Abstract
A 68-year-old woman with end-stage Stevens–Johnson syndrome developed cystoid macular edema (CME) 3 months following Boston keratoprosthesis type II (KPro-II) implantation and treated with single-dose injection of triamcinolone acetonide (TA) in the inferior peribulbar region. After 14 days, CME resolved completely, and she regained 20/30 vision. Seven months later, she developed recurrent CME. She was again treated with a similar peribulbar injection of TA. CME was resolved completely after 2 weeks with full visual and anatomical recovery. Here, we present a case of recurrent CME following KPro-II implantation responsive to peribulbar injection of TA, which may be the only effective treatment option.
Keywords: Boston keratoprosthesis type II implantation, cystoid macular edema, peribulbar triamcinolone injection
The Boston keratoprosthesis type II (KPro-II) is considered to be the last-resort intervention to salvage some functional vision for severe end-stage cicatricial ocular surface diseases, such as Stevens–Johnson syndrome (SJS) or ocular cicatricial pemphigoid (OCP).[1] The KPro-II is similar to the KPro-I device but requires a permanent complete tarsorrhaphy at the end of the procedure through which optic of the device protrudes. Additional surgeries, such as extracapsular cataract surgery (ECCE), pars plana vitrectomy (PPV), and Ahmed glaucoma valve (AGV) implantation are performed along with KPro-II implantation. Since the United States Food and Drug Administration approval for marketing in 1992, so far, more than 200 Boston KPro-II implantations have been done worldwide.[2,3] The reported incidence of CME following Boston KPro-I is ranging from 0% to 33% and the treatment includes: topical nonsteroidal anti-inflammatory drugs (NSAIDs), posterior sub-Tenon's triamcinolone acetonide (TA), subconjunctival bevacizumab, and intravitreal injection of triamcinolone acetonide (TA) or bevacizumab.[4,5] The reported incidence of CME following Boston KPro-II implantation was 8.3%,[2] but, without any treatment guidelines in the literature. The treatment as described for 'CME following Boston KPro Type-I' (in which eyelids remain open just like after conventional penetrating keratoplasty) is not possible in KPro-II because of the eyelids which are permanently closed. So, it possesses a unique challenge for the treating physician. Here, we describe the first case report of recurrent CME following Boston Type-II implantation responsive to a single dose of peribulbar injection of TA on both occasions.
Case Report
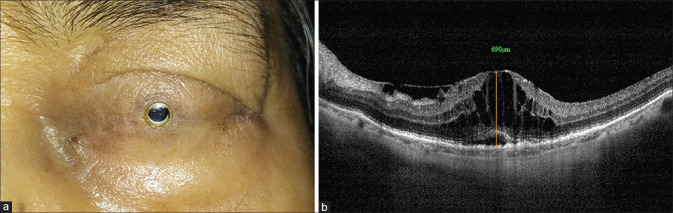
A 68-year-old woman was suffering from chronic SJS sequalae for the last 28 years. During the disease, she lost her right eye about 10 years back due to a corneal ulcer with no perception of light. She presented to us about 1 year back with complete loss of vision in the left eye (LE) for 2 years. On examination, her vision in the LE was only light perception (PL). She had total symblepharon and keratinization of the cornea. Intraocular pressure (finger tension) was normal. B scan ultrasonography (USG) showed a normal posterior segment. She is otherwise healthy, nonhypertensive and nondiabetic individual. She was operated for Boston KPro-II implantation in August 2018 [Fig. 1a]. This was combined with ECCE, PPV, and AGV implantation via pars plana route. The postoperative period was uneventful. On the 7th postoperative day, her best-corrected visual acuity (BCVA) was 20/30. In the first instance, she developed CME at 3 months following the surgery. Conventional treatment for CME was not possible as her eyelids were completely closed. At that time, she was treated differently with 1 ml (40 mg/ml) injection of TA in the inferior peribulbar region. After 2 weeks, her vision improved to 20/30 with resolved CME.[6] There was no increase in intraocular pressure as checked by finger tension. She was followed up regularly at the monthly interval and maintained BCVA of 20/30 with normal macular optical coherent tomography (OCT) findings till 9 months.
Figure 1.
(a) Appearance of the left eye: 10 months after Boston keratoprosthesis Type-II implantation. (b) Macular optical coherence tomography (OCT) showed large cystoid macular edema. Central macular thickness (CMT) was 690 microns
At 10 months follow-up, she presented again with a similar episode of decreased vision without any other symptoms. On examination, her BCVA had dropped to 20/200, Boston KPro-II optic was clear without any retroprosthetic membrane and intraocular pressure (finger tension) was normal. Macular OCT showed a recurrence of CME with a central macular thickness (CMT) of 690 microns [Fig. 1b]. She was treated with a peribulbar injection of TA similar to the last episode. After 2 weeks, her vision again improved to 20/30. Repeat macular OCT showed dramatic resolution of CME and macula looked normal with CMT of 182 microns [Fig. 2]. After 6 months of repeat treatment, her BCVA was maintained at 20/30 with normal macular OCT findings. Intraocular pressure was normal.
Figure 2.
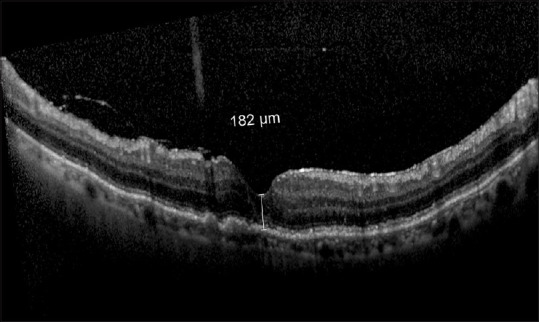
Normal macular OCT after 2 weeks of triamcinolone injection in the inferior peribulbar region. CMT reduced to 182 microns
Discussion
This one-eyed otherwise healthy lady was operated for Boston KPro-II implantation about 16 months back. Postoperatively, during the first year, she developed recurrent CME (two times).
The pathophysiology of KPro-associated CME is presumed to be related to postoperative inflammation, similar to pseudophakic or aphakic CME, and therefore is treated similarly.[7,8]
In any case of CME following KPro-II, it is not possible to treat with conventional management as the eyelids are completely closed at the end of the surgery. There is no scope to give topical NSAIDs. It is also not wise to open the lids for any conventional treatment and then close them. Lee et al. first described CME as a postoperative complication following Boston KPro-II implantation but did not mention about the treatment guidelines.[2]
We treated our patient twice with the same regimen—1 ml (40 mg/ml) of TA injection in the inferior periorbital region with 24 G needle. We did not approach the superior peribulbar region as the AGV was implanted in the superotemporal region during KPro-II surgery. For both episodes, 2 weeks postinjection, the visual and anatomic improvements were dramatic. In 1997, Thach et al. reported the use of retrobulbar injection of corticosteroids in refractory CME in medical treatment.[9] But they used dexamethasone, not triamcinolone, which is now used via different routes for the treatment of CME.
Conclusion
To best of our knowledge, this is the first report to describe the use of single peribulbar injection of triamcinolone acetonide for the treatment of recurrent CME following Boston KPro-II implantation. This is a novel approach to deliver an injection of TA at the posterior part of the globe to treat CME, which was not described earlier.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Duignan ES, Ní Dhubhghaill S, Malone C, Power W. Long-term visual acuity, retention and complications observed with the type-I and type-II Boston keratoprostheses in an Irish population. Br J Ophthalmol. 2016;100:1093–7. doi: 10.1136/bjophthalmol-2015-307443. [DOI] [PubMed] [Google Scholar]
- 2.Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-term visual outcomes and complications of Boston keratoprosthesis type II implantation. Ophthalmology. 2017;124:27–35. doi: 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Iyer G, Srinivasan B, Agarwal S, Ravindran R, Rishi E, Rishi P, et al. Boston Type 2 keratoprosthesis - midterm outcomes from a tertiary eye care centre in India. Ocul Surf. 2019;17:50–54. doi: 10.1016/j.jtos.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Muakkassa NW, Klein KA, Hamrah P, Reichel E. Subconjunctival bevacizumab for the treatment of keratoprosthesis- associated cystoid macular edema. Ophthalmic Surg Lasers Imaging Retina. 2016;47:276–9. doi: 10.3928/23258160-20160229-11. [DOI] [PubMed] [Google Scholar]
- 5.Goldman DR, Hubschman J-P, Aldave AJ, Chiang A, Huang JS, Boueges JL, et al. Postoperative posterior segment complications in eyes treated with the Boston type I keratoprosthesis. Retina. 2013;33:532–41. doi: 10.1097/IAE.0b013e3182641848. [DOI] [PubMed] [Google Scholar]
- 6.Basak SK, Basak S. Cystoid macular edema following Boston keratoprosthesis type II: Treatment. Ophthalmology. 2019;126:966. doi: 10.1016/j.ophtha.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Zur D, Loewenstein A. Postsurgical cystoid macular edema. Dev Ophthalmol. 2017;58:178–90. doi: 10.1159/000455280. [DOI] [PubMed] [Google Scholar]
- 8.Guo S, Patel S, Baumrind B, Johnson K, Levinsohn D, Marcus E, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60:123–37. doi: 10.1016/j.survophthal.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Thach AB, Dugel PU, Flindall RJ, Sipperley JO, Sneed SR. A comparison of retrobulbar versus sub-Tenon's corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology. 1997;104:2003–8. doi: 10.1016/s0161-6420(97)30065-7. [DOI] [PubMed] [Google Scholar]