–C) (45). When used, large-scale treatment of hypothyroidism with LT3 was well accepted by patients. For example, during 1968–1978, hypothyroid patients in Brazil were routinely treated with LT3 alone, given that LT4 was not commercially available and DTE was considered unreliable at the time. The LT3 doses of up to 75 mcg/day were adjusted clinically and given two to three times a day, rapidly reversing symptoms of hypothyroidism (102). Physicians were well aware of the possible ADRs associated with LT3 replacement therapy and lower doses were given to those patients at higher risk. Serum TSH was used to adjust the dose but, at that time, sensitivity of the assay overlapped with the normal reference range. Therefore, patients on LT3 were routinely examined for signs and symptoms of thyrotoxicosis, and the LT3 dose adjusted down to 50 mcg/day as needed. When, some years later, LT4 and more sensitive TSH assays became available, patients were switched to daily tablets of LT4, but ∼25% of the patients insisted on continuing therapy with LT3 (102). The LT3 is no longer commercially available in Brazil.
An analysis of multiple T3-dependent parameters was conducted in 10 hypothyroid patients (aged 20–67 years; 7 females) treated consecutively with increasing doses of LT3 (10, 20, 25 and 50 mcg/day, single dose), each dosage given during at least 4 weeks, for a total of 16 weeks (18). On LT3, 20–25 mcg/day, cardiac systolic time intervals and serum creatine phosphokinase activity had normalized, but BMR, total cholesterol concentration, and serum TSH levels had not. As the dose of LT3 was increased to 50 mcg/day, mean basal serum TSH levels decreased from 55 to 16 μU/mL, bringing all parameters to within normal reference range, except for total cholesterol that remained above 200 mg/dL. Notably, only 50 mcg/day LT3 normalized the 24-hour-integrated serum concentration of T3; no ADRs were reported (18).
More recent studies indicate that switching from LT4 to LT3 can be accomplished without major changes in thyroid-responsive parameters or the occurrence of ADRs. In the study of 18 hypothyroid patients (aged 24–56 years; 16 females) with normal serum TSH levels, their regular LT4 regimen was switched to 15 mcg/day LT3 for 2 weeks and then to 30 or 45 mcg/day for 6 weeks (51). Although serum TSH remained slightly elevated throughout treatment with LT3, no changes in heart rate, temperature, blood pressure, or respiratory rate were observed throughout the study (51). A subsequent study focused only on serum TSH levels to guide a crossover study of 10 adult (51.3 ± 3.4 years old; 9 females) hypothyroid patients who were treated with LT3 40 ± 11 mcg/day or LT4 (115 ± 39 mcg/day) for 4 weeks. At the end of the LT3 arm, fasting serum total T3 was 185 ± 100 ng/dL and serum TSH was 1.48 ± 0.78 μU/L. Monitoring heart rate and blood pressure revealed no differences versus the LT4 arm; none of the patients reported ADRs (103). Similar results were obtained while studying a group of 14 hypothyroid adults (49.3 ± 8 years old; 13 females) receiving a similar dose of LT3 (104).
Summary
While LT3 administration rapidly restores euthyroidism and normalizes serum TSH in hypothyroid patients, there is no reason as to why a hypothyroid patient should be treated with LT3 alone. The doses of LT3 needed to achieve clinical and biochemical euthyroidism (30–45 mcg/day) cause serum T3 to peak above the reference range 2–3 hours after administration. This does not acutely affect thyroid-responsive parameters or results in cardiovascular ADRs. However, the available trials are few, only enrolled a limited number of patients, and lasted a few weeks; no data are available about bone density or turnover markers. The treatment of hypothyroidism with LT3 alone is not practical and loss of clinical effect occurs rapidly if therapy were to lapse. The AACE, ATA, BTA, and NICE guidelines are not supportive of LT3 therapy for the treatment of hypothyroidism (Table 1) (10,29,30,105). Currently, LT3 is recognized in the treatment of myxedema coma and it briefly suppresses serum TSH levels in a number of diagnostic and treatment conditions (10,106).
LT4 and LT3 combination therapy for hypothyroidism
Dampened enthusiasm for DTE to treat hypothyroidism over-standardization concerns (78) led physicians to progressively turn to synthetic LT4 and/or LT3 preparations that eventually became the standard of care (78,79,86). It is fascinating that, in the 1960s, T4 and T3 were seen as two independent hormones produced by the thyroid gland. Whereas each hormone was able to reproduce the metabolic actions of endogenous thyroid secretion, there were noticeable differences when either was used individually to treat patients with hypothyroidism. Thus, at that time, neither molecule achieved a dominant position as the replacement drug of choice. The LT4 was seen as a slow-acting molecule with more prolonged effects than natural thyroid secretions or DTE. Therapy with LT4 required administration of doses that would result in disproportionally high PBI (T4), since the metabolic contribution provided by T3 was felt to be lacking (107). Conversely, LT3 actions exhibited rapid onset but brief duration, producing brisk but short-lived clinical changes. In addition, the mean PBI levels in patients on full therapeutic LT3 replacement doses (75–125 mcg LT3/day) were very low. The fact that LT4 or LT3 restored euthyroidism but neither fully normalized PBI was seen as a dissociation between the metabolic state of the patient and the circulating TH levels. Thus, the idea that TH replacement had to be made with combinations of LT4 and LT3 in physiologic proportions was born. Indeed, liotrix, a combination of LT4 and LT3, was formulated with the intention of circumventing this disconnect. This era, of course, came to an end with the discovery that T4 and T3 are not independent molecules and that humans convert the pro-hormone T4 to its active metabolite T3 via tissue-specific deiodination (84,85).
One of the first clinical trials of combination therapy was designed to define the ratio at which LT4 and LT3 should be combined to treat hypothyroidism (78). Using clinical parameters to define euthyroidism, the crossover study of 21 hypothyroid patients who were treated with different LT4/LT3 combinations for periods of 6–8 weeks concluded that 175–200 mcg/day LT4 combined with 25–50 mcg/day LT3 maintained an athyreotic patient in clinical euthyroidism (78). However, the authors acknowledged that “It is difficult to measure precisely what has been termed clinical euthyroidism. Such an appraisal requires not only objective measurements but also determination of the patient's well-being which can be estimated only by interpretation of subjective responses. There is certainly a wide spectrum of euthyroidism and small deviations from the norm are at present not accountable to objective methods. Patients were considered clinically euthyroid if they evidenced no signs or symptoms of thyroid lack or excess.” (78). In another similar trial that also did not use serum TSH to guide replacement doses, patients on combination therapy were found to have a higher number of ADRs. However, in this case, TH replacement doses were higher, 160–240 mcg/day LT4 and 40–60 mcg/day LT3 (108).
With the benefit of measuring serum TSH and TH levels to define euthyroidism, 16 clinical trials studied hypothyroid patients placed on combination therapy given as a single daily dose (Supplementary Table S1) (109–124). Overall, the studies include a total of 888 patients (23–79 years old; 763 females) who received LT4 (75–228 mcg/day) and LT3 (3–21.4 mcg/day) for 10–48 weeks. The doses were adjusted by replacing a certain amount of LT4 for LT3 or by using a fixed LT4/LT3 ratio: (i) Eight trials replaced 50 mcg LT4/day for 10–25 mcg LT3/day (109–112,114,115,120,121); (ii) eight trials used a fixed LT4/LT3 ratio that varied from 3:1 to 21:1 (113,116–119,122–124). Results were compared with patients taking LT4 alone. As expected, patients on combination therapy exhibited 5–40% lower fasting serum T4 levels and 3–161% higher serum T3 levels; the magnitude of these differences depended on the LT3:LT4 molar ratio used. When compared with the LT4 group or LT4 arm, serum TSH fluctuated minimally, remaining within the normal reference range. Heart rate and blood pressure were reported in 12 trials (109,110,112–118,123): Heart rate remained largely unaffected by combination therapy, while systolic blood pressure increased by 4–12 mmHg in two studies (113,116). Urinary deoxypyridinoline levels (121) and serum osteocalcin levels (116,118) were higher in patients on combination therapy, but they were well within normal reference ranges. The ADRs included (i) AF in one patient (LT3 6.5 mcg/day and LT4 123 mcg/day for 24 weeks) with suppressed serum TSH (113); (ii) premature atrial beats in one patient (116); (iii) tachycardia, nervousness, and heat intolerance in two patients (124); and vague symptoms 10% more likely (115). In all other instances, the frequency of ADRs was similar in the LT4+LT3 and LT4 groups.
Another group of four trials (125–128) comparing mono and combined therapy for hypothyroidism evaluated similar parameters except that LT3 was given twice daily (Supplementary Table S2). Overall, 88 patients (25–75 years old, 74 females) were studied while receiving LT4 (117.5–224 mcg/day) and LT3 (6.25–12.5 mcg B.I.D.) for 15–36 weeks (125–128). At the end, serum TSH was 7–90% lower in three studies and 3% higher in one study. Serum T4 was 18–38% lower when compared with the LT4 arm; serum T3 was 15–74% higher. No ADRs were reported except for one patient who dropped out due to tremulousness, fatigue, and poor performance (125) and four patients due to palpitation (127). Body weight, heart rate, lipid levels, and blood pressure were not different when compared with LT4 therapy (125,127).
In a specific attempt to identify adverse outcomes for patients on LT3, cardiovascular, skeleton, and mental outcomes were assessed through an observational study during 1997 and 2014 in the Scottish town of Tayside (129). The total follow-up was 280,334 person-years with a mean follow-up of 9.3 years (SD 5.6) and a maximum follow-up of 17.3 years. Compared with the nearly 34,000 patients taking only LT4, those using LT4+LT3 (n = 327) or LT3 alone (n = 73) had no increased mortality or morbidity risk due to cardiovascular disease, AF, or fractures after adjusting for age; the number of prescriptions for bisphosphonates or statins were similar. Remarkably, there was an increased risk of new prescriptions for antipsychotic medication (HR 2.26 [CI 1.64–3.11], p < 0.0001), proportional to the number of LT3 prescriptions (129). This, of course, needs to be assessed in other series but it adds to the idea that treatment with TH may unpredictably interfere with psychiatric outcomes (129).
Summary
Trials following almost a 1000 patients observed for as long as 1 year indicate that the combination of LT4 and LT3 maintains clinical and biochemical euthyroidism in hypothyroid patients. To initiate combination therapy, a given amount of the regular LT4 dose was replaced for LT3, while maintaining serum TSH within the normal range. The peaks of serum T3 observed after the LT3 tablets only minimally affected serum TSH, heart rate, and blood pressure; the frequency of ADRs was similar to patients taking LT4. Bone turnover markers were studied in two trials, and they remained within normal range. The Tayside study, although retrospective, provides no evidence that long-term combination therapy poses a risk for the cardiovascular or skeleton systems. At this time, the AACE and Latin American Thyroid Society guidelines do not support the use of combination therapy as a form of TH replacement in patients with primary hypothyroidism (105,130). At the same time, the ATA, BTA, ETA, and NICE guidelines take a more permissive stance. They recommend against the routine use of combination therapy but recognize that a trial of LT4+LT3 can be attempted on a case-by-case basis for those patients who remain symptomatic on LT4 alone (Table 1) (9,10,29,30).
Clinical trials in which liothyronine therapy was used in euthyroid individuals
A search in PubMed reveals that TH, including LT3, has been used in a variety of conditions other than hypothyroidism. In many instances, this practice is controversial, and in some cases flat out condemned by the ATA and AACE clinical guidelines (10,11). Nevertheless, making it clearly known that this is not being recommended, as controversy and restrictions exist for such types of unconventional TH usage, we feel that the inclusion of these studies here is justified as the results can be useful for the purposes of this discussion.
The LT3 has been used in patients with major depressive disorder as an adjuvant to the conventional treatment with anti-depressants, even if the patients are euthyroid (Supplementary Table S3) (131–144). We identified 12 prospective clinical trials conducted between 1970s and 2018 (Supplementary Table S2) in which a regimen with LT3 was used. Overall, 364 adult (<65 years old) patients (200 females) were treated with 25–50 mcg/day LT3 for 2–16 weeks. The ADRs to LT3 were not considered a primary outcome in any of the studies and only five reported specifically on palpitation, heart rate, and tremor, with the remainder only mentioning nonspecific ADRs (Supplementary Table S3). Remarkably, in none of these 12 studies did the association of LT3 result in ADRs (131–140,143,144). A subsequent meta-analysis that included eight studies (five clinical trials described earlier plus three observational studies) failed to identify differences in ADRs in LT3 versus control patients (145). Along these lines, a second meta-analysis of six trials confirmed that daily administration of 25 mcg LT3 during 3–4 weeks was well tolerated by patients, with no specific ADRs reported (142). Also, in a much older population (22 patients; 81 ± 8 years old), treatment with LT3 (25 mcg/day) for 4 weeks was associated with a 25% elevation in fasting serum T3 and an 85% reduction in serum TSH (dropped to the lower limit of the reference range). Notably, the study specifically looked for signs of agitation as well as heat intolerance and cardiovascular or gastrointestinal symptoms, but no ADRs were observed (146). In a separate study, 60 patients (46 ± 11 years old; 36 females) received LT3 at 25 or 50 mcg/day for 8 weeks (141). As a result, serum T3 levels increased by ∼25 and ∼50% in the 25- and 50 mcg-patient groups, respectively. In the 25 mcg-patients, ADRs were not different than in patients who received placebo (18%). Only in the 50 mcg-patient group, about 30% of the patients exhibited ADRs (palpitation, sweating, and/or nervousness) that either interfered with their normal functioning or outweighed the therapeutic benefit (141).
The LT3 has been used in obese patients with different metabolic objectives, for example to accelerate weight loss and reduce serum cholesterol levels (Supplementary Table S4). We identified a clinical trial that included eight euthyroid, morbidly obese patients who were instructed to take one LT3 tablet (25 mcg/tablet) three times daily for 30 weeks, increasing the dose by one tablet daily each week to maintain weight loss for as long as they did not develop ADRs (147). Overall, patients received a mean of 11 tablets per day (range 6–20). They lost ∼25 lbs, and serum total cholesterol levels dropped by ∼80 mg/dL. As far as ADRs were concerned, two individuals dropped out of the study due to nervousness and increased heart rate, and hyperglycemia was detected in one patient with a family history of diabetes; all other individuals developed tachycardia (+18 bpm), a mild elevation in systolic blood pressure (+9 mmHg), and a reduction in diastolic blood pressure (−13 mmHg) (147). A group of 6 similar randomized trials analyzed weight loss and other metabolic parameters in 60 obese individuals (17–68 years old; 40% females) who were given LT3 (25–225 mcg/day) in combination with caloric restriction for 3 days to 12 weeks (148–153). Treatment with LT3 accelerated weight loss, which was associated with a rise in urinary nitrogen, an indication that lean body mass was being lost. The ADRs prompted four patients to drop out of the study. In the patients who completed the studies, the ADRs occurred in patients taking 225 mcg/day LT3 daily: 21 accelerated heart rate, with 1 patient developing AF; 6 patients reported tremor; 1 patient reported weakness; and 1 patient indicated nervousness (148–153). In a separate clinical trial, LT3 was given to nine adult individuals (32.2 ± 8.6 years old; six females) subsequent to a period of caloric restriction and weight loss (154), given that serum T3 levels are known to decrease after weight loss (155). Patients were treated with 25 mcg/day LT3 given either fasting in the morning or 12.5 mcg/day B.I.D. during 5 weeks. Doses were titrated to maintain T3 serum levels within 80–220 ng/dL (blood collected at 8 AM fasting). Treatment with LT3 increased serum T3 levels by ∼20% (102 ± 14 to 121 ± 13 ng/dL), whereas serum TSH levels dropped by ∼75% (1.95 ± 0.65 to 0.51 ± 0.20 μU/L). The LT3 treatment minimally accelerated epinephrine and norepinephrine urinary excretion, but no ADRs were reported (154).
Summary
The administration of a wide range of LT3 doses to psychiatric or morbidly obese euthyroid patients illustrated that an LT3 dose of 25 mcg/day was well tolerated for several months, with no ADRs. A number of parameters, including cardiovascular function, were closely monitored and found not to be affected. However, higher doses of LT3 were clearly associated with cardiovascular and metabolic ADRs, even as they achieved the desired outcome in some cases. This is a point of concern given the great interest in the utilization of LT3 for many nonthyroidal conditions. A search on ClinicalTrials.gov revealed a large number of ongoing clinical trials with LT3, not only for hypothyroidism but also for the novel or adjunctive management of fibromyalgia, heart failure, acute respiratory distress syndrome, Alzheimer's disease, and other conditions (44). Even if positive outcomes are achieved with a relatively low dose of LT3, it is possible that the scope and/or threshold for ADRs are different for each clinical condition. Thus, safety must be carefully addressed and defined in each case, even if beneficial outcomes are achieved. The ATA and AACE guidelines recommend against the use of TH therapy for conditions other than hypothyroidism (10,11).
Conclusions
-
1.
There is universal consensus among all clinical guidelines that newly diagnosed hypothyroid patients should be treated with LT4. Persistent symptoms by some hypothyroid patients on LT4 monotherapy are not uncommon and should prompt an investigation of other conditions that may present with similar symptoms. A trial of combination therapy with LT4+LT3 can be considered for those patients who have unambiguously not benefited from LT4.
-
2.
Various LT3/LT4 ratios were tested in clinical trials. Mathematical modeling and observations in these trials indicate that reducing the LT4 dose by 25 mcg/day and adding 2.5–7.5 mcg LT3 once or twice a day is a reasonable starting point (nota bene, the thyroid only secretes ∼5 mcg T3/day), provided that serum TSH is kept within normal range. Transient episodes of hypertriiodothyroninemia with these doses of LT3 are unlikely to reach outside the normal reference range and have not been associated with increased incidence of ADRs when compared with LT4 alone. If serum T3 levels are needed, blood samples should be obtained while patients are fasting and approximately three hours post-dose.
-
3.
The DTE is a non-FDA approved form of combination therapy in which the LT3/LT4 ratio is relatively higher. The mean daily dose needed to restore euthyroidism and bring serum TSH back to the reference range contains ∼11 mcg T3; some patients may require higher DTE doses, which can bring the daily T3 dose to ∼24 mcg. Whereas these doses are more likely to transiently elevate serum T3 above the normal reference range, ADRs have not been reported in clinical trials in which serum TSH was maintained within the normal range.
-
4.
Clinical guidelines alert for potential safety concerns of combination therapy, such as the presence of supraphysiologic serum T3 levels and a paucity of long-term safety outcome data. However, the data obtained from (i) all published clinical trials, which total ∼1000 patients followed for up to ∼1 year, and (ii) an observational study of 400 patients with a mean follow-up of ∼9 years, do not indicate increased mortality or morbidity risk due to cardiovascular disease, AF, or fractures after adjusting for age when compared with patients taking only LT4. Of note, most clinical trials to date only recruited uncomplicated hypothyroid patients, namely, those who were not pregnant or breast-feeding, patients with cardiovascular preconditions, or patients concomitantly receiving other drugs, including therapy with sympathomimetics that could amplify many of the T3-dependent biological effects. An analysis of parameters to be considered in future clinical trials is available (156).
-
5.
The LT3 alone has been used in the treatment of hypothyroidism, but it is rarely seen these days. The doses that restore clinical euthyroidism and normalize serum TSH range between 30 and 45 mcg/day; these doses will bring serum T3 levels well above the upper limit of the normal reference range for several hours. Even though LT3 monotherapy has not been associated with ADRs, the trials are few, lasted only a few weeks, and include a relatively small number of patients. We are not aware of any conceptual or practical reason that justifies long-term treatment of hypothyroid patients with LT3 alone.
Supplementary Material
Acknowledgment
The authors thank Dr. Ronald Cohen for great discussions and suggestions.
Author Disclosure Statement
A.C.B. is a consultant for Allergan, Inc. and Synthonics, Inc.; S.P. is chairman of the scientific board for Synthonics, Inc.; and the other authors have nothing to disclose.
Funding Information
National Institutes of Health DK58538 and DK116396.
Supplementary Material
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. 2017. Hypothyroidism. Lancet 390:1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toft AD. 1994. Thyroxine therapy. N Engl J Med 331:174–180 [DOI] [PubMed] [Google Scholar]
- 3. Oppenheimer JH, Braverman LE, Toft A, Jackson IM, Ladenson PW. 1995. A therapeutic controversy. Thyroid hormone treatment: when and what? J Clin Endocrinol Metab 80:2873–2883 [DOI] [PubMed] [Google Scholar]
- 4. Helfand M, Crapo LM. 1990. Monitoring therapy in patients taking levothyroxine. Ann Intern Med 113:450–454 [DOI] [PubMed] [Google Scholar]
- 5. Mandel SJ, Brent GA, Larsen PR. 1993. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med 119:492–502 [DOI] [PubMed] [Google Scholar]
- 6. McDermott MM, Ridgway EC 2008 Hypothyroidism In: Cooper DS (ed) Medical Management of Thyroid Disease. Informa Healthcare, New York, pp 145–201 [Google Scholar]
- 7. Thayakaran R, Adderley NJ, Sainsbury C, Torlinska B, Boelaert K, Sumilo D, Price M, Thomas GN, Toulis KA, Nirantharakumar K. 2019. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ 366:l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, Brix TH, Hegedus L. 2018. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 28:566–574 [DOI] [PubMed] [Google Scholar]
- 9. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J 1:55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone Replacement. 2014. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. 2012. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Practice 18:988–1028 [DOI] [PubMed] [Google Scholar]
- 12. Ito M, Miyauchi A, Morita S, Kudo T, Nishihara E, Kihara M, Takamura Y, Ito Y, Kobayashi K, Miya A, Kubota S, Amino N. 2012. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol 167:373–378 [DOI] [PubMed] [Google Scholar]
- 13. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. 2011. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 6:e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson SJ, McAninch EA, Bianco AC. 2016. Is a normal TSH synonymous with “Euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 101:4964–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. 2016. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid 26:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stock JM, Surks MI, Oppenheimer JH. 1974. Replacement dosage of L-thyroxine in hypothyroidism. A re-evaluation. N Engl J Med 290:529–533 [DOI] [PubMed] [Google Scholar]
- 17. Gorman CA, Jiang NS, Ellefson RD, Elveback LR. 1979. Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J Clin Endocrinol Metab 49:1–7 [DOI] [PubMed] [Google Scholar]
- 18. Ridgway EC, Cooper DS, Walker H, Daniels GH, Chin WW, Myers G, Maloof F. 1980. Therapy of primary hypothyroidism with L-triiodothyronine: discordant cardiac and pituitary responses. Clin Endocrinol 13:479–488 [DOI] [PubMed] [Google Scholar]
- 19. McAninch EA, Rajan KB, Miller CH, Bianco AC 2018 Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab [Epub ahead of print]; DOI: 10.1210/jc.2018-01361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee YK, Lee H, Han S, Jung H, Shin DY, Nam KH, Chung WY, Lee EJ. 2019. Association between thyroid-stimulating hormone level after total thyroidectomy and hypercholesterolemia in female patients with differentiated thyroid cancer: a retrospective study. J Clin Med 8:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito M, Miyauchi A, Hisakado M, Yoshioka W, Ide A, Kudo T, Nishihara E, Kihara M, Ito Y, Kobayashi K, Miya A, Fukata S, Nishikawa M, Nakamura H, Amino N. 2017. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid 27:484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor P, Bianco AC. 2019. Urgent need for further research in subclinical hypothyroidism. Nat Rev Endocrinol 15:503–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saravanan P, Visser TJ, Dayan CM. 2006. Psychological well-being correlates with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab 91:3389–3393 [DOI] [PubMed] [Google Scholar]
- 24. Wiersinga WM. 2017. Therapy of endocrine disease: T4 + T3 combination therapy: is there a true effect? Eur J Endocrinol 177:R287–R296 [DOI] [PubMed] [Google Scholar]
- 25. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, Kopp PA, Ross DS, Samuels MH, Sawka AM, Taylor PN, Jonklaas J, Bianco AC. 2018. An online survey of hypothyroid patients captured predominantly dissatisfied individuals. Thyroid 28:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akirov A, Fazelzad R, Ezzat S, Thabane L, Sawka AM. 2019. A systematic review and meta-analysis of patient preferences for combination thyroid hormone treatment for hypothyroidism. Front Endocrinol (Lausanne) 10:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weetman AP. 2006. Whose thyroid hormone replacement is it anyway? Clin Endocrinol 64:231–233 [DOI] [PubMed] [Google Scholar]
- 28. Ladenson PW. 2002. Psychological wellbeing in patients. Clin Endocrinol 57:575–576 [DOI] [PubMed] [Google Scholar]
- 29. Okosieme O, Gilbert J, Abraham P, Boelaert K, Dayan C, Gurnell M, Leese G, McCabe C, Perros P, Smith V, Williams G, Vanderpump M. 2016. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol 84:799–808 [DOI] [PubMed] [Google Scholar]
- 30. NIfHaCaE 2019 Thyroid Disease: assessment and Management. Available at www.nice.org.uk/guidance/ng145 (accessed January31, 2020)
- 31. ClinCalc.com The top 300 of 2019. Available at https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed January31, 2020).
- 32. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 33. Zavacki AM, Harney JW, Li J, Gereben B, Fekete C, Bianco AC. 2007. TEB4 Is an Endoplasmic Reticulum Ubiquitin Ligase That Mediates Type 2 Iodothyronine Deiodinase (D2) Degradation 89th Annual Meeting of the Endocrine Society. Vol OR7-1, Toronto, Canada [Google Scholar]
- 34. Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GW, Bocco B. 2019. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev 40:1000–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ. 2008. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 93:2300–2306 [DOI] [PubMed] [Google Scholar]
- 36. Laurberg P, Vestergaard H, Nielsen S, Christensen SE, Seefeldt T, Helleberg K, Pedersen KM. 2007. Sources of circulating 3,5,3′-triiodothyronine in hyperthyroidism estimated after blocking of type 1 and type 2 iodothyronine deiodinases. J Clin Endocrinol Metab 92:2149–2156 [DOI] [PubMed] [Google Scholar]
- 37. Abdalla SM, Bianco AC. 2014. Defending plasma T3 is a biological priority. Clin Endocrinol 81:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christoffolete MA, Arrojo e Drigo R, Gazoni F, Tente SM, Goncalves V, Amorim BS, Larsen PR, Bianco AC, Zavacki AM. 2007. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology 148:954–960 [DOI] [PubMed] [Google Scholar]
- 39. Galton VA, Schneider M, Clark AS, Germain DL. 2009. Life without T4 to T3 conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fliers E, Bianco AC, Langouche L, Boelen A. 2015. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 3:816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van den Berghe G, de Zegher F, Baxter RC, Veldhuis JD, Wouters P, Schetz M, Verwaest C, Van der Vorst E, Lauwers P, Bouillon R, Bowers CY. 1998. Neuroendocrinology of prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab 83:309–319 [DOI] [PubMed] [Google Scholar]
- 42. Idrees T, Price JD, Piccariello T, Bianco AC. 2019. Sustained release T3 therapy: animal models and translational applications. Front Endocrinol (Lausanne) 10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gabay M. 2014. The drug quality and security act. Hosp Pharm 49:615–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Medicine NLo. ClinicalTrials.gov Available at ClinicalTrials.gov (accessed January31, 2020)
- 45. Van Tassell B, Wohlford GFt, Linderman JD, Smith S, Yavuz S, Pucino F, Celi FS. 2019. Pharmacokinetics of L-triiodothyronine in patients undergoing thyroid hormone therapy withdrawal. Thyroid 29:1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jonklaas J, Burman KD, Wang H, Latham KR. 2015. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit 37:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hays MT. 1970. Absorption of triiodothyronine in man. J Clin Endocrinol Metab 30:675–676 [DOI] [PubMed] [Google Scholar]
- 48. Ban Y, Iino S, Hamada N, Momotani N, Minura T, Nishikawa Y, Ito K. 1979. Studies on the changes with time in the concentrations of serum triiodothyronine, thyroxine and thyrotropin after the oral administration of various thyroid hormones (author's transl) [in Japanese]. Nihon Naibunpi Gakkai Zasshi 55:1038–1050 [DOI] [PubMed] [Google Scholar]
- 49. Surks MI, Schadlow AR, Oppenheimer JH. 1972. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest 51:3104–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jonklaas J. 2016. Risks and safety of combination therapy for hypothyroidism. Expert Rev Clin Pharmacol 9:1057–1067 [DOI] [PubMed] [Google Scholar]
- 51. Jonklaas J, Burman KD. 2016. Daily administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid 26:770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saberi M, Utiger RD. 1974. Serum thyroid hormone and thyrotropin concentrations during thyroxine and triiodothyronine therapy. J Clin Endocrinol Metab 39:923–927 [DOI] [PubMed] [Google Scholar]
- 53. Martin NM, Small CJ, Lee JL, Ellis S, Dhillo WS, Smith KL, Kong WM, Frost GS, Bloom SR. 2007. Low-dose oral tri-iodothyronine does not directly increase food intake in man. Diabetes Obes Metab 9:435–437 [DOI] [PubMed] [Google Scholar]
- 54. Brent GA. 2012. Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hones GS, Rakov H, Logan J, Liao XH, Werbenko E, Pollard AS, Praestholm SM, Siersbaek MS, Rijntjes E, Gassen J, Latteyer S, Engels K, Strucksberg KH, Kleinbongard P, Zwanziger D, Rozman J, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Klein-Hitpass L, Kohrle J, Armstrong DL, Grontved L, Bassett JHD, Williams GR, Refetoff S, Fuhrer D, Moeller LC. 2017. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc Natl Acad Sci U S A 114:E11323–E11332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McAninch EA, Miller BT, Ueta CB, Jo S, Kim BW. 2015. Thyroid hormone at near physiologic concentrations acutely increases oxygen consumption and extracellular acidification in LH86 hepatoma cells. Endocrinology 156:4325–4335 [DOI] [PubMed] [Google Scholar]
- 57. Lev-Ran A. 1983. Part-of-the-day hypertriiodothyroninemia caused by desiccated thyroid. JAMA 250:2790–2791 [PubMed] [Google Scholar]
- 58. Lovejoy JC, Smith SR, Bray GA, DeLany JP, Rood JC, Gouvier D, Windhauser M, Ryan DH, Macchiavelli R, Tulley R. 1997. A paradigm of experimentally induced mild hyperthyroidism: effects on nitrogen balance, body composition, and energy expenditure in healthy young men. J Clin Endocrinol Metab 82:765–770 [DOI] [PubMed] [Google Scholar]
- 59. Lovejoy JC, Smith SR, Zachwieja JJ, Bray GA, Windhauser MM, Wickersham PJ, Veldhuis JD, Tulley R, de la Bretonne JA. 1999. Low-dose T(3) improves the bed rest model of simulated weightlessness in men and women. Am J Physiol 277:E370–E379 [DOI] [PubMed] [Google Scholar]
- 60. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 61. Williams GR, Bassett JHD. 2018. Thyroid diseases and bone health. J Endocrinol Invest 41:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anderson JL, Jacobs V, May HT, Bair TL, Benowitz BA, Lappe DL, Muhlestein JB, Knowlton KU, Bunch TJ. 2020. Free thyroxine within the normal reference range predicts risk of atrial fibrillation. J Cardiovasc Electrophysiol 31:18–29 [DOI] [PubMed] [Google Scholar]
- 63. Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CM, Trompet S, Arking DE, Geelhoed B, Guo X, Kleber ME, Lin HJ, Lin H, MacFarlane P, Selvin E, Shaffer C, Smith AV, Verweij N, Weiss S, Cappola AR, Dorr M, Gudnason V, Heckbert S, Mooijaart S, Marz W, Psaty BM, Ridker PM, Roden D, Stott DJ, Volzke H, Benjamin EJ, Delgado G, Ellinor P, Homuth G, Kottgen A, Jukema JW, Lubitz SA, Mora S, Rienstra M, Rotter JI, Shoemaker MB, Sotoodehnia N, Taylor KD, van der Harst P, Albert CM, Chasman DI. 2019. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol 4:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JR, Weedon MN, Singleton A, Hernandez D, Evans J, Durant C, Ferrucci L, Melzer D, Saravanan P, Visser TJ, Ceresini G, Hattersley AT, Vaidya B, Dayan CM, Frayling TM. 2008. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab 93:3075–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCarrison R. 1917. Myxedema: The Thyroid Gland in Health and Disease. William Wood & Company, New York [Google Scholar]
- 66. Jackson AS. 1926. Diagnosis and Treatment of Myxedema and Cretinism Goiter and Other Disease of the Thyroid Gland. Paul B. Hoeber, Inc., New York [Google Scholar]
- 67. Hartsock CL 1932 Clinical Aspects of Hypothyroidism. In: Crile G (ed) Diagnosis and Treatment of Diseases of the Thyroid Gland. W. B. Saundres Co., Philadelphia, pp 86–100 [Google Scholar]
- 68. Means JH 1948 Myxedema: diagnosis, treatment, special features and complications. In: Means JH (ed) The Thyroid and Its Diseases. J.B. Lippincott Co., Philadelphia, pp 241–273 [Google Scholar]
- 69. McGavack TH 1951 Hypothyroidism: II. After maturity. Adult myxedema. In: McGavack TH (ed) The Thyroid. The C. V. Mosby Co, St Louis, pp 374–417 [Google Scholar]
- 70. Williams RH 1955 The thyroid. In: Williams RH (ed) Textbook of Endocrinology. W. B. Saunders Co., Philadelphia, pp 99–230 [Google Scholar]
- 71. Van Middlesworth L 1968 Evaluation of the measurement of response of the ankle jerk in thyroid disease. In: Astwood EB, Cassidy CE (eds) Clinical Endocrinology, vol. II. Grune & Straton, New York, pp 291–295 [Google Scholar]
- 72. Werner SC 1971 Hypothyroidism. In: Werner SC, Ingbar SH (eds) The Thyroid. Harper & Row Publishers, New York, pp 714–715 [Google Scholar]
- 73. Means JH 1937 Mixedema. In: Means JH (ed) The Thyroid and Its Diseases. J. B. Lippincott Co., Philadelphia, pp 228–261 [Google Scholar]
- 74. Sloan EP 1936 Hypothyroidism. In: Sloan EP (ed) The Thyroid. Charles C. Thomas, Springfield, IL, pp 353–375 [Google Scholar]
- 75. Wolf W 1939 The thyroid gland. In: Wolf W (ed) Endocrinology in Modern Practice. W. B. Saunders Co., Philadelphia, pp 238–335 [Google Scholar]
- 76. Means JH, DeGroot LJ, Stanbury JB. 1963. Adult Hypothyroid States: The Thyroid and Its Diseases. McGraw-Hill Book Co., New York [Google Scholar]
- 77. Kaufman SC, Gross TP, Kennedy DL. 1991. Thyroid hormone use: trends in the United States from 1960 through 1988. Thyroid 1:285–291 [DOI] [PubMed] [Google Scholar]
- 78. Wool MS, Selenkow HA. 1965. Physiologic combinations of synthetic thyroid hormones in myxedema. Clin Pharmacol Ther 6:710–715 [DOI] [PubMed] [Google Scholar]
- 79. Taylor S. 1961. Thyroid extract. Lancet 1:332–333 [DOI] [PubMed] [Google Scholar]
- 80. Stephenson NR. 1967. The standardization of desiccated thyroid. Ann Intern Med 67:211–212 [DOI] [PubMed] [Google Scholar]
- 81. Blumberg KR, Mayer WJ, Parikh DK, Schnell LA. 1993. Liothyronine and levothyroxine in Armour thyroid. J Pharm Sci 76:346–347 [DOI] [PubMed] [Google Scholar]
- 82. Macgregor AG. 1961. Why does anybody use thyroid B.P.? Lancet 1:329–332 [DOI] [PubMed] [Google Scholar]
- 83. Catz B, Ginsburg E, Salenger S. 1962. Clinically inactive thyroid U.S.P. A preliminary report. N Engl J Med 266:136–137 [DOI] [PubMed] [Google Scholar]
- 84. Braverman LE, Ingbar SH, Sterling K. 1970. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic subjects. J Clin Invest 49:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Braverman LE, Vagenakis A, Downs P, Foster AE, Sterling K, Ingbar SH. 1973. Effects of replacement doses of sodium L-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest 52:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hamburger JI 1969 Management of hypothyroidism. In: Hamburger JI (ed) Diagnosis and Management of Common Thyroid Problems. Charles C. Thomas, Springfield, IL, pp 104–111 [Google Scholar]
- 87. Mateo RCI, Hennessey JV. 2019. Thyroxine and treatment of hypothyroidism: seven decades of experience. Endocrine 66:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McDermott MM, Ridgway EC 2001 Diagnosis and treatment of hypothyroidism In: Cooper DS (ed) Medical Management of Thyroid Disease. Marcel Dekker, Inc., New York, pp 135–186 [Google Scholar]
- 89. Utiger RD. 1971. Thyrotrophin radioimmunoassay: another test of thyroid function. Ann Intern Med 74:627–629 [DOI] [PubMed] [Google Scholar]
- 90. Sawin CT, Hershman JM, Fernandez-Garcia R, Ghazvinian S, Ganda OP, Azukizawa M. 1978. A comparison of thyroxine and desicatted thyroid in patients with primary hypothyroidism. Metabolism 27:1518–1525 [DOI] [PubMed] [Google Scholar]
- 91. McAninch EA, Bianco AC. 2016. The history and future of treatment of hypothyroidism. Ann Intern Med 164:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pharmacopeia 1985 U.S., 21st Revision U.S.P. Convention, Rockville, MD, pp 1893–1895 [Google Scholar]
- 93. Jackson IM, Cobb WE. 1978. Why does anyone still use desiccated thyroid USP? Am J Med 64:284–288 [DOI] [PubMed] [Google Scholar]
- 94. Werner SC 1978 Hypothyroidism. In: Werner SC, Ingbar SH (eds) The Thyroid. Harper & Row Publishers, New York, pp 965–970 [Google Scholar]
- 95. Penny R, Frasier SD. 1980. Elevated serum concentrations of triiodothyronine in hypothyroid patients. Values for patients receiving USP thyroid. Am J Dis Child 134:16–18 [DOI] [PubMed] [Google Scholar]
- 96. Sawin CT, Castelli WP, Hershman JM, McNamara P, Bacharach P. 1985. The aging thyroid. Thyroid deficiency in the Framingham study. Arch Intern Med 145:1386–1388 [PubMed] [Google Scholar]
- 97. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. 2013. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab 98:1982–1990 [DOI] [PubMed] [Google Scholar]
- 98. Tariq A, Wert Y, Cheriyath P, Joshi R. 2018. Effects of long-term combination LT4 and LT3 therapy for improving hypothyroidism and overall quality of life. South Med J 111:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. By the American Geriatrics Society Beers Criteria Update Expert Panel 2019. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc 67:674–694 [DOI] [PubMed] [Google Scholar]
- 100. Asper SP Jr., Selenkow HA, Plamondon CA. 1953. A comparison of the metabolic activities of 3,5,3-L-triiodothyronine and L-thyroxine in myxedema. Bull Johns Hopkins Hosp 93:164–198 [PubMed] [Google Scholar]
- 101. Blackburn CM, McConahey WM, Keating RF, Albert A. 1954. Calorigenic effects of single intravenous doses of L-triiodothyronine and L-thyroxine in myxedematous persons. J Clin Invest 33:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Maciel RMB 1977 Development of a thyroglobulin radioimmunoassay and its application on the follow-up of patients with differentiated thyroid cancer. [PhD Thesis]. Sao Paulo, SP: Department of Medicine, Federal University of Sao Paulo [Google Scholar]
- 103. Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. 2010. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol 72:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. 2011. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab 96:3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA. 2012. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 22:1200–1235 [DOI] [PubMed] [Google Scholar]
- 106. American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 107. Ingbar SH, Woeber KA 1974 The thyroid gland In: Williams RH (ed) Textbook of Endocrinology. W. B. Saunders, Philadelphia, pp 95–232 [Google Scholar]
- 108. Smith RN, Taylor SA, Massey JC. 1970. Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J 4:145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr.. 1999. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 340:424–429 [DOI] [PubMed] [Google Scholar]
- 110. Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ Jr.. 2002. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves' disease. Endocrine 18:129–133 [DOI] [PubMed] [Google Scholar]
- 111. Bunevicius R, Prange AJ. 2000. Mental improvement after replacement therapy with thyroxine plus triiodothyronine: relationship to cause of hypothyroidism. Int J Neuropsychopharmacol 3:167–174 [DOI] [PubMed] [Google Scholar]
- 112. Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, Dhaliwal SS, Chew GT, Bhagat MC, Cussons AJ. 2003. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 88:4543–4550 [DOI] [PubMed] [Google Scholar]
- 113. Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Dabers T, Kirsch G, Sanger E, Engel G, Hamm AO, Nauck M, Meng W. 2004. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14: 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol 60:750–757 [DOI] [PubMed] [Google Scholar]
- 114. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. 2005. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab 90:805–812 [DOI] [PubMed] [Google Scholar]
- 115. Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. 2005. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract 11:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. 2005. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 90:2666–2674 [DOI] [PubMed] [Google Scholar]
- 117. Escobar-Morreale HF, Botella-Carretero JI, Gomez-Bueno M, Galan JM, Barrios V, Sancho J. 2005. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med 142:412–424 [DOI] [PubMed] [Google Scholar]
- 118. Regalbuto C, Maiorana R, Alagona C, Paola RD, Cianci M, Alagona G, Sapienza S, Squatrito S, Pezzino V. 2007. Effects of either LT4 monotherapy or LT4/LT3 combined therapy in patients totally thyroidectomized for thyroid cancer. Thyroid 17:323–331 [DOI] [PubMed] [Google Scholar]
- 119. Slawik M, Klawitter B, Meiser E, Schories M, Zwermann O, Borm K, Peper M, Lubrich B, Hug MJ, Nauck M, Olschewski M, Beuschlein F, Reincke M. 2007. Thyroid hormone replacement for central hypothyroidism: a randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. J Clin Endocrinol Metab 92:4115–4122 [DOI] [PubMed] [Google Scholar]
- 120. Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. 2009. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol 161:895–902 [DOI] [PubMed] [Google Scholar]
- 121. Fadeyev VV, Morgunova TB, Melnichenko GA, Dedov II. 2010. Combined therapy with L-thyroxine and L-triiodothyronine compared to L-thyroxine alone in the treatment of primary hypothyroidism. Hormones (Athens) 9:245–252 [DOI] [PubMed] [Google Scholar]
- 122. Trimboli P, Centanni M, Virili C. 2016. Liquid liothyronine to obtain target TSH in differentiated thyroid cancer patients. Endocr J 63:563–567 [DOI] [PubMed] [Google Scholar]
- 123. Kaminski J, Miasaki FY, Paz-Filho G, Graf H, Carvalho GA. 2016. Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch Endocrinol Metab 60:562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Krysiak R, Szkrobka W, Okopien B. 2018. Sexual function and depressive symptoms in young women with hypothyroidism receiving levothyroxine/liothyronine combination therapy: a pilot study. Curr Med Res Opin 34:1579–1586 [DOI] [PubMed] [Google Scholar]
- 125. Clyde PW, Harari AE, Getka EJ, Shakir KM. 2003. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 290:2952–2958 [DOI] [PubMed] [Google Scholar]
- 126. Solter D, Solter M. 2012. Benefit of combined triiodothyronine (LT(3)) and thyroxine (LT(4)) treatment in athyreotic patients unresponsive to LT(4) alone. Exp Clin Endocrinol Diabetes 120:121–123 [DOI] [PubMed] [Google Scholar]
- 127. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, Momtazi S, Musavinasab N, Hayatbakhsh MR. 2009. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res 34:80–89 [DOI] [PubMed] [Google Scholar]
- 128. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. 2003. Does a combination regimen of thyroxine (T4) and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 88:4551–4555 [DOI] [PubMed] [Google Scholar]
- 129. Leese GP, Soto-Pedre E, Donnelly LA. 2016. Liothyronine use in a 17 year observational population-based study—the tears study. Clin Endocrinol 85:918–925 [DOI] [PubMed] [Google Scholar]
- 130. Brenta G, Vaisman M, Sgarbi JA, Bergoglio LM, Andrada NC, Bravo PP, Orlandi AM, Graf H, Task Force on Hypothyroidism of the Latin American Thyroid Society. 2013. Clinical practice guidelines for the management of hypothyroidism [in Portuguese]. Arq Bras Endocrinol Metab 57:265–291 [DOI] [PubMed] [Google Scholar]
- 131. Steiner M RM, Elizur A, Blum I, Atsmon A, Davidson S. 1978. Failure of L-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 23:655–659 [Google Scholar]
- 132. Joffe RT, Singer W. 1990. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 32:241–251 [DOI] [PubMed] [Google Scholar]
- 133. Joffe RT. 1988. T3 and lithium potentiation of tricyclic antidepressants. Am J Psychiatry 145:1317–1318 [DOI] [PubMed] [Google Scholar]
- 134. Walshaw PD, Gyulai L, Bauer M, Bauer MS, Calimlim B, Sugar CA, Whybrow PC. 2018. Adjunctive thyroid hormone treatment in rapid cycling bipolar disorder: a double-blind placebo-controlled trial of levothyroxine (L-T4) and triiodothyronine (T3). Bipolar Disord 20:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mohagheghi A, Arfaie A, Amiri S, Nouri M, Abdi S, Safikhanlou S. 2015. Preventive effect of liothyronine on electroconvulsive therapy-induced memory deficit in patients with major depressive disorder: a double-blind controlled clinical trial. Biomed Res Int 2015:503918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Garlow SJ, Dunlop BW, Ninan PT, Nemeroff CB. 2012. The combination of triiodothyronine (T3) and sertraline is not superior to sertraline monotherapy in the treatment of major depressive disorder. J Psychiatr Res 46:1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Posternak M, Novak S, Stern R, Hennessey J, Joffe R, Prange A Jr., Zimmerman M. 2008. A pilot effectiveness study: placebo-controlled trial of adjunctive L-triiodothyronine (T3) used to accelerate and potentiate the antidepressant response. Int J Neuropsychopharmacol 11:15–25 [DOI] [PubMed] [Google Scholar]
- 138. Cooper-Kazaz R, Apter JT, Cohen R, Karagichev L, Muhammed-Moussa S, Grupper D, Drori T, Newman ME, Sackeim HA, Glaser B, Lerer B. 2007. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry 64:679–688 [DOI] [PubMed] [Google Scholar]
- 139. Abraham G, Milev R, Stuart Lawson J. 2006. T3 augmentation of SSRI resistant depression. J Affect Disord 91:211–215 [DOI] [PubMed] [Google Scholar]
- 140. Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, Alpert JE, Warden D, Luther JF, Niederehe G, Lebowitz B, Shores-Wilson K, Rush AJ. 2006. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 163:1519–1530; quiz 1665. [DOI] [PubMed] [Google Scholar]
- 141. Appelhof BC, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Huyser J, Schene AH, Tijssen JG, Wiersinga WM. 2004. Triiodothyronine addition to paroxetine in the treatment of major depressive disorder. J Clin Endocrinol Metab 89:6271–6276 [DOI] [PubMed] [Google Scholar]
- 142. Altshuler LL, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, Leight KL, Whybrow PC. 2001. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 158:1617–1622 [DOI] [PubMed] [Google Scholar]
- 143. Gitlin MJ, Weiner H, Fairbanks L, Hershman JM, Friedfeld N. 1987. Failure of T3 to potentiate tricyclic antidepressant response. J Affect Disord 13:267–272 [DOI] [PubMed] [Google Scholar]
- 144. Coppen A, Whybrow PC, Noguera R, Maggs R, Prange AJ Jr.. 1972. The comparative antidepressant value of L-tryptophan and imipramine with and without attempted potentiation by liothyronine. Arch Gen Psychiatry 26:234–241 [DOI] [PubMed] [Google Scholar]
- 145. Aronson R, Offman HJ, Joffe RT, Naylor CD. 1996. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 53:842–848 [DOI] [PubMed] [Google Scholar]
- 146. Mori T, Inoue D, Kosugi S, Miyamoto M, Nishino K, Sagawa H, Akamizu T, Yokota T, Nakamura H, Namikawa M, Imura H. 1988. Effects of low dose L-triiodothyronine administration on mental, behavioural and thyroid states in elderly subjects. Endocrinol Jpn 35:585–592 [DOI] [PubMed] [Google Scholar]
- 147. Gwinup G, Poucher R. 1967. A controlled study of thyroid analogs in the therapy of obesity. Am J Med Sci 254:416–420 [DOI] [PubMed] [Google Scholar]
- 148. Gardner DF, Kaplan MM, Stanley CA, Utiger RD. 1979. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med 300:579–584 [DOI] [PubMed] [Google Scholar]
- 149. Pasquali R, Baraldi G, Biso P, Piazzi S, Patrono D, Capelli M, Melchionda N. 1984. Effect of ‘physiological’ doses of triiodothyronine replacement on the hormonal and metabolic adaptation to short-term semistarvation and to low-calorie diet in obese patients. Clin Endocrinol (Oxf) 21:357–367 [DOI] [PubMed] [Google Scholar]
- 150. Koppeschaar HP, Meinders AE, Schwarz F. 1983. Metabolic responses in grossly obese subjects treated with a very-low-calorie diet with and without triiodothyronine treatment. Int J Obes 7:133–141 [PubMed] [Google Scholar]
- 151. Moore R, Grant AM, Howard AN, Mills IH. 1980. Treatment of obesity with triiodothyronine and a very-low-calorie liquid formula diet. Lancet 1:223–226 [DOI] [PubMed] [Google Scholar]
- 152. Bray GA, Melvin KE, Chopra IJ. 1973. Effect of triiodothyronine on some metabolic responses of obese patients. Am J Clin Nutr 26:715–721 [DOI] [PubMed] [Google Scholar]
- 153. Hollingsworth DR, Amatruda TT Jr., Scheig R. 1970. Quantitative and qualitative effects of L-triiodothyronine in massive obesity. Metabolism 19:934–945 [DOI] [PubMed] [Google Scholar]
- 154. Rosenbaum M, Goldsmith RL, Haddad F, Baldwin KM, Smiley R, Gallagher D, Leibel RL. 2018. Triiodothyronine and leptin repletion in humans similarly reverse weight-loss-induced changes in skeletal muscle. Am J Physiol Endocrinol Metab 315:E771–E779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Gluer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. 2015. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 102:807–819 [DOI] [PubMed] [Google Scholar]
- 156. Cappola AR 2020 Design of the optimal trial of combination therapy. Front Endocrinol [Epub ahead of print]; DOI: 10.3389/fendo.2020.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.