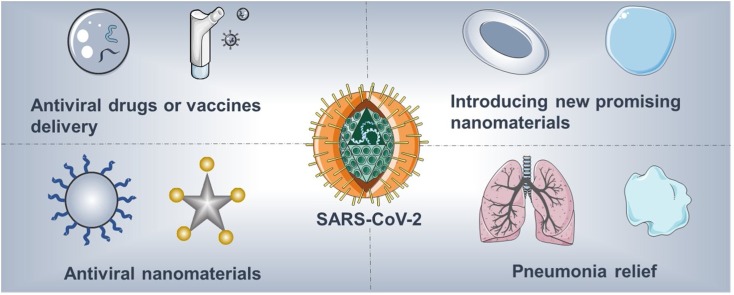
Graphical abstract
Keywords: COVID-19, Antiviral drugs, Vaccines, Clinical trials, Nanotechnology
Abstract
In just a few months, SARS-CoV-2 and the disease it causes, COVID-19, created a worldwide pandemic. Virologists, biologists, pharmacists, materials scientists, and clinicians are collaborating to develop efficient treatment strategies. Overall, in addition to the use of clinical equipment to assist patient rehabilitation, antiviral drugs and vaccines are the areas of greatest focus. Given the physical size of SARS-CoV-2 and the vaccine delivery platforms currently in clinical trials, the relevance of nanotechnology is clear, and previous antiviral research using nanomaterials also supports this connection. Herein we briefly summarize current representative strategies regarding nanomaterials in antiviral research. We focus specifically on SARS-CoV-2 and the detailed role that nanotechnology can play in addressing this pandemic, including i) using FDA-approved nanomaterials for drug/vaccine delivery, including further exploration of the inhalation pathway; ii) introducing promising nanomaterials currently in clinical trials for drug/vaccine delivery; iii) designing novel biocompatible nanomaterials to combat the virus via interfering in its life cycle; and iv) promoting the utilization of nanomaterials in pneumonia treatment.
Introduction
The COVID-19 pandemic is not a unique case in the history of viruses but one representative of the new century, which is sobering news for humans. At this time of writing, over 40,000,000 patients have been infected SARS-CoV-2, with over 1,100,000 deaths, according to the World Health Organization (WHO) [1]. With an in-depth understanding of the SARS-CoV-2, including knowing its gene sequence [2], analyzing the structure of key viral proteins by cryogenic electron microscopy (cryo-EM) [3,4], undertaking pathway analysis of its variation [5] and transmission [6], as well as exploring the origin of the virus [7], potential antiviral drugs and vaccines should advance rapidly. However, the ever-increasing pandemic, in terms of numbers of people and geographic areas, rapid transmission, repeated infections, and genomic variation in SARS-CoV-2 [5], presents difficult obstacles to the development of antiviral drugs and vaccines, although they can also be seen as stimulating challenges for researchers.
Generally, current trials relevant to COVID-19 encompass antiviral drugs, drugs to modulate the immune response, neutralizing antibodies, and vaccines. Based on the life cycle of SARS-CoV-2 and its structure, antiviral drugs work through inhibiting RNA polymerase (Remdesivir [8], Favipiravir with Tocilizumab [9]), inhibiting viral protease (Ivermectin, [10] Lopinavir/Ritonavir [11],), blocking membrane fusion (Leronlimab [12], rhACE2 [13], Hydroxychloroquine [14] and Arbidol Hydrochloride [15]), and broad-spectrum antiviral effects (EIDD-2801 [16]).
However, vaccines are probably a stronger weapon in this war. Examples currently include the BNT162 mRNA from BioNTech. Inc., [17] mRNA-1273 from Moderna. Inc. [18], INO-4800 from Inovio. Inc., [19], and ChAdOx1 nCoV-19 [20] from the University of Oxford. Despite the differences in working principles, the success of any vaccine in terms of stability and efficacy depends on the delivery platform: mRNA-1273 needs lipid particles, INO-4800 requires the CELLECTRA® portable device, and ChAdOx1 nCoV-19 requires its non-replicating adenovirus vector.
Among vaccine delivery platforms, lipid particles and non-replicating adenovirus vectors have similar nano size, attracting the attention of nanotechnologists. Not only that, but the latest clinical results on antiviral drugs such as Remdesivir [21] and Hydroxychloroquine [22] are not very satisfactory, while the results of mRNA vaccine trials are much more exciting; Phase 2/3 clinical trial data on BNT162 mRNA and Phase 3 clinical trial data on mRNA-1273 has proven the safety and efficacy of these agents [17,18]. Some other drugs also being utilized to mitigate SARS-CoV-2-induced lung injury include dexamethasone [23], ruxolitinib [24], fostamatinib [25], and nebulized heparin [26]. Scientists have also proposed the use of dexamethasone nano-formulations in the treatment of COVID-19 via targeting hyper-activated immune cells through inhalation or intravenous injection [27].
Not only the above technologies, but also neutralizing antibodies like BGB-DXP592 [28], LY3819252 [29], REGN10933/REGN10987 monoclonal antibodies [30] and antibody fragments (INOSARS) [31] etc. were developed for COVID-19 treatment and vaccination. The antibody fragments called nanobodies (for their nano-scale size) are yet another connection to nanotechnology. As the clinical trial results of the first wave of COVID drugs have yet to be been announced, now would be an appropriate time to push forward on other technologies, including nanotechnology [[32], [33], [34]].
We recently shared our views on tackling COVID-19 from a materials science perspective [35]. In the current opinion we specifically highlight the potential role of nanotechnology in treatments for COVID-19. Although nanotechnology has been introduced into antiviral research before, its prospects for success with SARS-CoV-2 are better than one might imagine, considering the following factors: 1) SARS-CoV-2 and nanomaterials are similar in size, setting the stage for direct contact/”combat”; 2) SARS-CoV-2 (60−140 nm) [36] is also near in size to most current FDA-approved nanomaterials; 3) nanomaterials can decrease the side effects of current antiviral drugs; 4) nanomaterials can co-deliver multiple drugs; 5) nanomaterials can enhance the stability of mRNA vaccines; and 6) nanotechnology facilitates controlled/targeted drug release. In this perspective, we focus on the most promising antiviral research involving nanotechnology and offer relevant paths forward in the current “war” against SARS-CoV-2 and future threats posed by other infectious viruses.
Nanotechnology versus the virus
Decades of rapid development have witnessed the widespread application of nanotechnology in the biomedical field [37,38]. Although it is not being widely applied in current antiviral research, its potential is unquestionable. To summarize, the advantages of nanotechnology in antiviral research include the following: 1) promotes the delivery of water-insoluble drugs [39]; 2) enhances the circulation time of drugs in vivo [40]; 3) achieves co-delivery of drugs [40]; 4) improves drug utilization efficiency and reduce side effects through targeting antibody modification [41]; 5) protects DNA and mRNA vaccines, overcoming bottlenecks for in vivo applications [42]; and 6) the physicochemical properties of nanomaterials can also be employed directly against viruses [43]. In this section, we will focus on the aspects of nanotechnology that have the greatest potential for clinical transformation or that have already been applied in the clinic, including in the delivery of antiviral drugs and vaccines, and the design of nanomaterials directly effective against viruses.
Delivery of drugs and vaccines
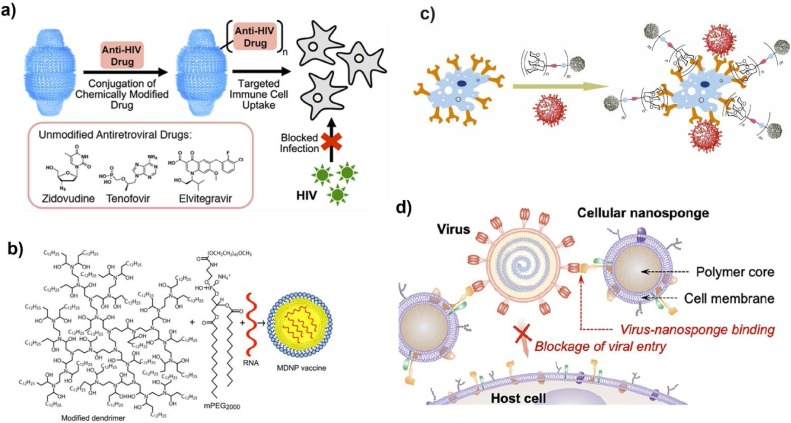
The FDA-approved nanomaterials liposomes [44] and polylactic-co-glycolic acid (PLGA) [45] are already in mature use in the delivery of drugs (and not only antiviral drugs). As described before, FDA-approved nanomaterials offer unique advantages for antiviral drug delivery, and potential nanomaterial candidates are still emerging. For example, to guarantee high loading efficiency, biocompatible porous metal-organic-framework nanomaterials were utilized as nanocarriers to deliver drugs including retrovirals to treat acquired immune deficiency syndrome (AIDS) [46], releasing the drugs gradually. Through chemical bioconjugation, lipid-coated PLGA nanomaterials first target T helper cells, which express the CD4+ (cluster of differentiation 4) protein on their surface [47]. Subsequent nanomaterials were designed to release encapsulated latency-reversing agents (LRAs) sustainably, ensuring synergistic effects against human immunodeficiency virus type 1 (HIV-1). In addition, nanoparticles were engineered to offer the controlled release of anti-HIV drugs when triggered by external energy. Owing to the magnetoelectricity capability of these nanoparticles, the bond between anti-HIV drugs and the nanoparticles can be broken as required with the application of a uniform magnetic field, releasing the drugs in a controlled manner [48]. Three antiretroviral drugs were also chemically conjugated to human vault nanoparticles, which are intracellular ribonucleoprotein particle complexes that can be easily engulfed by immune-related cells, ensuring the targeted delivery of antiretroviral drugs against HIV type 1 ( Fig. 1 a) [49].
Fig. 1.
Representative strategies against viruses using nanotechnology. a) Antiviral drug delivery: design drug-conjugated human vault nanoparticles against HIV-1 infection, Adapted from Ref. [49]. Copyright 2019 American Chemical Society; b) Vaccine delivery: modified dendrimer nanoparticles (MDNP) with lipid-anchored polyethylene glycol-2000 (PEG-2000) for antiviral mRNA delivery, Adapted from Ref. [52]. Copyright 2016 National Academy of Sciences; c) Multivalent binding strategy: virus-like glycodendrinanoparticles were introduced to capture virus and block infection, Adapted from Ref. [68]. Copyright 2018 Nature Publishing Group; d) Host cell membrane-mimicking strategy: the nanodecoy carrying host cell membrane for adsorbing Zika virus, and representative transmission electron microscope (TEM) image of one nanodecoy that binds the Zika virus (indicated by red arrows). Scale bar, 50 nm. Adapted from Ref. [71]. Copyright 2020 American Chemical Society.
Moving into the era of “universal” influenza vaccines, pulmonary surfactant (PS)-biomimetic liposomes were designed for heterosubtypic immunity potentiation regulated by cytotoxic T lymphocytes that express the CD8+ protein [50]. Moreover, the PS assisted in the delivery of liposomes to alveolar epithelial cells and achieved further immune activation, which ensured good treatment results in vivo. To improve immunogenicity and prevent the humoral immunity of DNA vaccines, dual-functional fullerene nanomaterials with an HIV virus-like morphology were fabricated to deliver the vaccine to efficiently stimulate immune response [51].
Since the RNA interference (RNAi) strategy was proposed, a number of siRNA- or mRNA-based vaccines have been carefully designed. Combining them with nanomaterials improves the stability of RNA to the levels necessary for in vivo applications. For instance, adjuvant-free dendrimer nanomaterials were created for mRNA vaccine delivery; only one dose was necessary to sustain immunity and combat not only H1N1 influenza but also Ebola virus ( Fig. 1b) [52].
In response to the lethal Zika virus, mRNA vaccines were mentioned in many research projects and some clinical trials. As an example, scientists first embedded the mRNA vaccines designed to protect against the Zika virus into lipid-nanomaterials, and then evaluated the immune response in vivo [53]. The exciting results drew global attention. Coincidentally, the positive results of recent clinical trials by Moderna. Inc. using an anti-Zika mRNA vaccine encapsulated in lipid-nanomaterials [54] provide strong encouragement for the advancement of mRNA vaccines against SARS-CoV-2. Recent data from Pfizer Inc. and BioNTech SE has confirmed improvements in the safety and dose-dependent efficiency of BNT162b2 mRNA vaccines, supporting their potential for ongoing Phase 2/3 large-scale evaluation [17,55]. The mRNA-1273 vaccine against SARS-CoV-2 is in Phase 3 clinical trials to determine its safety and efficiency for COVID-19 prevention up to 2 years after the second dose [18].
In other work, the endogenous untranslated regions (UTRs) of mRNAs were further engineered to enhance the generation of SARS-CoV-2 antigens. In addition, using TT3 nanoparticles for the delivery of mRNA vaccine improved performance over FDA-approved lipid nanoparticles [56].
Besides these vaccine examples, nanotechnology also shows potential in developing adjuvants for vaccines that promote antibody expression. As traditional aluminum hydroxide (alum) adjuvants, presenting as plate-like microgels with a positive charge, are likely to attach to the membrane rather than be internalized by the dendritic cells, an oil/water interphase of particulate alum via Pickering emulsion (rather than a surfactant-stabilized emulsion) was generated, which not only absorbed plenty of antigens but also promoted the uptake of loaded antigens by dendritic cells, improving the immune response induced by vaccines while maintaining safety [57].
Antiviral nanomaterials
In addition to serving as a delivery platform for antiviral drugs or vaccines, carefully designed nanomaterials themselves can also directly fight viruses [58,59]. First, understanding the virus replication cycle is central to a suitable antiviral strategy. Although we may eventually come to understand viral life cycles differently, the current model includes attachment, entry, biosynthesis, assembly of new viruses, and release; viral inhibition is possible within each of those steps. In this area, the exciting potential of nanomaterials has just begun to be tapped.
The advent of DNA origami technology has also enriched the original nanomaterial library, while its application in antiviral research is still in its early stages. By designing the DNA nanoarchitecture to have a specific star shape, the activity of the dengue virus can be inhibited through spatial pattern interaction [60]. Specifically, the nanoarchitecture was modified with ED3-targeting aptamers for recognizing ED3 clusters on the viral surface.
Moreover, using heparan sulfate proteoglycan (HSPG), gold nanomaterials were employed as broad-spectrum viral inhibitors. Unlike previous HSPG substances, newly synthesized mercaptoundecane sulfonic (MUS) acid-containing ligands are capable of binding viral attachment ligands even after dilution, producing forces that deform viruses [61]. Also, HSPG was incorporated into nanogels with various degrees of flexibility to help prevent virus entry [62]. Furthermore, MUS acid-modified cyclodextrins have also been the subject of broad-spectrum antiviral research [63], as the modified MUS acid can mimic HSPG to generate a virucidal reaction.
In addition to the above nanomaterials, mercaptobenzoic acid-modified gold nanomaterials were also used for HIV inhibition via the multivalent binding approach [64]. Based on the same antiviral strategy, new types of giant globular glycofullerenes were designed against artificial Ebola virus infection [65]. Another candidate using nanostructured glycan architecture with suitable space between ligands, composed of multivalent 6′-sialyllactose-polyamidoamine (6SL–PAMAM) conjugates, was designed for influenza A virus infection inhibition [66]. Most recently, bacteriophage capsids carrying ligands that tightly bind the influenza virus were also introduced to prevent its entry through a defined multivalent strategy [67]. Similarly, the glycodendrinanoparticles assembled by high-valency glycodendrimeric components were generated to mimic pathogens and block viral infection ( Fig. 1c) [68]. The good polyvalency effects on the nanoparticles' surface area suggest a universal strategy to inhibit viral infection. Via cell membrane technology [69], Zika virus host cells’ membrane-derived vesicles were added to the surface of gelatin nanomaterials [70]. These “nanodecoys” capture the Zika virus from intended targeting sites and silence viral infection. More recently, plasma membranes derived from human lung epithelial type II cells or human macrophage cell membrane-derived nanosponges were utilized to mimic the host cell surface and capture the SARS-CoV-2 for neutralization ( Fig. 1d) [71]. Inspired by the above examples, we have high hopes for the future of nanotechnology in antiviral research.
Outlooks
The maturity of antiviral research has always depended on time, effort, and following the right direction. Based on our deepening understanding of SARS-CoV-2 acquired just over the last few months, we have witnessed the continuous emergence of clinical trials against SARS-CoV-2. Predictably, some are exciting while others are unsatisfactory. Throughout this process, nanotechnology has consistently demonstrated its potential, and we can look forward to its bright future (Fig. 2).
Fig. 2.
Outlooks from nanotechnology against SARS-CoV-2.
Nanomaterials for the delivery of antiviral drugs and vaccines
Even with the similarities between SARS-CoV-2 and other viruses and our accumulated understanding of antiviral mechanisms, the existing drugs used to treat COVID-19 currently have unsatisfactory efficiency, as well as side effects. We anticipate that FDA-approved nanomaterials including liposomes, PLGA nanoparticles, etc. can be used to encapsulate antiviral drugs to achieve long-term circulation, sustained release, and co-delivery of multiple drugs for better treatment efficiency. Modified with antibodies that target the proteins in SARS-CoV-2, nanomaterials can simultaneously enhance drug utilization while reducing side effects, thus ensuring better treatment outcomes. The cryo-EM structure of potential drugs and the mechanism of their interaction with the life cycle of the virus (e.g. their channels into the RNA polymerase that the SARS-CoV-2 needs for replication) can be visualized with Å resolution for better understanding to improve drug design [72]. Drug combinations can be further tested and co-delivered by nanomaterials. Besides, it is well-known that in patients with severe COVID-19, the hypoxia caused by pneumonia [73] suppresses the delivery efficiency of antiviral drugs. Perhaps nanomaterials will soon be used to co-deliver angiogenesis factors (or other drugs to help relieve hypoxia) and antiviral drugs to generate better outcomes. The inhalation of antiviral drug-loaded nanomaterials is another promising direction. For the delivery of vaccines, current FDA-approved nanomaterials can also be modified with targeting antibodies or bionic molecules such as PS for superior immune activation.
Introducing new promising nanomaterials for delivery
When deciding whether to introduce potential new candidates for drug and vaccine delivery, it is wise to investigate the nanomaterials currently employed in clinical trials [74]. Since the discovery of exosomes, their potential has been demonstrated in the field of biomedicine. Exosomes with nano-size can be derived from cells, have unparalleled biocompatibility, and also carry out some of the same functions as cells [75]. One promising direction would be to apply exosomes to the delivery of antiviral drugs or vaccines. With features similar to exosomes, nanomaterials mimicking host cell membranes, bacterial membranes, and inactivated viral envelopes may become the next candidate delivery platform. In addition, self-assembly proteins and peptide-based nanomaterials are also promising delivery candidates.
Designing suitable nanomaterials to inhibit SARS-CoV-2
Based on the life cycle of SARS-CoV-2, and inspired by previous research, we put forward the following possibilities: i) modified DNA or RNA origami nanostructures could be designed to inhibit attachment on host cells; ii) HSPG-mimicking nanomaterials or nanomaterials modified with the multivalent ligands could be utilized for inhibition of the infusion process; iii) external Zn-based biocompatible nanomaterials may inhibit RNA polymerase through zinc ion release [76]; iv) membrane-targeting photosensitizers/nanomaterials may induce lipid oxidation to prohibit viral fusion via 1O2 generation; [77] and v) viral membrane-targeting photothermal nanomaterials (such as gold) may also destroy virus via local hyperthermia [78], as heat has been employed for virus inactivation for many years. In addition, nanomaterials with the antiviral capabilities mentioned above could also be embedded in personal protective equipment (PPE), yielding antiviral PPE. For instance, we could combine existing eco-friendly and mass-production methods [79] to synthesize nanofibrous materials with the above antiviral capabilities as components of PPE [80].
Introducing nanomaterials to relieve pneumonia symptoms
Addressing pneumonia, the main symptom of COVID-19 infection, urgently requires the incorporation of nanotechnology. From using nanomaterials to deliver anti-inflammatory drugs, introducing antioxidant nanomaterials, providing inhalation methods, fabricating platelet-derived nanomaterials that are actively targeted to inflammatory sites, and offering the capability for controlled drug release, to utilizing oxygen-generation nanomaterials, such strategies may not only provide timely solutions but also inspire future explorations. For example, platelet-derived nanomaterials can be introduced to encapsulate [5-(p-fluorophenyl)-2-ureido]thiophene-3-carboxamide (TPCA-1) to target the pneumonia site and calm cytokine storm [81]. Moreover, antioxidant nanomaterials such as cerium dioxide nanoparticles can also be utilized to eliminate reactive oxygen species (ROS) in the inflammatory site [82].
Other technologies could also be combined with nanotechnology to facilitate and promote its application in COVID-19 treatments. For example, artificial intelligence, which has been explored for COVID-19 diagnosis [83] and the identification of drugs that could be repurposed to treat COVID-19 [84], should also be considered to help isolate (or find) suitable drugs/vaccines-loaded nanomaterials, therapeutic nanomaterials, and nanomaterials that could provide pneumonia relief.
With the deepening of our understanding of SARS-CoV-2 and related research advancements, we hope and believe that nanotechnology can offer timelier and more effective approaches to dealing with SARS-CoV-2, not to mention other emerging viruses in the future.
Author contributions
All authors contributed to the discussion and writing of this manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work is supported by Harvard Medical School/Brigham and Women’s Hospital Department of Anesthesiology-Basic Scientist Grant (No. 2420 BPA075, W.T.), and Center for Nanomedicine Research Fund (No. 2019A014810, W.T.). W.T. is a recipient of the Khoury Innovation Award (No. 2020A003219) and American Heart Association (AHA) Collaborative Sciences Award (No. 2018A004190). W.T. also received a start-up package (for three years) from the Department of Anesthesiology, Perioperative and Pain Medicine to establish his independent research laboratory at Harvard Medical School and Brigham and Women’s Hospital. We thank our department for this generous support. Z.T. is supported by the China Postdoctoral Science Foundation (2019M663060).
Biographies

Zhongmin Tang obtained his B.E. degree from Shandong University (2014) and received his Ph.D. degree from Shanghai Institute of Ceramics, Chinese Academy of Sciences (2019). During the Ph.D. study, he was focusing on the development of various nanomaterials with energy conversion properties and their corresponding biomedical applications including cancer theranostics and bacterial killing. Currently, Dr. Tang is a Postdoctoral Fellow at Brigham and Women’s Hospital, Harvard Medical School. In the Center for Nanomedicine, he is now developing a novel nanotechnology platform for cancer therapy.

Dr. Xingcai Zhang, Harvard/MIT Research Fellow/Science Writer/Editorial (Advisory) Board Member for Springer Nature, Elsevier, Materials Today, Royal Society of Chemistry, Wiley/Nature Nano Ambassador with 5 STEM degrees/strong background in sustainable Nature-derived/inspired/mimetic materials for biomed/sensing/catalysis/energy/environment applications, with more than 80 high-impact journal publications in Nature Reviews Materials (featured cover paper), etc.

Yiqing Shu received her B.E. degree from the School of Chemical engineering and technology, Northwest University, China, in 2014. Now she is a Ph.D., Faculty of Information Technology, Macau University of Science and Technology, Macao. Her current research interests focus on exploring the nonlinear photonics of low-dimensional materials.

Ming Guo is an associate professor at the Department of Mechanical Engineering at Massachusetts Institute of Technology, where he holds class’ 54 career development professorship. Before joining MIT, Ming obtained his Ph.D. in Applied Physics at Harvard University, and B.S. in Engineering Mechanics at Tsinghua University. His group works on developing tools to probe and understand how mechanics and biology impact each other in a multicellular system and how these properties coordinate in space and time to together sculpt the evolution of multicellular physiological and pathological processes. Dr. Guo is an Alfred Sloan Fellow in Physics.

Han Zhang, is a full Professor and Director of Shenzhen Engineering Laboratory of phosphorene and optoelectronics, Shenzhen University. He has published over 300 peer-review research and invited review articles in the last 10 years. Most of his publications are correlated to the photonic and optoelectronic applications of two-dimensional monoelemental materials (Xenes). The related works have been published in many prestigious journals such as PNAS, Physics Reports, Nature Communications etc, which have received >36, 000 citations, with an H-index of 105. He has been awarded as Highly Cited Researcher by Clarivate Analytics from 2018 to 2020, RSC and OSA Fellow.

Wei Tao received his Ph.D. from Tsinghua University (2015). He held a Postdoctoral Fellowship at Harvard Medical School and Brigham and Women’s Hospital (2016–2018). Subsequently, he was promoted to be Instructor in 2018. In 2020, his promotion to Assistant Professor gained approval from the Harvard Medical School Chairs. Prof. Tao’s research interest is in the development of functional biomaterials, revealing nano-bio interactions, and exploring their biomedical applications. He currently serves as the Principal Investigator on multiple awards/grants including the U.S. METAvivor Early Career Investigator Award, Harvard Anesthesia Department Basic Scientist Grant, Khoury Innovation Award, Center for Nanomedicine Research Fund, etc.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.nantod.2020.101019.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.2020. World Health Organization.https://covid19.who.int/ [Google Scholar]
- 2.Snapgene, https://www.snapgene.com/resources/coronavirus-resources/?resource=SARS-CoV-2_%28COVID-19%29_Genome (2020).
- 3.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster P., Forster L., Renfrew C., Forster M. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L., Parker M., Bonsall D., Fraser C. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04292899 NCT04292899 (2020).
- 9.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04310228 NCT04310228 (2020).
- 10.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT01570504 NCT01570504 (2020).
- 11.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04321174 NCT04321174 (2020).
- 12.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04347239 NCT04347239 (2020).
- 13.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04335136 NCT04335136 (2020).
- 14.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04342221 NCT04342221 (2020).
- 15.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04260594 NCT04260594 (2020).
- 16.Emory, https://news.emory.edu/stories/2020/04/covid_eidd2801_fda/index.html (2020).
- 17.Walsh E.E., Frenck R., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Thompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. medRxiv. 2020 [Google Scholar]
- 18.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04470427 NCT04470427 (2020).
- 19.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04336410 NCT04336410 (2020).
- 20.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04324606 NCT04324606 (2020).
- 21.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane J.C., Weaver J., Kostka K., Duarte-Salles T., Abrahao M.T.F., Alghoul H., Alser O., Alshammari T.M., Biedermann P., Burn E. medRxiv. 2020 [Google Scholar]
- 23.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04509973 NCT04509973 (2020).
- 24.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04362137 NCT04362137 (2020).
- 25.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04579393 NCT04579393 (2020).
- 26.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04530578 NCT04530578 (2020).
- 27.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., Koschmieder S., Brümmendorf T.H., Kiessling F., Metselaar J.M. Nat. Nanotechnol. 2020;15:622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04551898 NCT04551898 (2020).
- 29.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04497987 NCT04497987 (2020).
- 30.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04426695 NCT04426695 (2020).
- 31.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04514302 NCT04514302 (2020).
- 32.S. Bai, C. Sun, H. Yan, X. Sun, H. Zhang, L. Luo, X. Lei, P. Wan, X.J.S. Chen, 11 (2015) 5807-5813. [DOI] [PubMed]
- 33.Z. Huang, Z. Zhang, X. Qi, X. Ren, G. Xu, P. Wan, X. Sun, H.J.N. Zhang, 8 (2016) 13273-13279. [DOI] [PubMed]
- 34.D. Ma, Y. Li, H. Mi, S. Luo, P. Zhang, Z. Lin, J. Li, H.J.A.C. Zhang, 130 (2018) 9039-9043.
- 35.Tang Z., Kong N., Zhang X., Liu Y., Hu P., Mou S., Liljeström P., Shi J., Tan W., Kim J.S., Cao Y., Langer R., Leong K.W., Farokhzad O.C., Tao W. Nat. Rev. Mater. 2020;5:847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.C. Xing, Z. Xie, Z. Liang, W. Liang, T. Fan, J.S. Ponraj, S.C. Dhanabalan, D. Fan, H.J.A.O.M. Zhang, 5 (2017) 1700884.
- 38.J. Zheng, Z. Yang, C. Si, Z. Liang, X. Chen, R. Cao, Z. Guo, K. Wang, Y. Zhang, J.J.A.P. Ji, 4 (2017) 1466-1476.
- 39.Liu Z., Robinson J.T., Sun X., Dai H. J. Am. Chem. Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farokhzad O.C., Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 41.Davis M.E., Zuckerman J.E., Choi C.H.J., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim M.E., Lee Y.-l., Zhang Y., Chu J.J.H. Biomaterials. 2012;33:1912–1920. doi: 10.1016/j.biomaterials.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 44.FDA, https://www.fda.gov/media/70837/download (2018).
- 45.FDA, https://www.fda.gov/media/125184/download (Accessed year 2020).
- 46.Horcajada P., Chalati T., Serre C., Gillet B., Sebrie C., Baati T., Eubank J.F., Heurtaux D., Clayette P., Kreuz C. Nat. Mater. 2010;9:172–178. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 47.Cao S., Slack S.D., Levy C.N., Hughes S.M., Jiang Y., Yogodzinski C., Roychoudhury P., Jerome K.R., Schiffer J.T., Hladik F. Sci. Adv. 2019;5:eaav6322. doi: 10.1126/sciadv.aav6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nair M., Guduru R., Liang P., Hong J., Sagar V., Khizroev S. Nat. Commun. 2013;4:1707. doi: 10.1038/ncomms2717. [DOI] [PubMed] [Google Scholar]
- 49.Fulcher J.A., Tamshen K., Wollenberg A.L., Kickhoefer V.A., Mrazek J., Elliott J., Ibarrondo F.J., Anton P.A., Rome L.H., Maynard H.D., Deming T., Yang O.O. Bioconjug. Chem. 2019;30:2216–2227. doi: 10.1021/acs.bioconjchem.9b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Li P., Yu Y., Fu Y., Jiang H., Lu M., Sun Z., Jiang S., Lu L., Wu M.X. Science. 2020;367:eaau0810. doi: 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L., Liu Y., Chen Z., Li W., Liu Y., Wang L., Ma L., Shao Y., Zhao Y., Chen C. Adv. Mater. 2013;25:5928–5936. doi: 10.1002/adma.201300583. [DOI] [PubMed] [Google Scholar]
- 52.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D., Sidik S.M., Lourido S., Langer R., Bavari S. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04064905 NCT04064905 (2020).
- 55.Clinicaltrials, https://clinicaltrials.gov/ct2/show/NCT04380701 NCT04380701 (2020).
- 56.Zeng C., Hou X., Yan J., Zhang C., Li W., Zhao W., Du S., Dong Y. Adv. Mater. 2020;32 doi: 10.1002/adma.202004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng S., Cao F., Xia Y., Gao X.D., Dai L., Yan J., Ma G. Adv. Mater. 2020;32 doi: 10.1002/adma.202004210. [DOI] [PubMed] [Google Scholar]
- 58.L. Wu, Z. Xie, L. Lu, J. Zhao, Y. Wang, X. Jiang, Y. Ge, F. Zhang, S. Lu, Z.J.A.O.M. Guo, 6 (2018) 1700985.
- 59.J. Zheng, X. Tang, Z. Yang, Z. Liang, Y. Chen, K. Wang, Y. Song, Y. Zhang, J. Ji, Y.J.A.O.M. Liu, 5 (2017) 1700026.
- 60.Kwon P.S., Ren S., Kwon S.-J., Kizer M.E., Kuo L., Xie M., Zhu D., Zhou F., Zhang F., Kim D. Nat. Chem. 2020;12:26–35. doi: 10.1038/s41557-019-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cagno V., Andreozzi P., D’Alicarnasso M., Silva P.J., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J. Nat. Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 62.Dey P., Bergmann T., Cuellar-Camacho J.L., Ehrmann S., Chowdhury M.S., Zhang M., Dahmani I., Haag R., Azab W. ACS Nano. 2018;12:6429–6442. doi: 10.1021/acsnano.8b01616. [DOI] [PubMed] [Google Scholar]
- 63.Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vuković L. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax9318. eaax9318-9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bowman M.-C., Ballard T.E., Ackerson C.J., Feldheim D.L., Margolis D.M., Melander C. J. Am. Chem. Soc. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muñoz A., Sigwalt D., Illescas B.M., Luczkowiak J., Rodríguez-Pérez L., Nierengarten I., Holler M., Remy J.-S., Buffet K., Vincent S.P. Nat. Chem. 2016;8:50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 66.Kwon S.-J., Na D.H., Kwak J.H., Douaisi M., Zhang F., Park E.J., Park J.-H., Youn H., Song C.-S., Kane R.S. Nat. Nanotechnol. 2017;12:48. doi: 10.1038/nnano.2016.181. [DOI] [PubMed] [Google Scholar]
- 67.Lauster D., Klenk S., Ludwig K., Nojoumi S., Behren S., Adam L., Stadtmüller M., Saenger S., Zimmler S., Hönzke K. Nat. Nanotechnol. 2020;15:373–379. doi: 10.1038/s41565-020-0660-2. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro-Viana R., Sanchez-Navarro M., Luczkowiak J., Koeppe J.R., Delgado R., Rojo J., Davis B.G. Nat. Commun. 2012;3:1303. doi: 10.1038/ncomms2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu C.-M.J., Fang R.H., Wang K.-C., Luk B.T., Thamphiwatana S., Dehaini D., Nguyen P., Angsantikul P., Wen C.H., Kroll A.V. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao L., Wang W., Meng Q.-F., Tian M., Cai B., Wang Y., Li A., Zan M., Xiao F., Bu L.-L. Nano Lett. 2018;19:2215–2222. doi: 10.1021/acs.nanolett.8b03913. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Griffiths A., Zhang L. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. https://pubs.acs.org/doi/abs/5510.1021/acs.nanolett.5570c02278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin W., Mao C., Luan X., Shen D.-D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M. Science. 2020;368:1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanley B., Lucas S.B., Youd E., Swift B., Osborn M., Clin J. Pathology. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 74.C. Xing, W. Huang, Z. Xie, J. Zhao, D. Ma, T. Fan, W. Liang, Y. Ge, B. Dong, J.J.A.P. Li, 5 (2018) 621-629.
- 75.Théry C., Zitvogel L., Amigorena S. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 76.Kaushik N., Subramani C., Anang S., Muthumohan R., Nayak B., Ranjith-Kumar C., Surjit M. J. Virol. 2017;91:e00754–00717. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vigant F., Santos N.C., Lee B. Nat. Rev. Microbiol. 2015;13:426–437. doi: 10.1038/nrmicro3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isaacs A., Lindenmann J. Proc. R. Soc. London, B-Biol. Sci. 1957;147:258–267. [Google Scholar]
- 79.Chen S., Zhang S., Galluzzi M., Li F., Zhang X., Yang X., Liu X., Cai X., Zhu X., Du B. Chem. Eng. J. 2019;358:634–642. [Google Scholar]
- 80.Si Y., Zhang Z., Wu W., Fu Q., Huang K., Nitin N., Ding B., Sun G. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aar5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Q., Fan Q., Xu J., Bai J., Han X., Dong Z., Zhou X., Liu Z., Gu Z., Wang C. Matter. 2020;3:287–301. doi: 10.1016/j.matt.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serebrovska Z., Swanson R.J., Portnichenko V., Shysh A., Pavlovich S., Tumanovska L., Dorovskych A., Lysenko V., Tertykh V., Bolbukh Y., Dosenko V. Biomed. Pharmacother. 2017;92:69–77. doi: 10.1016/j.biopha.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 83.Mei X., Lee H.-C., Diao K.-y., Huang M., Lin B., Liu C., Xie Z., Ma Y., Robson P.M., Chung M. Nat. Med. 2020;26:1224–1228. doi: 10.1038/s41591-020-0931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ke Y.-Y., Peng T.-T., Yeh T.-K., Huang W.-Z., Chang S.-E., Wu S.-H., Hung H.-C., Hsu T.-A., Lee S.-J., Song J.-S. Biomed. J. 2020;157:641–650. doi: 10.1016/j.bj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.