Abstract
Objective
Recent research on open-label placebos, or placebos administered without deception or concealment, suggests that they can be effective in a variety of conditions. The current research sought to unpack the mechanisms underlying the treatment efficacy of open-label placebos.
Method
A health care provider induced an allergic reaction in 148 participants via a histamine skin prick test. Participants were then exposed to 1 of 4 conditions additively leveraging various mechanisms of open-label placebo treatments: a supportive patient-provider relationship, a medical ritual, positive expectations, and a rationale about the power of placebos.
Results
There were no main effects of condition on allergic responses. However, participant beliefs about placebos moderated the effect of open-label placebo treatment condition on physiological allergic reactions: the condition including all 4 components of open-label placebos (a supportive patient-provider relationship, a medical ritual, positive expectations, and a rationale about the power of placebos) significantly reduced physiological allergic reaction among participants with a strong belief in placebos compared with participants in the control group.
Conclusion
Participants’ beliefs about placebos interact with information from the provider to reduce physiological allergic reactions in response to an open-label placebo treatment. This study underscores the importance of measuring and understanding how participants’ beliefs influence outcomes of treatment, and furthers our understanding of when and how open-label placebo treatments work.
Keywords: placebo, open-label placebo, beliefs, expectation, allergies
Research has demonstrated that placebo effects lead to clinically significant benefits in most conditions, including pain, anxiety, depression, Parkinson’s disease, asthma, allergies, immune deficiencies, Alzheimer’s disease, and recovery from surgery (Finniss, Kaptchuk, Miller, & Benedetti, 2010; Price, Finniss, & Benedetti, 2008). Yet, these placebo effects are often deemed irrelevant in clinical practice. One reason for this is that placebos are assumed to hinge on patients falsely believing they are taking a real medication when they are in fact taking a placebo. In other words, it is assumed that patients need to believe that the placebo pill is an active medication for healing effects to occur.
In recent years, however, several studies have begun examining the effects of placebos administered to patients without deception (“open-label” placebos). When open-label placebos are administered, patients are aware they are receiving an inert placebo treatment. In one such study, patients suffering from chronic irritable bowel syndrome (IBS) met with a physician who prescribed them placebo pills (blue and maroon gelatin capsules filled with avicel, a common inert filler used in pharmaceuticals) that were openly labeled as placebos (Kaptchuk et al., 2010). Patients took these pills twice daily for 2 weeks. Compared with a no-treatment control group who had the same quantity and quality of interactions with the physician, participants who took the open-label placebo pills had significantly improved IBS symptoms at the end of the study period (Kaptchuk et al., 2010). This finding—that nondeceptive, open-label placebos can lead to significant clinical benefits—has been supported in several studies of other conditions, including attention-deficit-hyperactivity disorder (ADHD), allergic rhinitis, migraines, and back pain (Carvalho et al., 2016; Kam-Hansen et al., 2014; Sandler & Bodfish, 2008; Sandler, Glesne, & Bodfish, 2010; Schaefer, Harke, & Denke, 2016; Schaefer, Sahin, & Berstecher, 2018). These studies demonstrate that the power of placebo effects does not rely wholly on the deceptive belief that one is taking an active medication.
What is responsible for the effects found in open-label placebo studies? Investigating the driving mechanisms of open-label placebo effects would be an important step toward harnessing those mechanisms alongside active medications in clinical practice. Although research indicates that the psychological and social elements that contribute to traditional placebo effects also influence active treatments, little has been done to utilize these forces deliberately in clinical care (Crum, Leibowitz, & Verghese, 2017). Understanding the mechanisms of open-label placebo treatments, where deception is removed, could help providers leverage the power of the placebo effect in clinical care.
Traditional placebo effects are presumed to be driven by three mechanisms: (a) a supportive patient-provider relationship, (b) conditioning processes driven by therapeutic rituals, and (c) positive expectations that the medication will produce some healing benefit (Finniss et al., 2010; Kaptchuk et al., 2010; Kaptchuk & Miller, 2015; Locher et al., 2017; Price et al., 2008). Similar mechanisms could be driving the effects found in open-label placebo studies (e.g., Carvalho et al., 2016; Kaptchuk et al., 2010; Kelley, Kaptchuk, Cusin, Lipkin, & Fava, 2012). The necessary ingredients for the first two mechanisms (the positive patient-provider relationship and medical ritual) are present in both traditional placebo trials and open-label placebo studies (Finniss et al., 2010). However, patient expectations about treatment differ between traditional and open-label placebo treatments. In traditional placebo trials, expectations are set under the premise that patients are either definitely or possibly taking active medications designed to treat a specific condition. This is certainly the case in traditional research on placebo effects, when providers deceptively tell patients that placebo treatments are real medications. This is also often the case in randomized controlled trials involving placebo arms, when providers tell patients there is a chance they are taking the active medication and enumerate the immense promise of that medication (Finniss et al., 2010). In both cases, positive expectations are predicated on the belief that patients are or could be taking an active medication.
In open-label placebo trials these same expectations cannot be set because patients know their placebo treatment contains no active ingredients. However, positive expectations are still communicated to the patient in open-label placebo treatment. Patients in these studies often know that the placebo condition is the treatment of interest (Kaptchuk et al., 2010; Locher et al., 2017), which may signal to patients that the researcher expects some benefit from the placebo treatment. Furthermore, open-label placebo paradigms use an additional mechanism to instill positive expectations in patients: a rationale about the power of placebos. The rationale in open-label placebo studies, thus, serves as a fourth mechanism, enabling positive expectations to take root in response to openly inert placebo medications. The most commonly used protocol for open-label placebos is modeled after the first open-label placebo study by Kaptchuk et al. (2010). In this protocol, providers explain that patients will receive an inert substance, like a sugar pill, and then providers discuss four specific points: “1) the placebo effect is powerful, 2) the body can automatically respond to taking placebo pills like Pavlov’s dogs who salivated when they heard a bell, 3) a positive attitude helps but is not necessary, and 4) taking the pills faithfully is critical” (Kaptchuk et al., 2010, p. 2). Together, these discussion points constitute a “persuasive rationale” for why an inert placebo treatment could provide real benefit (Kaptchuk et al., 2010). The goal of this rationale is to convey the message that placebo effects work and that the researchers and clinicians expect them to benefit the patient (Kaptchuk et al., 2010). This rationale serves to elicit the positive expectations that are presumed to be present in traditional placebo paradigms. As such, the ultimate impact of this rationale may be to transfer patients’ expectations of healing from belief in the power of active medications to belief in the power of the placebo effect.
Previous open-label placebo research alludes to the potential mechanisms (relationship, ritual, and expectations set via persuasive rationale) that generate open-label effects. Because a supportive patient-provider relationship leads to beneficial outcomes, previous open-label placebo studies controlled for this relationship, ensuring that participants across conditions had the same quality and quantity of interactions with a caring physician (Carvalho et al., 2016; Derksen, Bensing, & Lagro-Janssen, 2013; Hojat et al., 2011; Kaptchuk et al., 2010; Pereira, Figueiredo-Braga, & Carvalho, 2016; Rakel et al., 2011). In these studies, however, both the ritual and the expectations differ between conditions. Only the open-label placebo intervention group—but not the control group—receives a medical ritual (pills or other treatment) and the positive expectations that follow from the rationale about the power of placebos (Carvalho et al., 2016; Finniss et al., 2010; Kelley et al., 2012).
Only a few studies have experimentally explored the degree to which relationship, ritual, and expectations set via rationale about the power of placebos independently serve as mechanisms driving open label placebo effects. These studies have led to mixed conclusions: at least one study found that the addition of a rationale about the power of placebos made no difference in the efficacy of an open-label placebo treatment for allergic rhinitis (Schaefer et al., 2018). However, another study found that participants who received the open-label placebo treatment with a rationale reported diminished self-reported pain intensity compared with participants who received the open-label placebo treatment without the rationale (Locher et al., 2017). Overall, however, no studies have examined how a rationale about the power of placebos could influence the effects of open-label placebo treatments. We suspect the rationale is important because it instills or reinforces the specific belief in the power of placebos. Previous research suggests that specific beliefs about particular medications powerfully drive expectations and health outcomes. For example, branded medications work better than generic medications, presumably because the brand itself is tied to stronger, specific beliefs about the power of these medications (Kam-Hansen et al., 2014). The role of the rationale in establishing the belief that placebo effects are a powerful force for healing may be similarly important.
To our knowledge, no previous open-label placebo studies have assessed participant beliefs in the power of placebos as either an outcome of the rationale or a moderator of open-label placebo effects. Furthermore, no previous study has attempted to tease apart the mechanisms of open-label placebos (the patient-provider relationship, medical ritual, and positive expectations set via rationale) or understand whether the rationale in open-label placebo treatments changes participant beliefs about the power of placebos. Open-label placebo research has not yet clarified the minimum necessary ingredients that must be present to produce open-label placebo effects, or what the rationale in open-label placebo treatments is actually doing. Revealing the driving factors behind open-label placebo effects would be a significant contribution to the theoretical literature on placebo effects, as the relative power of expectations (set explicitly by the provider and implicitly by the rationale about the power of placebos) and conditioning (elicited by use of medical rituals) has been a subject of much debate in the placebo literature (Finniss et al., 2010). Additionally, understanding which components drive open-label placebo effects could encourage clinicians to leverage those forces alongside active medications and treatments. For example, if medical ritual alone is the driving factor, the implication is that medical practitioners should focus on prescribing or providing rituals such as prescribing de facto placebos in the form of vitamins (Tilburt, Emanuel, Kaptchuk, Curlin, & Miller, 2008). If both the medical ritual and positive expectations set by the provider were necessary for eliciting positive outcomes, physicians should be advised to set positive expectations for patients explicitly. Finally, if all components were necessary to elicit positive outcomes, providers would want to instill positive beliefs about the efficacy of a the treatment by explaining why and how treatments were likely to be effective in addition to setting explicit positive expectations and prescribing medical rituals (Locher et al., 2017). In this way, unpacking the most important components of open-label placebo effects will bring us closer to applying lessons from placebo literature to clinical practice, thereby improving the power of biomedical drugs and treatments.
Hypotheses
Past research has utilized four mechanisms together to evoke open-label placebo responses: (a) the supportive patient-provider relationship, (b) medical ritual, and (c) positive expectations elicited by (d) a compelling rationale. The present study utilized a controlled laboratory setting to test the mechanisms of an open-label placebo treatment for an induced allergic reaction (Howe, Goyer, & Crum, 2017) by incrementally adding one additional component of open-label placebo treatment for each of four conditions (see Figure 1). We hypothesized that the condition including all four components would most effectively diminish allergic reactions because it would provide patients with a familiar medical ritual, set positive expectations for them, and change their beliefs about the power of placebos, instilling the belief that placebos can be an effective treatment for allergic reactions. The goal of the condition including all four components was to change participants’ beliefs about placebos, helping participants to see placebos as a source of potential healing. Consequently, we hypothesized that participants in this condition would believe in placebos more strongly than participants in other conditions. We hypothesized that each of these four mechanisms would produce some cumulative benefit as they were added across conditions, and we hypothesized that the change in participants’ beliefs about the power of placebos elicited by the rationale would be critical.
Figure 1.

Visual depiction of four study conditions. In the Control condition, participants were exposed to a warm and competent provider. In the Ritual condition, participants were exposed to a warm and competent provider who administered an open-label placebo (OLP) cream (a medical ritual) for their allergic reaction. In the Expectation condition, participants were exposed to a warm and competent provider who administered an OLP cream (a medical ritual) for their allergic reaction and set positive expectations that it would reduce their itchiness and irritation. In the Rationale condition, participants were exposed to a warm and friendly provider who administered an OLP cream (a medical ritual) for their allergic reaction, set positive expectations that it would reduce their itchiness and irritation, and provided a rationale for how OLPs can treat allergic reactions.
Method
Participants
A total of 148 participants (63.5% women; 43.2% White; Mean age = 24.55) were recruited from a psychology subject pool of mostly undergraduate students, university staff, or alumni of Stanford University. Participants were recruited for a study investigating “the characteristics that influence variation in allergic response” and were compensated with either a $20 Amazon eGift card or course credit for one hour of participation. Participants completed a prescreening survey to exclude them if they had recently taken antihistamines, and provided written, informed consent before study participation. Data analyses included all participants who completed the study and were over age 18. Participants were 43.2% White, 5.4% African American, 29.8% Asian, 7.4% Hispanic, and 11.5% other. 66.2% of the sample was between the ages of 18 and 22. Sample size was based on previous research using the same paradigm to detect allergic response and calculated, using the G*Power tool, 20 participants per cell as necessary for >85% power to detect a medium effect size (Faul, ErdFelder, Lang, & Buchner, 2007; Howe et al., 2017).
Design
Participants were recruited for a study on “allergic response” and were scheduled for a 1-hr session in a simulated doctor’s office housed in the Psychology Department at Stanford University. Previous research has shown placebos significantly influence allergic reaction and treatment response (Bartels, Van Laarhoven, Van De Kerkhof, & Evers, 2016; Booth, Petrie, & Brook, 1995; Czubalski & Zawisza, 1976; Goebel, Meykadeh, Kou, Sched-lowski, & Hengge, 2008; Jewett, Fein, & Greenberg, 1990; Kamenica, Naclerio, & Malani, 2013; Schaefer et al., 2016, 2018). For example, placebo injections can lead to allergic responses (Jewett et al., 1990), expectations of allergy relief induced via exposure to advertising influence the efficacy of treatments for allergic reactions (Kamenica et al., 2013), a placebo cream administered by a warm and competent doctor can reduce allergic reactions in healthy lab participants (Howe et al., 2017), and open-label placebos can be effective in treating allergic rhinitis (Schaefer et al., 2016, 2018).
To assess the impact of various components of open-label placebos on physiological reactions, allergic response to a histamine skin prick was used. Skin prick testing is a common procedure used to diagnose allergies (Chiriac, Bousquet, & Demoly, 2013). In this procedure, allergists prick the patient’s skin with a very small needle that has been soaked in the suspected allergen, and the resulting allergic reaction—a red, raised bump called a “wheal”—is compared to a skin prick with histamine, which causes an allergic reaction in almost all individuals (Eigenmann & Sampson, 1998; Howe et al., 2017). For the present study, only a histamine skin prick test was used to elicit allergic reactions in participants. Past research on allergic reactions suggests this paradigm is appropriate to assess the effects of open-label placebo treatments, both because of the ability to assess the effects of the skin prick test and subsequent treatment via objective physiological measures and because of the responsiveness of allergic reactions to placebo treatments (Howe et al., 2017). Across four conditions, the information and treatment participants received in response to their allergic reaction was manipulated to leverage the components present in previous studies of open-label placebos.
Condition 1: Control.
In the “Control” condition, participants were exposed to a supportive patient-provider relationship. This condition was designed to provide participants with a positive interaction with the provider. In the Control condition (N = 40), the provider merely examined patients’ allergic reactions without saying anything about it or giving patients cream or other treatment.
Condition 2: Ritual.
In the “Ritual” condition, participants were exposed to a supportive patient-provider relationship and a medical ritual. This condition was designed to provide participants with a specific medical ritual related to the allergic reaction: in this case, the open-label placebo treatment. In the Ritual condition (N = 36), the provider examined participants’ allergic reactions and said: “OK, I’m going to give you some skin cream for your arm. I’m not sure if this will be familiar to you or not, but this cream is a placebo treatment. That means it doesn’t have any active ingredients in it.” The provider then gently rubbed a small drop of unscented hand lotion onto the participants’ forearms, directly on top of the allergic reaction site.
Condition 3: Expectation.
In the “Expectation” condition, participants were exposed to a supportive patient-provider relationship, a medical ritual, and positive expectations. This condition was designed to leverage patient expectations by explicitly instilling in patients a general belief that their reaction would feel better in the near future. In the Expectation condition (N = 35), the provider examined participants’ allergic reactions and said: “OK, I’m going to give you some skin cream for your arm. I’m not sure if this will be familiar to you or not, but this cream is a placebo treatment. That means it doesn’t have any active ingredients in it.” The provider then gently rubbed a small drop of unscented hand lotion onto the participants’ forearms, directly on top of the allergic reaction site. After applying the lotion, the provider set positive expectations for participants by saying: “From this point forward your allergic reaction will start to diminish, and your rash and irritation will go away.”
Condition 4: Rationale.
In the “Rationale” condition, participants were exposed to a supportive patient-provider relationship, a medical ritual, positive expectations, and a persuasive rationale. This condition was designed to provide participants with a persuasive rationale about the power of placebos, thereby evoking in participants a belief in placebos as a powerful treatment and implicitly instilling specific expectations that the placebo treatment would elicit a healing response. In the Rationale condition (N = 37), the provider examined participants’ allergic reactions and said: “OK, I’m going to give you some skin cream for your arm. I’m not sure if this will be familiar to you or not, but this cream is a placebo treatment. That means it doesn’t have any active ingredients in it.” The provider then continued to explain the mechanisms by which a placebo cream, containing no active ingredients, might be effective in reducing an allergic reaction, using a pamphlet entitled “Allergic Response: The Science Behind the Reaction,” to assist in his explanation. The provider explained:
We know that placebo creams are effective for allergic reactions like this; it will make your reaction go away more quickly. There are many reasons why this works. [open pamphlet and refer to relevant parts] One is that it creates positive expectations that you will heal. But these don’t have to be conscious expectations: we know the human body can actually learn to respond physiologically to objects and situations it associates with healing, like taking a pill, seeing a doctor, or applying a cream. That is the placebo effect. We know this cream also works by engaging the parasympathetic nervous system to reduce stress, which reduces inflammation throughout the body. These are the big reasons this placebo cream treatment has been shown to be effective; there are probably also others.
The provider then gently rubbed a small drop of unscented hand lotion onto participants’ forearms, directly on top of the allergic reaction site. After applying the lotion, the provider set positive expectations for participants by saying: “From this point forward your allergic reaction will start to diminish, and your rash and irritation will go away.” For the pamphlet used in this condition, see the online supplemental material available online.
See Figure 1 for a visual depiction of these conditions.1 This design allowed us to separate the effect of general positive expectations (Expectation condition) from the placebo-specific expectations elicited by the rationale about the power of placebos (Rationale condition). The Rationale condition most closely replicates previous open-label placebo studies. In each condition, the provider followed a protocol and script. The full scripts and protocols utilized by the provider and research assistant in this study are publicly available on the Open Science Framework at: https://osf.io/krd8w/. The Stanford University Institutional Review Board (IRB) approved all procedures.
Procedure
After consenting and completing the prescreen survey to ensure inclusion eligibility, participants met a White male research assistant (“the provider”) in his mid-20s, who was wearing a white lab coat and stethoscope. Participants were told he was a physician from the university’s Allergy Research Center. The provider then conducted a basic health screening, which included asking participants about their health background and taking participant height, weight, blood pressure, and a saliva sample. Participants completed a questionnaire assessing how itchy or irritated their forearms felt at the site where the skin prick test was to be conducted (0 = not at all to 100 = extremely), and how itchy or irritated they expected their arms would feel in 3 min, after the skin prick test.2 The provider then conducted the allergy skin prick test by pricking participants in the forearm with a Quintip stainless steel lancet soaked in a solution of 10 mg/mL histamine dihydrochloride.
Participants were randomized to condition using a random number generator. To ensure consistency across conditions at baseline, the provider was blind to participants’ conditions during the initial health screening and the skin prick test. After conducting the skin prick test, the provider informed participants that a research assistant would take regular measurements of their allergic reaction as it progressed. He then went into a separate room and opened a computer file revealing the participants’ randomly assigned condition. The research assistant, who was blind to participant conditions, then measured participants’ allergic reactions using a standardized allergy testing ruler while participants again completed a questionnaire assessing their current and expected itchiness and irritation.
At approximately 6 min after the skin prick test, the provider reentered the room to deliver the intervention according to the conditions described above (see Figure 1). The provider finished delivering the intervention by 9 min post-skin prick test, at which point the research assistant who was blind to condition reentered the room to administer the current and expected itchiness and irritation questionnaire and measure participants’ allergic reactions at subsequent intervals. All measurements of physiological allergic reaction were, thus, double-blind. At the end of the study, participants filled out a final questionnaire asking about their study experience and their beliefs in placebos. For additional details about the procedures used in this study, see the online supplemental material. For ease of replication, scripts, protocols, full questionnaires, and data used in the study are available at: https://osf.io/krd8w/.
Measures
Primary outcome measure: Physiological allergic reaction.
Participants’ physiological allergic reactions were assessed via the size of the wheal (raised bump). The wheal was measured by the research assistant, who was blind to participant conditions, using a standardized allergy testing ruler at five time-points, at intervals of 3 min with a break between T1 and T2 for the provider to deliver the open-label placebo intervention (T1 = baseline measurement 3 min post-skin prick test, T2 = 9 min post-skin prick test and directly after provider intervention, T3 = 12 min post-skin prick test and 3 min post-intervention, T4 = 15 min post-skin prick test and 6 min post-intervention, T5 = 18 min post-skin prick test and 9 min post-intervention;3 for additional details on wheal measurements, see the online supplemental material).
Manipulation check measures: Expectation and belief in placebo.
To assess participant expectations, participants rated their expectations for how their forearm would feel 3 min in the future (0 = quite a bit worse than now, 100 = quite a bit better than now) before the skin prick test and at each time point. To assess participants’ belief in placebos, participants filled out a questionnaire where they completed measures assessing their experience in the study and a four-item measure of belief in placebos (1 = strongly disagree, 6 = strongly agree; α = .59) after the final measurement at 18 min post-skin prick test. These four items were averaged into a composite “placebo belief” measure, and included items such as “Placebo effects are a part of all active medications,” and “Placebo effects can occur in all illnesses and conditions.” For additional details on the placebo items, see the online supplemental materials.
Results
Manipulation Check: Effect of Condition on Expectation and Belief in Placebos
Analyses were conducted using multilevel regression, with time nested in participants at Level-1 and controlling for baseline measures of expectations (the average of ratings at Minute 3 and Minute 6) at Level-2. These analyses demonstrated that immediately after the provider encounter (9-min post-skin prick test) participants in the Expectations and Rationale conditions who were exposed to positive expectations from the provider expected to feel less itchy 3 min in the future than participants in the control and ritual conditions, who did not receive positive expectations from the provider (MControl + Ritual = 55.15 vs. MExpectation + Rationale = 69.15; B = 14, SE = 2.83, Z = 4.95, p < .001, 99% confidence interval [CI] [8.46, 19.54], d = .73; see Figure 2). This manipulation check indicates that including positive expectations in two of the four conditions successfully manipulated participants’ expectations about how they would feel in the future.
Figure 2.
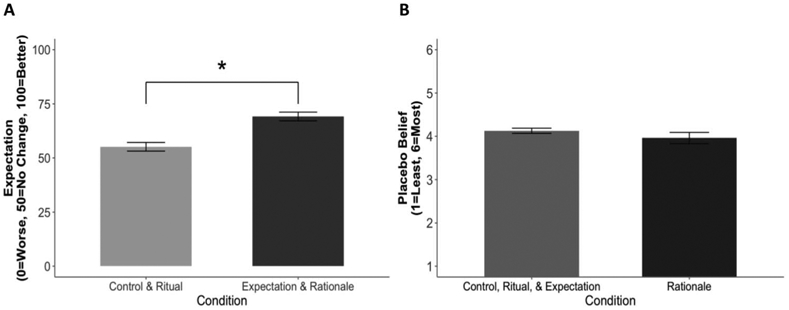
Participants’ ratings of placebo belief and expectation of how their arms would feel 3 min in the future. Panel (A) displays mean expectations for how patients would feel 3 min in the future, assessed at T2 (immediately after interacting with the provider at 9 min post-skin prick test), collapsed across conditions with and without expectations, controlling for baseline expectations, showing a main effect such that participants who were given positive expectations by the provider expected to feel better in the future than participants who were not given any expectations about how they would feel in the future. Panel (B) displays mean placebo belief, assessed at the end of the study via mean participant agreement with four items about the power and pervasiveness of placebo treatments, showing no difference between participants who were exposed to a placebo rationale and those who were not. SEs represent ±1 SE, N = 148. * p < .05.
Participants in the Rationale condition were exposed to information highlighting the efficacy of placebo treatments and detailing several mechanisms by which placebo creams can reduce allergic reactions. The goal of this condition was to change participants’ beliefs about placebos, helping participants to see placebos as a source of potential healing, leading participants in the Rationale condition to have higher belief in placebos than participants in other conditions. A one way analysis of variance (ANOVA) with a Tukey’s-b post hoc multiple comparison indicated that there was no difference in placebo beliefs by condition, F(3, 143) = 0.67, p = .569; Figure 2. This manipulation check suggests that our intervention was not successful in changing participants’ beliefs about placebos.
Analytic Strategy for Physiological Results
Following the strategy used by Howe et al., 2017, multilevel longitudinal modeling was used to assess changes in the size of participants’ allergic reactions to the skin prick test at four time points over the 9 min after the interaction with the provider. The wheal was analyzed at T2-T5 (9–18 min post-SPT) controlling for initial allergic reaction at T1. Additional details regarding the modeling strategy are available in the online supplemental materials.
Confirmatory analysis: Do the components of open-label placebos additively influence physiological allergic reactions?
First, we examined whether any of the conditions including an open-label placebo cream reduced physiological allergic reactions compared with the control group. We expected that post-intervention (T2-T5), the Rationale condition would significantly decrease physiological allergic reactions compared with a control group. However, our results found no difference between the physiological reactions in the Rationale condition compared with the control condition immediately post-intervention at T2 (z = −0.5, p = .61). Furthermore, no differences between these conditions emerged over time, indicating that this condition did not influence either the initial wheal size or its trajectory over time (z = 0.57, p = .57). Likewise, no differences in wheal size were observed between the control condition and the Ritual and Expectation conditions either immediately post-intervention at T2 or over the subsequent time course of the wheal trajectory (all ∣z∣s ≤ 0.21, all ps > .83). These results suggest that none of the mechanisms of open-label placebo treatments added significant benefit compared with a control condition in reducing allergic reactions in healthy laboratory participants.
Post hoc analysis: Do preexisting beliefs about placebos moderate the effect of open-label placebo treatment on physiological allergic reaction?
We hypothesized that a compelling rationale of the open-label placebo treatment would increase individuals’ belief in the power of placebos and subsequently reduce physiological allergic reactions. However, our manipulation check indicated that the Rationale condition was not successful in changing participants’ beliefs about placebos. Because participants’ beliefs in the Rationale condition did not differ from participants’ beliefs in other conditions after the interaction with the provider, the rationale showed no indication of changing participants’ beliefs. Thus, it is not surprising that this condition failed to provide additional benefit and reduce participants’ allergic reactions. However, this does not suggest that participant beliefs, or the rationale provided in previous open-label placebo studies, are not important. Rather than allergic reactions being influenced by the beliefs set by the study conditions, allergic reactions may have been influenced by the preexisting beliefs held by participants in the study. Because our rationale failed to change participants’ beliefs about placebos, we wondered if exposure to a placebo treatment and rationale instead activated participants’ preexisting beliefs about placebos. To test this possibility, we examined participants’ belief in placebos as a potential moderator of condition on allergic reaction. We found that placebo beliefs significantly moderated the effect of condition on physiological allergic reaction immediately postprovider interaction at T2 (B = −0.88, SE = 0.33, z = −2.65, p <.01, 99% CI [ − 1.53, −0.22], d = −0.74); participants in the Rationale condition with a strong belief in placebos (1 SD above the mean), exhibited significantly reduced allergic reactions compared with the control condition (Madj = 6.31 vs. 6.94, B = −0.63, SE = 0.30, z = −2.13, p = .03, 99% CI [ −1.21, −0.05], d = −0.53; see Figure 3). This effect was not significant for participants who did not believe in the power of placebos (1 SD below the mean; Madj = 6.55 vs. 6.02, B = 0.52, SE = 0.31, z = 1.71, p = .09, 99% CI [−0.08, 1.13]; see Figure 3). In the Rationale Condition, there was no interaction between beliefs about placebos and condition on slope of the allergic reaction over time (B = −0.04, SE = 0.03, z = −1.34, p = .18, 99% CI [−0.09, 0.02]), suggesting that belief in placebos influenced the initial size of the allergic reaction post-intervention in the Rationale Condition and this initial difference was maintained over time. Placebo beliefs did not significantly moderate the effect of condition on allergic reaction for participants in the Ritual or Expectation conditions compared with the control conditions.
Figure 3.
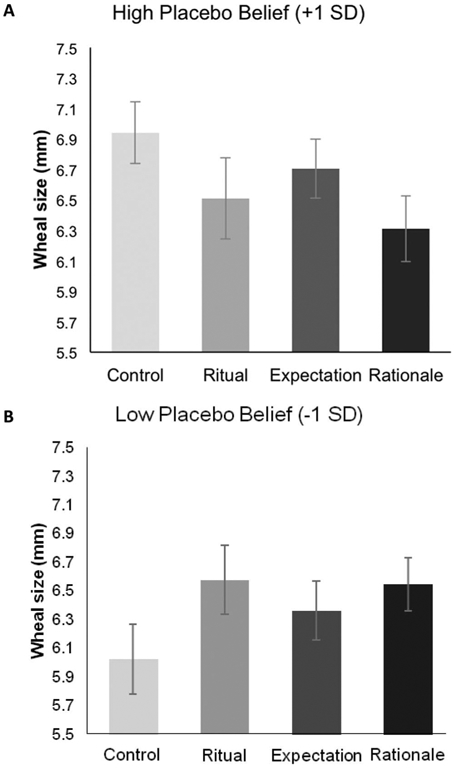
Interaction between participants’ allergic reactions and their belief in placebos. Participants’ allergic reactions were measured via wheal size by a research assistant blind to participant condition. Panels (A) and (B) display adjusted means at T2 (immediately after interacting with the provider at 9 min post-skin prick test) derived from a multilevel longitudinal model controlling for wheal size at T1 (3 min post-skin prick test). Panel (A) shows participants high on the belief in placebos scale (+1 SD), and panel (B) shows participants low on the belief in placebos scale (−1 SD). SEs represent ±1 SE. * p < .05.
Discussion
This study contributes to our understanding of the mechanisms of open-label placebo effects by underscoring the importance of participants’ beliefs in producing open-label placebo effects. This study demonstrated that participants’ beliefs about placebos interacted with the components in the Rationale condition to reduce physiological allergic reactions. We hypothesized that the Rationale condition would be the most effective in diminishing allergic reactions because it would provide patients with a familiar medical ritual, set positive expectations for them, and change their beliefs about the power of placebos, instilling the belief that placebos can be an effective treatment for allergic reactions. Yet, our manipulation check showed no differences in placebo beliefs by condition, suggesting that our rationale was not sufficiently persuasive to change participants’ beliefs about placebos. Thus, we did not find that patients in the Rationale condition had a smaller allergic reaction than participants in the other groups. In fact, there was no difference in allergic reaction size between any of the conditions and the control condition, suggesting our study did not find support for an effect of an open-label placebo treatment on allergic reactions. However, we did find that the Rationale condition reduced allergic reactions compared with the control condition when patients strongly believed in placebos. This suggests that the rationale may have activated the belief in placebos, connecting the open-label placebo treatment to the improvement of the allergic reaction. Belief in the power of placebos may not only be helpful for producing positive health outcomes in response to open-label placebo treatments, it may actually be required.
The results of this study suggest that patient beliefs are an important variable influencing the effect of open-label placebo treatments. Yet, patient beliefs have largely been overlooked in the literature on open-label placebos. Future open-label placebo research should not only include measures of participants’ preexisting beliefs about the power of placebos, but should also work to design rationales that are persuasive enough to generate a belief in the power of placebos in people who may be skeptical initially.
There may be several reasons why the rationale about the power of placebos in our study was unsuccessful in changing participant beliefs. First, the time course of our intervention: providing patients with the medical ritual, positive expectations, and the rationale was allotted only 3 min. This timing allowed the study provider to deliver the intervention between wheal measurements by the research assistant, who was, thus, able to remain blind to participants’ conditions. However, this rationale was shorter than in previous open-label placebo studies, many of which involved participants meeting with providers twice over the course of 2 or 3 weeks (Carvalho et al., 2016; Kaptchuk et al., 2010; Schaefer et al., 2016, 2018). It is possible that a short, one-time interaction is not enough to make the rationale for open-label placebo treatments sufficiently persuasive. That said, future research should be mindful of the possible impact that interaction length may have on patient outcomes. Although in the current study, the rationale condition was only 3 min, the other conditions were even shorter (the control condition was 40 s, the ritual condition was 1 min and 20 s, and the expectation condition was 1 min and 30 s). Future research should explore the optimal interaction length needed to effectively alter placebo beliefs as well as ensure that the information provided in the interaction—and not the interaction length—is driving the effects.
Another reason we may not have replicated previous open label placebo effects is that our study examined the effects of an open-label placebo treatment on a laboratory-induced allergic reaction in healthy participants. We believe this may constitute a particularly conservative test of this effect, because the allergic reaction in this paradigm was relatively unstressful and lasted only a short period of time and, thus, the provided rationale may not have been viewed as sufficiently important or relevant for healthy participants to attend to closely. Most open-label placebo studies have been conducted with clinical populations, and it is common for clinical patients who enroll in open-label placebo studies to do so only after trying several unsuccessful treatments. Patients in these trials may have been particularly hopeful or eager for an effective treatment and may have attended to explanations from the provider more closely. Participants in our study, however, were unlikely to be emotionally invested in the success of the open-label placebo treatment. Finally, 66% of our participants were college aged, most of whom were participating in the study for course credit. Thus, it is possible that this participant population may have been more knowledgeable or skeptical about placebo treatments than the general population.
Although placebo beliefs did not moderate the effect of the Ritual or Expectation conditions on allergic reaction compared with the control condition, that does not indicate that beliefs were not at play in these conditions. Rather, the influence of these beliefs coupled with the Ritual and Expectation without a rationale may not have been strong enough to reduce allergic reactions significantly. Whether it is possible for the combined effect of medical rituals, expectations, and beliefs about placebos to produce significant clinical benefit in response to open-label placebo treatment should be investigated in future research.
To our knowledge, the only other study using a similar, lab-induced paradigm to study the effects of open-label treatment on healthy participants found no differences in objective outcomes between groups, and one additional study assessing an open-label placebo treatment on wound healing also found no effects on objective physiological outcomes (Locher et al., 2017; Mathur, Jarrett, Broadbent, & Petrie, 2018). Why, then, have several other studies found successful open-label placebo effects leveraging the same components tested here (Carvalho et al., 2016; Kaptchuk et al., 2010)? No previous open-label placebo studies have explicitly measured participant beliefs about placebos, but it is likely that previous open-label placebo studies have inadvertently recruited participants who already believe placebos are powerful and pervasive. Previous open-label placebo studies have recruited patients for “novel mind-body treatment[s]” (Carvalho et al., 2016; Kaptchuk et al., 2010; Locher et al., 2017). In many cases, participants were informed in advance of the study, during screening, that they would be receiving open-label placebos as their treatment (Carvalho et al., 2016; Kaptchuk et al., 2010; Kelley et al., 2012). While we cannot be certain, as no previous open-label placebo studies reported beliefs about placebos at baseline or follow-up, it seems likely that these studies recruited a particular subset of the population: those who are open to the idea of receiving placebo treatments. Although physicians did communicate during these studies that belief in the placebo is not necessary, these recruitment strategies may have inadvertently tapped into a population with preexisting positive beliefs about placebos. The present study, however, recruited participants under the guise that they were participating in a study about allergic reactions, and recruitment materials mentioned nothing about placebos or mind-body treatments. This participant population was, thus, unlikely to have preexisting beliefs that placebos are powerful. Therefore, examining the participants in the present study who believe strongly in placebos may most closely replicate the populations in previous open-label placebo studies.
One limitation of this study is that participants’ beliefs about placebos were measured after the intervention. This design ensured that participants were not alerted to the purposes of the study, or the fact that the study involved placebos, ahead of time. However, because of this timing it is possible that, rather than beliefs influencing the trajectory of the allergic reaction, the trajectory of the allergic reaction influenced participant beliefs, such that those participants whose reactions diminished then believed more strongly in the power of placebos. Future studies should find ways to measure participants’ beliefs in placebos both at baseline and at the end of the study in ways that do not alert participants to the true intent of the study.
Previous open-label placebo studies have used the clinically accepted guidelines for each of the medical conditions assessed, including IBS, migraines, allergic rhinitis, and back pain (Carvalho et al., 2016; Goebel et al., 2008; Kaptchuk et al., 2010; Kelley et al., 2012; Sandler & Bodfish, 2008; Sandler et al., 2010). Yet, clinical diagnoses for these conditions necessarily rely on subjective assessment on the part of patient and clinician. Because previous research suggests that placebos primarily influence self-report outcomes, these conditions may be particularly likely to benefit from open-label placebo treatments (Kaptchuk & Miller, 2015; Locher et al., 2017). The present study utilized a double-blind design to assess the outcomes of an open-label placebo cream on physiological allergic reaction, as measured objectively by the size of the allergic rash. This procedure ruled out the possibility that improvement was because of subjective discernment on the part of patient or provider. Previous research also indicates that placebo effects may be higher in clinical settings than in healthy participants in experimental paradigms (Vase, Petersen, Riley, & Price, 2009; Vase, Riley, & Price, 2002). With this in mind, assessing objective health outcomes in healthy patients makes this a particularly conservative test of open-label placebo efficacy.
This study is the first to thoroughly unpack the mechanisms of open-label placebo effects on a double-blind, physiological outcome. Our results indicate that, for open-label placebos to be effective, several components are needed, including not only those provided in the clinical interaction, such as a medical ritual, positive expectations, and a compelling rationale, but also a previously unmeasured factor the patient brings to the interaction: beliefs about the power of placebos. This study revealed patient beliefs as a hidden variable influencing the effect of open-label placebo treatments. More research is needed to understand how patient beliefs about placebos influence placebo research, and open-label placebo studies. In particular, future open-label placebo studies should measure participant beliefs about placebos to assess whether (a) participants recruited for these studies have a higherthan-average belief in placebos and (b) placebo belief moderates the effect of open-label placebo treatments on patient health outcomes. The implications of this research should also be considered in the broader context of clinical trials. In these trials, individuals’ beliefs about placebos may vary based on their knowledge of and prior experiences with placebos, and if and how the placebo arm of a trial is explained to them. These beliefs may in turn strengthen or weaken placebo effects, thereby influencing the observed effect size in randomized controlled trials. Thus, understanding variability in placebo response—which this study demonstrates is likely influenced by placebo beliefs—may be critical to the validity of randomized controlled trials.
Broadly, this study reveals the important ways patient beliefs about a treatment influence the efficacy of that treatment. As medicine moves toward treatment plans that are increasingly customized for each patient, patient beliefs about treatment options should not be overlooked. Information provided in a health care context interacts with patient beliefs to influence treatment outcomes. The present study identified patient beliefs about placebos as an overlooked, underresearched factor shaping the effect of open-label placebo treatments: the combined ritual, expectation, and rationale effectively reduced physiological allergic reaction when patients believed in the power of placebos. By revealing patient beliefs as an important factor that is potentially crucial to the success of open-label placebo treatments, the present study sheds light on both the mechanisms contributing to open-label placebo effects and the often-underestimated importance of patients’ beliefs in influencing treatment outcomes.
Supplementary Material
Acknowledgments
This research was supported by the Sean N. Parker Allergy Center and the Joan Butler Ford Stanford Graduate Fellowship. We also thank Kris Evans for his invaluable support with data collection, Elijah Zenger for his help with materials, and Ted Kaptchuk, Lauren Howe, Isaac Handley-Minor, Erika Weisz, and Sean Zion for their guidance.
Footnotes
An additional condition, which included positive expectations but no open-label placebo cream, was also assessed to address a separate experimental question. Data from that condition and the control condition described here are reported in: Leibowitz, Hardebeck, Goyer, and Crum (2018). Physician assurance reduces patient symptoms in US adults: an experimental study. Journal of General Internal Medicine, 1–2.
Based on a previous study using this paradigm that found no effects of placebo cream on self-reported itch (Howe et al., 2017), we did not consider itch to be a primary outcome in our study and did not make any a priori hypotheses regarding itch. Like Howe et al., we found no differences in self-reported itch by condition at any time point, and there was likewise no significant moderating effect of placebo beliefs on the relationship between condition and skin itchiness. However, some trends in our results suggest that for the high placebo believers only, the Rationale condition may have reduced itch as compared with the control group. Complete results and a discussion of these effects can be found in the online supplemental materials.
At these time points the flare (redness surrounding the wheal) was also measured using the same procedures described for the wheal, and participants’ itch or irritation was assessed via questionnaire. Following Howe et al. (2017), we discuss results pertaining to the wheal, as this was the site of open-label placebo cream application and is the focus of most research on allergic reactions. Information on all measures included in this study is reported in the online supplemental materials.
Supplemental materials: http://dx.doi.org/10.1037/hea0000751.supp
Contributor Information
Kari A. Leibowitz, Department of Psychology, Stanford University
Emerson J. Hardebeck, Department of Psychology, Stanford University; Department of Clinical Psychology, Antioch University Seattle.
J. Parker Goyer, Department of Psychology, Stanford University.
Alia J. Crum, Department of Psychology, Stanford University
References
- Bartels DJP, Van Laarhoven AIM, Van De Kerkhof PCM, & Evers AWM (2016). Placebo and nocebo effects on itch: Effects, mechanisms, and predictors. United Kingdom: European Journal of Pain. [DOI] [PubMed] [Google Scholar]
- Booth RJ, Petrie KJ, & Brook RJ (1995). Conditioning allergic skin responses in humans: A controlled trial. Psychosomatic Medicine, 57, 492–495. 10.1097/00006842-199509000-00012 [DOI] [PubMed] [Google Scholar]
- Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, & Kirsch I (2016). Open-label placebo treatment in chronic low back pain: A randomized controlled trial. Pain, 157, 2766–2772. 10.1097/j.pain.0000000000000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriac AM, Bousquet J, & Demoly P (2013). In vivo methods for the study and diagnosis of allergy In Adkinson NF Jr., Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, & O’Hehir RE Jr. (Eds.), Middleton’s allergy principles and practice (8th ed., Vol. 2–2, pp. 1119–1132). Philadelphia, PA: Elsevier. [Google Scholar]
- Crum AJ, Leibowitz KA, & Verghese A (2017). Making mindset matter. British Medical Journal, 356, j674 10.1136/bmj.j674 [DOI] [PubMed] [Google Scholar]
- Czubalski K, & Zawisza E (1976). The role of psychic factors in patients with allergic rhinitis. Acta Oto-Laryngologica, 81, 484–488. 10.3109/00016487609107504 [DOI] [PubMed] [Google Scholar]
- Derksen F, Bensing J, & Lagro-Janssen A (2013). Effectiveness of empathy in general practice: A systematic review. The British Journal of General Practice, 63, e76–e84. 10.3399/bjgp13X660814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann PA, & Sampson HA (1998). Interpreting skin prick tests in the evaluation of food allergy in children. Pediatric Allergy and Immunology, 9, 186–191. 10.1111/j.1399-3038.1998.tb00371.x [DOI] [PubMed] [Google Scholar]
- Faul F, ErdFelder E, Lang A-G, & Buchner A (2007). G Power 3.1 manual. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, & Benedetti F (2010). Biological, clinical, and ethical advances of placebo effects. Lancet, 375, 686–695. 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel MU, Meykadeh N, Kou W, Schedlowski M, & Hengge UR (2008). Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychotherapy and Psychosomatics, 77, 227–234. 10.1159/000126074 [DOI] [PubMed] [Google Scholar]
- Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, & Gonnella JS (2011). Physicians’ empathy and clinical outcomes for diabetic patients. Academic Medicine, 86, 359–364. 10.1097/ACM.0b013e3182086fe1 [DOI] [PubMed] [Google Scholar]
- Howe LC, Goyer JP, & Crum AJ (2017). Harnessing the placebo effect: Exploring the influence of physician characteristics on placebo response. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 36, 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DL, Fein G, & Greenberg MH (1990). A double-blind study of symptom provocation to determine food sensitivity. The New England Journal of Medicine, 323, 429–433. 10.1056/NEJM199008163230701 [DOI] [PubMed] [Google Scholar]
- Kamenica E, Naclerio R, & Malani A (2013). Advertisements impact the physiological efficacy of a branded drug. Proceedings of the National Academy of Sciences of the United States of America, 110, 12931–12935. 10.1073/pnas.1012818110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, & Burstein R (2014). Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science Translational Medicine, 6, 218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, … Lembo AJ (2010). Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS ONE, 5, e15591 10.1371/journal.pone.0015591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, & Miller FG (2015). Placebo effects in medicine. The New England Journal of Medicine, 373, 8–9. 10.1056/NEJMp1504023 [DOI] [PubMed] [Google Scholar]
- Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, & Fava M (2012). Open-label placebo for major depressive disorder: A pilot randomized controlled trial. Psychotherapy and Psychosomatics, 81, 312–314. 10.1159/000337053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz KA, Hardebeck EJ, Goyer JP, & Crum AJ (2018). Physician assurance reduces patient symptoms in US adults: an experimental study. Journal of general internal medicine, 33, 2051–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Frey Nascimento A, Kirsch I, Kossowsky J, Meyer A, & Gaab J (2017). Is the rationale more important than deception? A randomized controlled trial of open-label placebo analgesia. Pain, 158, 2320–2328. 10.1097/j.pain.0000000000001012 [DOI] [PubMed] [Google Scholar]
- Mathur A, Jarrett P, Broadbent E, & Petrie KJ (2018). Open-label placebos for wound healing: a randomized controlled trial. Annals of Behavioral Medicine, 52, 902–908. [DOI] [PubMed] [Google Scholar]
- Pereira L, Figueiredo-Braga M, & Carvalho IP (2016). Preoperative anxiety in ambulatory surgery: The impact of an empathic patientcentered approach on psychological and clinical outcomes. Patient Education and Counseling, 99, 733–738. 10.1016/j.pec.2015.11.016 [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, & Benedetti F (2008). A comprehensive review of the placebo effect: Recent advances and current thought. Annual Review of Psychology, 59, 565–590. 10.1146/annurev.psych.59.113006.095941 [DOI] [PubMed] [Google Scholar]
- Rakel D, Barrett B, Zhang Z, Hoeft T, Chewning B, Marchand L, & Scheder J (2011). Perception of empathy in the therapeutic encounter: Effects on the common cold. Patient Education and Counseling, 85,390–397. 10.1016/j.pec.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler AD, & Bodfish JW (2008). Open-label use of placebos in the treatment of ADHD: A pilot study. Child: Care, Health and Development, 34, 104–110. 10.1111/j.1365-2214.2007.00797.x [DOI] [PubMed] [Google Scholar]
- Sandler AD, Glesne CE, & Bodfish JW (2010). Conditioned placebo dose reduction: A new treatment in attention-deficit hyperactivity disorder? Journal of Developmental and Behavioral Pediatrics, 31, 369–375. 10.1097/DBP.0b013e3181e121ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Harke R, & Denke C (2016). Open-label placebos improve symptoms in allergic rhinitis: A randomized controlled trial. Psychotherapy and Psychosomatics, 85, 373–374. 10.1159/000447242 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Sahin T, & Berstecher B (2018). Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis. PLoS ONE, 13, e0192758 10.1371/journal.pone.0192758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, & Miller FG (2008). Prescribing “placebo treatments”: Results of national survey of U.S. internists and rheumatologists. British Medical Journal, 337, a1938 10.1136/bmj.a1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vase L, Petersen GL, Riley JL III, & Price DD (2009). Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain, 145, 36–44. 10.1016/j.pain.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Vase L, Riley JL III, & Price DD (2002). A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain, 99, 443–452. 10.1016/S0304-3959(02)00205-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


