Abstract
Purpose:
To validate the GO-specific quality of life (QOL) questionnaire in Hindi language and to determine the correlation of scores (visual functioning and appearance) with disease severity and activity.
Methods:
We recruited 114 consecutive patients with GO attending Endocrinology Clinic at tertiary care center. Eye examination was performed, and QOL was assessed by questionnaire.
Results:
The questionnaire was validated by 50 GO patients and test-retest reliability was performed in 15 patients. Hindi version GO-QOL was administered in 49 GO patients. GO was mild in 51.0% and sight-threatening in only 2.0% of cases. Orbitopathy was clinically active in only 10 (20.4%) cases. The GO-QOL scores (median) for visual function and appearance were 81.3 and 62.5, respectively. Patients with moderate-to-severe and sight-threatening GO had significantly lower median appearance scores (56.3 vs. 68.5, P = 0.01) compared to mild disease but no difference in visual scores. Patients with active disease had significant lower median visual function (53.1 vs. 85.7, P = 0.009) and psychosocial (appearance) scores (40.6 vs. 68.8, P = 0.03) compared to inactive disease. On multivariate regression analysis of GO-QOL scores, extraocular eye movement involvement (EOM), proptosis, and severity of eye disease were significantly associated with visual functioning while appearance was significantly associated only with the severity of eye disease.
Conclusion:
GO-QOL scores were significantly reduced in patients with GO.
Keywords: Graves orbitopathy, Hindi, quality of life, questionnaire, validation
Graves' orbitopathy (GO) is an autoimmune inflammatory disorder and one of the most common extrathyroidal manifestations of Graves' disease (GD).[1,2] GO manifests as chronic debilitating infiltrative eye disease characterized by proptosis, diplopia, reduced vision, lacrimation, redness, and orbital pain.[3] These symptoms can cause patients to suffer from severe disfigurement and can lead to functional restrictions. From a patient's perspective, clinical measures do not correlate with their physical, emotional, and psychological well-being in their daily life.[4] Hence, health-related QOL has become an important parameter in assessing clinical outcomes with treatment.
In 1998, Terwee et al. designed a disease-specific quality of life (QOL) questionnaire, which has 16 questions with two subscales measuring visual functioning and psychosocial (appearance) effects of a changed appearance.[5] This was applied in patients with GO in the Dutch language and was further tested and validated on Dutch patients.[6] Park et al. translated into English and assessed QOL in Australian patients with GO and found significant impairment in QOL.[7] German-language version of GO-QOL was developed by Ponto et al., which showed significantly lower scores in active and severe disease.[8] This questionnaire has been translated into 15 languages and is being used as one of the important outcome measures to evaluate treatment effects [http://www.eugogo.eu/downloads.html].
Reddy et al. found 28% prevalence of GO among North Indian patients with GD (n = 235) and clinically active and severe orbitopathy was uncommon when compared to Caucasians.[9] There are sparse data regarding QOL studies in GO in Indian patients. So, we have done a study to validate the questionnaire in the local language (Hindi) and assessed the QOL in GO. We also determined to study the correlation of GO-specific QOL scores (visual functioning and appearance) with disease severity and activity.
Methods
Methods and study subjects
A cross-sectional study was done in a tertiary hospital of northern India to study the quality of life in GO. We enrolled 114 consecutive newly referred patients with GO (age at onset ≥18 years) attending the Endocrinology Clinic at Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India. A diagnosis of GD was made clinically by the presence of thyrotoxicosis, diffuse goiter clinically, elevated thyroid hormones, suppressed thyroid stimulating hormone (TSH), and thyroid scintiscan evidence of diffuse increased uptake.[3]
A diagnosis of GO was made based on the American Academy of Ophthalmology Association criteria by Bartley and Gorman, 1995 as stated below.[10]
-
Eyelid retraction (upper eyelid) in conjunction with
- Thyroid dysfunction or
- Exophthalmos or
- Optic nerve dysfunction or
- Extraocular muscle involvement.
-
If eyelid retraction is absent, thyroid dysfunction in association with
- Exophthalmos or
- Optic nerve dysfunction or
- Extraocular muscle involvement.
The exclusion criteria included when the cause of thyrotoxicosis is unclear, the presence of chronic disorders mimicking various features of orbitopathy, and patients with reduced vision like cataract, glaucoma, and retinopathy.[11]
Study procedure
Ophthalmic assessment
Detailed eye and periorbital examination were performed as per the European Group on Graves' Orbitopathy (EUGOGO) recommendations in preliminary case record form.[12,13] Eye screening examination in all GD patients for GO was performed by a single endocrinologist (KD). A measurement ≥20 mm using a Hertel's exophthalmometer was diagnosed as exophthalmos.[14,15] Clinical activity of GO was classified as per clinical activity score (CAS) recommended by EUGOGO. A CAS of 0-2 was considered inactive and 3-7 active GO.[12,13] The severity of GO was classified into mild, moderate-to-severe, and sight-threatening based on the EUGOGO classification.[12]
Graves' orbitopathy quality of life questionnaire
The QOL was assessed by a self-administered disease-specific health-related quality of life questionnaire (GO-QOL), recommended by the EUGOGO. This questionnaire measures two different aspects of health-related quality of life (HRQL) namely visual functioning because of double vision and reduced visual acuity (questions 1 to 8) and psychosocial functioning because of a changed appearance (questions 9 to 16).[15]
The questionnaire was administered in an interview session or if patients need help, question and answer options were read to them in a neutral way to ensure that the answers reflect the patient's opinion. Answers were scored 1 to 3 points for each question from left to right. The questions 1 to 8 and questions 9 to 16 were added up to two raw scores from 8 to 24 points, and then transformed to two total scores from 0-100 by the following formula: total score = (raw score – 8)/16 * 100. Higher scores indicate a better QOL. All patients provided written informed consent. Institutional ethics committee approval was obtained prior to the start of the study.
Assays
Serum total T3, total T4, and TSH were estimated by chemiluminescence immunoassay (Immulite 1000, Siemens, USA). Serum TSH receptor antibodies (TRAb) concentration were estimated with a second-generation enzyme immunoassay (Medizym T.R.A., Medipan GmbH, Dahlewitz/Berlin, Germany). The value of TRAb ≥ 1.5 IU/l was considered positive. The analytical sensitivity of TRAb was 0.5 IU/l. Serum thyroid peroxidase antibodies (TPOAb) were measured using radioimmunoassay (Immunotech, Prague, Czech Republic). A cut-off value of TPOAb ≥ 35 IU/ml was considered positive. The analytical sensitivity of TPO Ab was 2 IU/ml.
Statistical methods
Continuous variables are reported as mean ± standard deviation (SD) or median (inter-quartile range). The Student's t-test or Mann–Whitney U test was used for comparison of continuous variables as appropriate. The Chi-square test was used to compare categorical variables. Mean QOL scores in each aspect were correlated with activity and severity of GO using Spearman correlation coefficient. A two-tailed P value <0.05 was considered significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software package (version 20.0; SPSS Inc., Chicago, IL, USA).
Results
Minor modifications were made in the original English version of GO-QOL to suit the Indian population and the questionnaire was translated into Hindi. Backward translation of the Hindi version was performed, and the content of the translated version remained unchanged. The Hindi version was validated in 50 Indians with GO and the test-retest reliability was performed after a gap of 2 weeks in a subset of 15 patients. Cronbach's alpha was 0.83 and 0.81 and intraclass correlation coefficients for visual function and psychosocial (appearance) function were 0.93 and 0.93, respectively, indicating a high degree of internal consistency and stability. Significant ceiling and floor effects were defined to be more than 15% of the responses at maximum and minimum value, respectively.
The Hindi version of GO-QOL questionnaire was distributed to 49 GO patients and all of them completed it with 100% response rate. Demographic details of GO patients are shown in Table 1. Among 49 patients, 28 (57.1%) were females. Twelve (24.5%) were smoker and all were male. A total of 92% of patients were on antithyroid medications and 43% had relapsed after treatment. TRAb was positive in 91% of GO patients and TPOAb positivity was detected in 79% of patients with GO.
Table 1.
Characteristics of patients with Graves’ disease with orbitopathy (n=49)
| Variables | Graves’ disease with orbitopathy |
|---|---|
| Age at onset (years) | 33 (26, 40) |
| Female (%) | 28 (57.1%) |
| GD duration (months) | 24 (9.5, 60) |
| Therapy for GD | |
| Anti-thyroid drugs | 45 (91.8%) |
| Radioiodine ablation | 1 (2%) |
| Surgery | 3 (6.1%) |
| No treatment | 1 (2%) |
| Relapse after treatment | 21 (42.9%) |
| Severity of eye disease | |
| Mild | 25 (51.0%) |
| Moderate-severe | 23 (46.9%) |
| Sight-threatening | 1 (2.0%) |
| Clinically active disease | 10 (20.4%) |
| TRAb titer (IU/l) | 20.4 (4, 58) |
| TRAb positivity (>1.5 IU/l) | 44 (90.7%) |
| TPOAb titers (IU/ml) | 283 (81, 1000) |
| TPOAb positivity (>35 IU/ml) | 39 (78.6%) |
Median (inter-quartile range), n (%), GD: Graves’ disease, GO: Graves’ orbitopathy, TRAb: thyrotropin receptor antibody, TPOAb: Thyroid peroxidase antibody
Lower (71.4%) and upper eyelid retraction (65.3%) was the most common manifestation. Extra-ocular muscle involvement (18.4%) and optic nerve dysfunction (2%) were uncommon. Unilateral involvement of the eye was seen in 7 (14.3%) patients. GO was mild in severity in 25 (51%) patients, moderate-to-severe in 23 (47%) patients, sight-threatening (due to optic nerve dysfunction) in only 1 (2%), and none of the patient had corneal involvement. Most patients (79.6%) had clinically inactive GO. Only 10 (20.4%) patients had active GO.
The frequencies of responses to each of the questions are shown in Table 2. The most frequently limited activities in visual functioning were watching television and reading. Most patients had a change in appearance and being stared in streets as a limiting response. There were significant ceiling effects (22.4%) with visual functioning subscale and no ceiling effects with appearance scale. Also, there was no significant floor effect with both scales of GO-QOL.
Table 2.
Frequencies of responses to items on the visual functioning subscale and psychosocial (appearance) subscale (n=49)
| Limitations in carrying out the following activities (visual functioning) | Severely limited | Mildly limited | Not limited | Missing response |
|---|---|---|---|---|
| Driving two-wheeler vehicle | 4 | 14 | 35 | 47 |
| Performing domestic duties | 4 | 33 | 63 | 0 |
| Moving around the house | 6 | 18 | 76 | 0 |
| Walking outdoors | 8 | 16 | 76 | 0 |
| Reading | 14 | 31 | 41 | 14 |
| Watching television | 18 | 39 | 41 | 2 |
| Hobbies or pastimes | 14 | 25 | 61 | 0 |
| Hindered from doing something you wanted to do | 4 | 29 | 67 | 0 |
| Psychosocial consequences because of thyroid eye disease | Very much | A little | No | Missing response |
| Appearance has changed | 35 | 53 | 12 | 0 |
| Stared at in the streets | 51 | 37 | 12 | 0 |
| People react unpleasantly | 16 | 47 | 37 | 0 |
| Influence on self-confidence | 12 | 63 | 25 | 0 |
| Socially isolated | 20 | 29 | 51 | 0 |
| Influence on making friends | 10 | 33 | 57 | 0 |
| Appear less often in mirrors than before | 14 | 29 | 57 | 0 |
| Mask changes in your appearance | 6 | 22 | 72 | 0 |
The total median score in the visual functioning scale was 81.3 and in appearance scale, score was 62.5. There was a positive correlation between both functional and appearance scales (r = 0.57, P = 0.000). Patients with moderately severe and sight-threatening GO had lower median psychosocial (appearance) scores (56.3 vs. 68.8, P = 0.01) as compared to mild disease. There was no statistically significant difference in the visual functioning scale with mild vs. moderate-to-severe and sight-threatening GO [Fig. 1]. Patients with active disease had significant lower median visual function (53.1 vs. 85.7, P = 0.009) and psychosocial (appearance) scores (40.6 vs. 68.8, P = 0.03) compared to inactive disease [Fig. 2].
Figure 1.
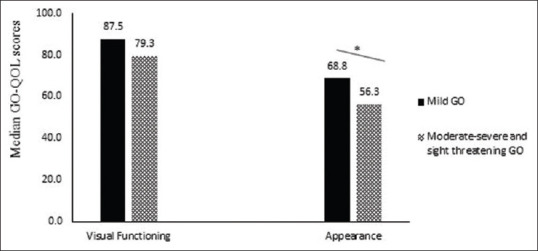
Disease specific quality of life scores (GO-QOL scores) in mild GO and moderately severe and sight threatening GO. GO - Graves' orbitopathy; QOL–Quality of life; GO-QOL– Disease specific Graves' orbitopathy quality of life scores. *p = 0.01
Figure 2.
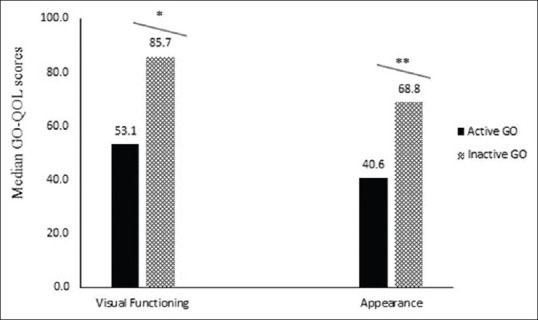
Disease specific quality of life scores (GO-QOL scores) in active GO and inactive GO. GO - Graves' orbitopathy; QOL–Quality of life; GO-QO –Disease specific Graves' orbitopathy quality of life scores. *p = 0.009. **p = 0.03
On multivariate regression analysis of GO-QOL scores, extraocular eye movement involvement (EOM), proptosis, and severity of eye disease were significantly associated with visual functioning while appearance was significantly associated only with the severity of eye disease. [Table 3]. There was no significant correlation between GO-QOL scores (both visual and appearance) with TRAb and TPOAb titers.
Table 3.
Multivariate linear regression analysis of GO-QOL scores
| Visual functioning | Appearance | |||
|---|---|---|---|---|
| Beta | P | Beta | P | |
| Age | -0.14 | 0.34 | -0.1 | 0.6 |
| Sex | -0.22 | 0.06 | 0.02 | 0.9 |
| EOM involvement | -0.51 | 0.000 | -0.22 | 0.18 |
| Proptosis | 0.38 | 0.002 | -0.02 | 0.9 |
| CAS score | -0.19 | 0.11 | 0.1 | 0.51 |
| Severity of eye disease | -0.32 | 0.02 | -0.38 | 0.02 |
| TPO | -0.02 | 0.83 | 0.07 | 0.7 |
| TRAb | -0.01 | 0.99 | -0.33 | 0.12 |
CAS: Clinical activity score, EOM: Extraocular muscle, TRAb: Thyrotropin receptor antibody; TPOAb: Thyroid peroxidase antibody
Discussion
GO is an extrathyroidal manifestation of GD, which not only affects general physical well-being but also causes limitations in QOL.[15] Previously, QOL was assessed by various multidomain health-related surveys (like MOS-SF24, HADS, POMS, and BDI), which had a disadvantage of being too general and unable to detect small, but clinically important changes.[16,17,18,19] Therefore, a disease-specific GO-QOL questionnaire was developed by Terwee et al. in the Dutch language which has two subscales (visual functioning and psychosocial consequences of changed appearance).[5] The patients with varying severity and clinical activity were included in this study. We translated the questionnaire into Hindi version, which was validated and had well preserved internal consistency (Cronbach's alpha was 0.83 and 0.81 for visual function and psychosocial function respectively) as compared with the Dutch GO-QOL survey and we found that QOL is impaired in GO, which correlates with disease activity and severity. So, the results of this study showed that the Hindi version of GO-QOL questionnaire is a valid tool for evaluating disease-specific QOL and subjective well-being of patients with GO.
The scores of visual functioning in our study was 81.3, which was higher than those of other studies like 54.7 in the Dutch survey, 59 in Australian survey, 72.5 in German survey, and scores in appearance was 62.5 in our study which was similar to other studies like 60.1 in the Dutch survey, 54.5 in Australian survey, and 71.3 in German survey.[5,7] These differences in visual functioning could be due to a large proportion of patients with mild and moderately severe orbitopathy.
The median visual functioning scores were lower in patients with diplopia and EOM involvement. In appearance scale, lower scores were seen in patients with moderately severe and sight-threatening disease than with mild disease, which might be due to the presence of proptosis and soft tissue involvement. Moreover, no correlation was found between appearance score and age or visual functioning score and age, also no correlation was found with sex. The study by Peng Zeng et al. had found a correlation with female sex, but not with age.[20]
Multivariate analysis in our study showed EOM involvement, proptosis, and severity of eye disease predicted the visual functioning scores, however, only severity of eye disease predicted appearance scores and similar results were seen in German, Korean, and Spanish study.[8,21,22] Significant ceiling effects (21.4%) were seen in the visual functioning subscale, which suggests that patients had a maximum response to this scale. Similar ceiling effects were also seen in the German study (27%). The possible reason would be as patient with mild, inactive disease having features like minimal proptosis or lid retraction does not experience any limitation in their visual functioning. This implies that QOL scale is less sensitive in evaluating mild disease severity and also in detecting small changes after treatment.[8] Also, no floor effects were seen, which shows that this tool is good at evaluating severe disease. This result was similar to the results obtained in the studies by I-Chan Lin et al. and Peng Zeng et al.[20,23]
In our study, we found a large number of missing responses to driving and reading. This may be due to the presence of the female population where they rarely use vehicles for driving and many of the patients being illiterate were unable to read, which might have led into a missing response. In our Indian population where large number of people are from rural background, we should use questions like limitations in using public transport or working in agricultural fields instead of above two questions in the visual functioning subscale.
The questionnaire was filled by or read out to patients in their outpatient department (OPD) visits and all patients had a 100% response rate, confirming the utility, easy to fill, and simple to understand format that should be used in a regular basis as an outcome measure. The current study highlights the fact that GO patients especially clinically active/severe disease with motility disorders felt limitations in visual and appearance of GO-QOL scores.
The following are strengths of the study. First, to the best of our knowledge, this is the first study from India where GO-QOL questionnaire was validated in Hindi and assessed. India is a developing country where patients are from rural background and GO-QOL scores may not be truly comparable with previous studies which are conducted in developed nations. Second, this study was conducted in the endocrinology department where we see predominantly GD with mild to moderate and large chunk of inactive GO patients. In previous studies, GO-QOL was assessed at tertiary eye care hospital catering patients with severe and active GO disease status, which might have overrepresented the disease.
There are few limitations to this study. First, the study is conducted at a tertiary care centre, which might cause referral bias. Second, this is a cross-sectional study and all treated and untreated patients were included. The effects of treatment on QOL were not assessed. Finally, the questionnaire was filled by patients after being examined by a doctor, which might cause bias in answering.
Conclusion
To conclude, we have validated the Hindi version GO-QOL questionnaire and found impaired QOL in GO patients. As recommended by EUGUGO, the QOL tool is an important parameter in providing patient-focused treatment plan and in follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, et al. Clinical features of dysthyroid optic neuropathy: A European Group on Graves' Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–8. doi: 10.1136/bjo.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TJ, Hegedüs L. Graves' disease. N Engl J Med. 2016;375:1552–65. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WM, George A, Kahaly Mainz J, Freiburg B. Graves' Orbitopathy A Multidisciplinary Approach-Questions and Answers. 2nd, revised edition. Basel: Printed in Switzerland by Reinhardt Druck; 2010. [Last accessed on 2019 Octr 14]. Available from: https://wwwkargercom/Article/PDF/320424 . [Google Scholar]
- 5.Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves' ophthalmopathy: The GO-QOL. Br J Ophthalmol. 1998;82:773–9. doi: 10.1136/bjo.82.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terwee CB, Gerding MN, Dekker FW, Prummel MF, van der Pol JP, Wiersinga WM. Test-retest reliability of the GO-QOL: A disease-specific quality of life questionnaire for patients with Graves' ophthalmopathy. J Clin Epidemiol. 1999;52:875–84. doi: 10.1016/s0895-4356(99)00069-4. [DOI] [PubMed] [Google Scholar]
- 7.Park JJ, Sullivan TJ, Mortimer RH, Wagenaar M, Perry-Keene DA. Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol. 2004;88:75–8. doi: 10.1136/bjo.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponto KA, Hommel G, Pitz S, Elflein H, Pfeiffer N, Kahaly GJ. Quality of life in a german graves orbitopathy population. Am J Ophthalmol. 2011;152:483–90e1. doi: 10.1016/j.ajo.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Reddy SVB, Jain A, Yadav SB, Sharma K, Bhatia E. Prevalence of Graves' ophthalmopathy in patients with Graves' disease presenting to a referral centre in north India. Indian J Med Res. 2014;139:99–104. [PMC free article] [PubMed] [Google Scholar]
- 10.Bartley GB, Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol. 1995;119:792–5. doi: 10.1016/s0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 11.Konuk O, Anagnostis P. Diagnosis and Differential Diagnosis of Graves'. Orbitopathy. 2017. [Last accessed on 2019 Oct 20]. pp. 74–92. Available from: https://wwwkargercom/DOI/101159/000475950 .
- 12.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves' Orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 13.European Group on Graves' Orbitopathy (EUGOGO) Wiersinga WM, Perros P, Kahaly GJ, Mourits MP, Baldeschi L, Boboridis K, et al. Clinical assessment of patients with Graves' orbitopathy: The European Group on Graves' Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol. 2006;155:387–9. doi: 10.1530/eje.1.02230. [DOI] [PubMed] [Google Scholar]
- 14.Kumari Sodhi P, Gupta VP, Pandey RM. Exophthalmometric values in a normal Indian population. Orbit. 2001;20:1–9. doi: 10.1076/orbi.20.1.1.2647. [DOI] [PubMed] [Google Scholar]
- 15.Wiersinga WM. Quality of life in Graves' ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:359–70. doi: 10.1016/j.beem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Farid M, Roch-Levecq AC, Levi L, Brody BL, Granet DB, Kikkawa DO. Psychological disturbance in graves ophthalmopathy. Arch Ophthalmol (Chicago, Ill 1960) 2005;123:491–6. doi: 10.1001/archopht.123.4.491. [DOI] [PubMed] [Google Scholar]
- 17.Kahaly GJ, Hardt J, Petrak F, Egle UT. Psychosocial factors in subjects with thyroid-associated ophthalmopathy. Thyroid. 2002;12:237–9. doi: 10.1089/105072502753600205. [DOI] [PubMed] [Google Scholar]
- 18.Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves' ophthalmopathy is markedly decreased: Measurement by the medical outcomes study instrument. Thyroid. 1997;7:885–9. doi: 10.1089/thy.1997.7.885. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Roh HS, Yoon JS, Lee SY. Assessment of quality of life and depression in Korean patients with Graves' ophthalmopathy. Korean J Ophthalmol. 2010;24:65–72. doi: 10.3341/kjo.2010.24.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng P, Fan SX, Li ZJ, Peng YY, Hu YX, Xu MT, et al. Evaluation of the Graves' orbitopathy-specific quality of life questionnaire in the mainland Chinese population. J Ophthalmol. 2019;2019:7602419. doi: 10.1155/2019/7602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delfino LC, Zunino A, Sapia V, Croome MDCS, Ilera V, Gauna AT. Related quality of life questionnaire specific to dysthyroid ophthalmopathy evaluated in a population of patients with Graves' disease. Arch Endocrinol Metab. 2017;61:374–81. doi: 10.1590/2359-3997000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi YJ, Lim HT, Lee SJ, Lee SY, Yoon JS. Assessing Graves' ophthalmopathy-specific quality of life in Korean patients. Eye. 2012;26:544–51. doi: 10.1038/eye.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin IC, Lee CC, Liao SL. Assessing quality of life in Taiwanese patients with Graves' ophthalmopathy. J Formos Med Assoc. 2015;114:1047–54. doi: 10.1016/j.jfma.2013.12.002. [DOI] [PubMed] [Google Scholar]


