Abstract
Aim
Previous studies have reported inconsistent results regarding the association between metformin use and clinical outcomes in diabetes mellitus (DM) patients with coronavirus disease 2019 (COVID-19). This study aimed to evaluate the association between metformin use and clinical outcomes in DM patients with COVID-19.
Methods
This retrospective study was based on claims data. All diseases, including COVID-19, were defined using International Classification of Diseases 10th Revision (ICD-10) codes. Patients were divided into three groups depending on metformin use: CON (those not taking DM medication); N-MFOM (those taking DM medications other than metformin); and MFOM (those taking metformin for DM). Ultimately, 1865 patients were included; CON, N-MFOM and MFOM groups comprised 1301, 95 and 469 patients, respectively.
Results
Kaplan–Meier analyses showed that MFOM patients had poorer survival rates than those in the CON group, but there were no significant differences in survival rates between MFOM and N-MFOM groups. Multivariate Cox regression analyses revealed more favourable survival in CON than in N-MFOM patients, but there was no statistically significant difference in MFOM vs the other groups. Also, there were no significant differences in rates of use of inotropes, extracorporeal membrane oxygenation, conventional oxygen therapy, high-flow nasal cannulas or mechanical ventilators, nor in the rates of acute kidney injury or cardiac events across all study groups.
Conclusion
No definite association could be found between metformin use and clinical outcomes, including survival. However, given the disproportionate participant numbers in our groups and small number of events, further studies are needed to determine whether the use of metformin has favourable or unfavourable effects in DM patients with COVID-19.
Keywords: Clinical outcome, Coronavirus disease, COVID-19, Diabetes mellitus, Metformin
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first diagnosed in Wuhan in December 2019, and continues to be an ongoing global pandemic. As of 6 August 2020, 18,575,326 patients were confirmed to have COVID-19 worldwide, according to the World Health Organization (WHO) [1]. South Korea has been affected by the COVID-19 outbreak; it had approximately 14,499 confirmed COVID-19 cases as of 6 August 2020, according to the Korea Centers for Disease Control Prevention (KDCA) [2]. Many epidemiological studies have shown that several risk factors are associated with poor prognoses in patients with COVID-19, and comorbidities such as diabetes mellitus (DM), chronic kidney disease (CKD) and heart disease are well-known risk factors for poor prognoses in COVID-19 patients [3].
Although the absolute number of confirmed cases is greater in the healthy population than in the population with underlying comorbidities, cumulative studies have elucidated the risk factors for poor outcomes among COVID-19 patients with specific comorbidities. DM is an important comorbidity in such patients as it is associated with a high mortality rate [4], and the association between DM medication and prognosis has been a major concern in both COVID-19 and DM patients [5]. In addition to its glucose-lowering properties, metformin has, for example, anti-inflammatory properties and can reduce the production of reactive oxygen species [6]. Among DM medications, metformin has been the centre of attention in much research, with a few studies focusing on the association between metformin use and clinical outcomes. Bramante et al. [7] found a favourable association between the use of metformin and survival in women, while Luo et al. [8] showed favourable outcomes in metformin users in general. However, two other studies found no significant associations between clinical outcomes and metformin use on multivariate analyses [9], [10]. Nevertheless, a meta-analysis of these four studies revealed a favourable survival rate for COVID-19 and DM patients taking metformin [6]. Therefore, further epidemiological research into the effects of metformin in COVID-19 patients could help to identify whether there is an association between metformin use and clinical outcomes, as well as help to further determine optimal treatment strategies for DM patients with COVID-19. Thus, the present study aimed to evaluate the association between metformin use and clinical outcomes in DM patients with COVID-19 using a nationwide, population-based dataset.
Methods
Data source
This retrospective study was based on claims data from the Health Insurance Review and Assessment Service (HIRA) of South Korea. The Korean national healthcare system and Medical Aid Program cover almost the entire South Korean population. The HIRA, as a government-affiliated organization, includes nearly all patients’ medical information—from diagnoses and past medical records to procedural data. Therefore, the HIRA was able to identify data for patients who underwent COVID-19 testing from 1 February 2020 to 15 May 2020 and to merge these data with those patients’ claims data over the last 3 years (from 1 January 2017 to 15 May 2020). These merged data became available to researchers after they were anonymized and de-identified [11], and the present retrospective study was conducted using these data. The study was approved by the Institutional Review Board (IRB) of Yeungnam University Medical Centre (IRB No: YUMC 2020-07-024), which waived the need to obtain informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Study population and variables
Of all the participants who underwent COVID-19 testing (n = 234,427), only those who tested positive were included (n = 7590). However, patients aged <18 years (n = 249), those without DM (n = 5410) and those who had type 1 DM (n = 66) were excluded. In the remainder, the following baseline characteristics were evaluated: age; gender; time of COVID-19 diagnosis; use of concomitant medications; and comorbidities. In addition, they were also assessed according to follow-up duration; death during the study period; use of inotropes, conventional oxygen therapy, high-flow nasal cannula (HFNC), mechanical ventilation (MV) and extracorporeal membrane oxygenation (ECMO); and the development of acute kidney injury (AKI), cardiac arrest, myocardial infarction (MI) and acute heart failure (AHF).
All diseases, including COVID-19, were defined using the International Classification of Diseases 10th Revision – Clinical Modification (ICD-10-CM) classification system. Patients with COVID-19 were classified based on the following ICD diagnostic codes: B342, B972, Z208, Z290, U18, U181, Z038, Z115, U071 or U072. Patients were considered to have DM if diagnosed with codes E10, E11, E12, E13 or E14 within 1 year prior to COVID-19 diagnosis. Type 1 DM was defined by code E10, and CKD was defined by codes N18, N19, N25.0, Z49.0-2, Z94.0, Z99.2, N16.5, I12, I13, E10.2, E11.2, E13.2, E14.2 or T861. Patients were divided into three groups according to metformin use: CON (participants not taking DM medications); N-MFOM (participants taking DM medications other than metformin); and MFOM (participants taking metformin for DM). All DM medications prescribed within 1 year of COVID-19 diagnosis were identified and evaluated using HIRA codes (Table S1; see supplementary materials associated with this article online). Metformin use was defined as a medication adherence ratio ≥80%, as previously described, while insulin use was defined as any insulin prescribed within 3 months of COVID-19 diagnosis [12], [13], [14].
The presence of comorbidities was evaluated from 1 year prior to diagnosis of COVID-19 and was defined by codes used by Quan et al. [15], [16]. In addition, the Charlson Comorbidity Index (CCI) score was also calculated. During follow-up, all clinical outcomes (except death) were defined using either the Electronic Data Interchange system or ICD codes from HIRA, which were as follows: M0040 for conventional oxygen therapy; M0046 for HFNC; M5850 or M5857–M5860 for MV; O1901–O1904 for ECMO; O7031–O7035 or O7051–O7055 for dialysis; I10, M5873–M5877 or M5880 for cardiac arrest; I21, I22, I252, M655x–M657x, OA631x–OA639x, OB631x–OB639x, OA641x, OA642x, OA647x, O0161x–O0171x or O1641x–O1647x for MI; and I110, I130, I132, I255, I420, I425, I428, I429, I43 or I50 for AHF. Inotrope use was defined as the use of norepinephrine, epinephrine, vasopressin, dopamine or dobutamine after diagnosis of COVID-19. Patients undergoing dialysis after COVID-19 diagnosis were considered to have AKI.
Statistical analyses
Data were analyzed using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA). Categorical variables are presented as numbers (n) and percentages (%), while continuous variables are presented as means ± standard deviation (SD). Pearson’s χ2 test or Fisher’s exact test was used to analyze categorical variables. For continuous variables, means were compared using one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc comparison. Survival estimates were calculated using Kaplan–Meier curve and Cox regression analyses. P values for comparison of survival curves were determined by log-rank test. Multivariate Cox regression analyses were adjusted for age, gender, CCI score and hypertension. In addition, logistic regression analyses were performed to evaluate independent variables for clinical outcomes. P < 0.05 was considered statistically significant.
Results
Clinical characteristics of participants
Ultimately, 1865 patients were included in our study. The CON, N-MFOM and MFOM groups consisted of 1301 (69.8%), 95 (5.1%) and 469 (25.1%) patients, respectively (Table 1 ). The CON group included the largest number of male patients, and patients in this group were also younger than those in the other two groups. The N-MFOM group had the highest CCI scores and proportions of patients with MI, congestive heart failure, cerebrovascular disease, dementia, mild liver disease, CKD and hypertension. Moreover, 0, 44 (46.3%) and 232 (49.5%) patients were using insulin in the CON, N-MFOM and MFOM groups, respectively (P < 0.001). However, there were no significant differences in the use of thiazolidinedione, sodium–glucose co-transporter type-2 (SGLT2) inhibitors, glucagon-like peptide (GLP)-1 receptor agonists and renin–angiotensin–aldosterone system (RAAS) blockers between the N-MFOM and MFOM groups, whereas the use of dipeptidyl peptidase (DPP)-4 inhibitors was significantly higher in the N-MFOM than in the MFOM group.
Table 1.
Baseline characteristics of the participants.
| CON (n = 1301) | N-MFOM (n = 95) | MFOM (n = 469) | P* | |
|---|---|---|---|---|
| Gender (male) | 813 (62.5) | 40 (42.1) | 243 (51.8) | <0.001 |
| Age (years) | 59.2 ± 16.5 | 67.4 ± 12.1a | 64.8 ± 11.4a | <0.001 |
| Current diabetic drugs (n) | – | 1.4 ± 0.6a | 1.6 ± 0.7a,b | <0.001 |
| Charlson Comorbidity Index score | 4.8 ± 2.6 | 7.0 ± 3.4a | 5.7 ± 2.8a,b | <0.001 |
| Follow-up duration (days) | 22.8 ± 14.3 | 23.3 ± 15.7 | 24.0 ± 14.6 | 0.282 |
| Myocardial infarction | 80 (6.1) | 13 (13.7) | 34 (7.2) | 0.017 |
| Congestive heart failure | 204 (15.7) | 27 (28.4) | 86 (18.3) | 0.004 |
| Peripheral vascular disease | 378 (29.1) | 41 (43.2) | 159 (33.9) | 0.005 |
| Cerebrovascular disease | 274 (21.1) | 34 (35.8) | 143 (30.5) | <0.001 |
| Dementia | 226 (17.4) | 27 (28.4) | 97 (20.7) | 0.014 |
| Chronic pulmonary disease | 789 (60.6) | 57 (60) | 264 (56.3) | 0.256 |
| Connective tissue disease | 198 (15.2) | 13 (13.7) | 62 (13.2) | 0.556 |
| Peptic ulcer disease | 631 (48.5) | 50 (52.6) | 226 (48.2) | 0.721 |
| Mild liver disease | 907 (69.7) | 76 (80) | 353 (75.3) | 0.013 |
| Hemiplegia | 71 (5.5) | 6 (6.3) | 19 (4.1) | 0.433 |
| Chronic kidney disease | 192 (14.8) | 45 (47.4) | 138 (29.4) | <0.001 |
| Any malignancy | 205 (15.8) | 17 (17.9) | 61 (13.0) | 0.272 |
| Moderate-to-severe liver disease | 15 (1.2) | 0 (0) | 2 (0.4) | 0.231 |
| Metastatic tumour | 20 (1.5) | 3 (3.2) | 7 (1.5) | 0.467 |
| Acquired immunodeficiency syndrome | 6 (0.5) | 0 (0) | 0 (0) | 0.271 |
| Hypertension | 692 (53.2) | 78 (82.1) | 346 (73.8) | <0.001 |
| Concomitant medications: | ||||
| Thiazolidinedione | – | 12 (12.6) | 38 (8.1) | 0.157 |
| DPP-4 inhibitor | – | 74 (77.9) | 303 (64.6) | 0.012 |
| SGLT2 inhibitor | – | 4 (4.2) | 30 (6.4) | 0.635 |
| GLP-1 receptor agonist | – | 0 | 1 (0.2) | 1.000 |
| RAAS blocker | – | 46 (48.4) | 228 (48.6) | 0.973 |
Data are expressed as n (%) for categorical variables, means ± standard deviation for continuous variables; * by one-way analysis of variance followed by Bonferroni post-hoc test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorical variables, for concomitant medication use in N-MFOM (patients taking diabetic medications other than metformin) vs MFOM (patients taking metformin) groups; aP < 0.05 vs CON (patients not taking diabetic drugs) group, bP < 0.05 vs N-MFOM group; DPP-4, dipeptidyl peptidase-4; SGLT2, sodium–glucose cotransporter type 2; GLP-1, glucagon-like peptide-1; RAAS, renin–angiotensin–aldosterone system.
Survival analyses
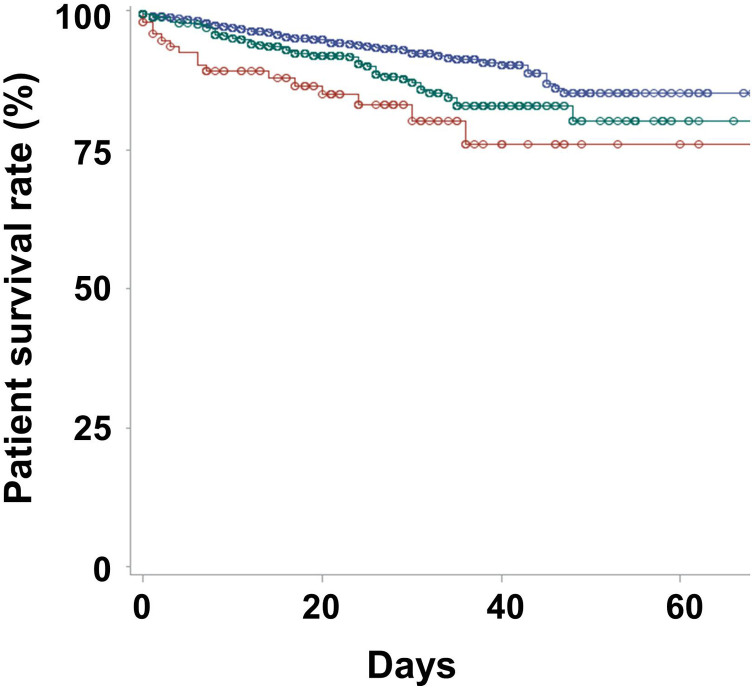
In total, 83 (6.4%), 16 (16.8%) and 51 (10.9%) patients died during follow-up in the CON, N-MFOM and MFOM groups, respectively (P < 0.001). Kaplan–Meier curves showed that the best survival was in the CON group and the poorest was in the N-MFOM group (P < 0.001 for trend; P < 0.001 for CON vs N-MFOM groups; P = 0.003 for CON vs MFOM groups; P = 0.577 for MFOM vs N-MFOM groups; Fig. 1 ). Univariate Cox regression analyses found that patients in the CON group had the highest survival rates (Table 2 ). Multivariate Cox regression analyses further revealed that patients in the CON group had better survival rates than those in the N-MFOM group, whereas there were no significant differences in survival rates between the N-MFOM and MFOM groups. In addition, the hazard ratio (HR) for use of DPP-4 inhibitors was 0.78 [95% confidence interval (CI): 0.48–1.27; P = 0.315] on univariate Cox regression analysis.
Fig. 1.
Kaplan–Meier survival curves according to antidiabetic medication use. In these survival graphs of the CON (blue; patients not taking diabetic drugs), N-MFOM (red; patients taking diabetic medications other than metformin) and MFOM (green; patients taking metformin) groups, circles indicate censored points. The 20-day survival rates were 94.8%, 84.9% and 91.9% in the CON, N-MFOM and MFOM groups, respectively (P < 0.001), and 40-day survival rates were 90.1%, 76.0% and 82.8%, respectively (P < 0.001).
Table 2.
Cox regression analysis of survival rates according to variables.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per 1-year increase) | 1.09 (1.08–1.11) | <0.001 | 1.09 (1.07–1.11) | < 0.001 |
| Gender (ref: female) | 1.65 (1.20–2.27) | 0.002 | 1.87 (1.35–2.59) | < 0.001 |
| CCI score (per 1-point increase) | 1.22 (1.17–1.28) | <0.001 | 1.09 (1.03–1.15) | 0.003 |
| Hypertension | 4.80 (2.90–7.96) | <0.001 | 1.55 (0.90–2.65) | 0.111 |
| Insulin use | 1.78 (1.29–2.46) | <0.001 | – | – |
| Groups: | ||||
| N-MFOM (ref: CON) | 2.60 (1.52–4.43) | <0.001 | 1.79 (1.04–3.10) | 0.036 |
| MFOM (ref: CON) | 1.62 (1.14–2.30) | 0.007 | 1.38 (0.97–1.97) | 0.076 |
| MFOM (ref: N-MFOM) | 0.62 (0.36–1.10) | 0.100 | 0.77 (0.44–1.35) | 0.052 |
Data are expressed as hazard ratio (HR) (95% confidence interval, CI); multivariate analysis adjusted for age, gender, Charlson Comorbidity Index (CCI) score, hypertension and group according to metformin use; ref: reference; CON, patients not taking diabetic drugs; MFOM, patients taking metformin; N-MFOM, patients taking diabetic medications other than metformin.
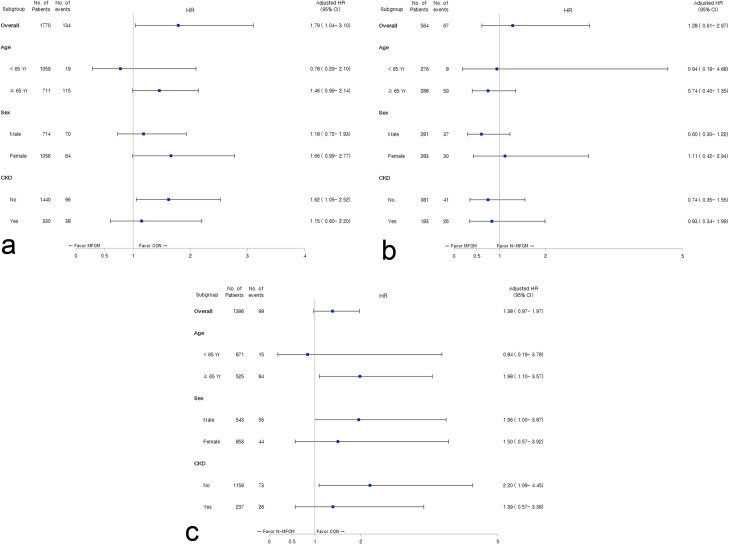
The numbers of patients aged <65 and ≥65 years in the CON, N-MFOM and MFOM groups were 827 and 474, 44 and 51, and 232 and 237, respectively. The numbers of male patients in the CON, N-MFOM and MFOM groups were 813 (62.5%), 40 (42.1%) and 243 (51.8%), respectively. The numbers of CKD patients in the CON, N-MFOM and MFOM groups were 192 (14.8%), 45 (47.4%) and 138 (29.4%), respectively. Multivariate Cox regression analyses according to subgroups by age, gender and presence of CKD showed that patients without CKD in the CON group had better survival rates than those in the N-MFOM and MFOM groups (Fig. 2 ). In addition, elderly patients in the CON group had better survival rates than those in the N-MFOM group.
Fig. 2.
Forest plots of the association between metformin use and survival according to age, gender (sex) and presence of chronic kidney disease (CKD): (A) CON (patients not taking diabetic drugs) vs MFOM (patients taking metformin); (B) N-MFOM (patients taking diabetic medications other than metformin) vs MFOM; and (C) CON vs N-MFOM. Multivariate analyses of age and presence of CKD subgroups were adjusted for age, gender, Charlson Comorbidity Index (CCI) scores and hypertension. Multivariate analyses for gender subgroups were adjusted for age, CCI score and hypertension. HR, hazard ratio; CI, confidence interval.
Differences in clinical outcomes by group
In total, 9 (0.7%), 3 (3.3%) and 8 (1.7%) patients developed AKI in the CON, N-MFOM and MFOM groups, respectively (P = 0.021; Table 3 ). Rates of conventional oxygen therapy and MV use were highest in the N-MFOM group. However, there were no significant differences in rates of inotrope use, ECMO use, cardiac arrest, MI and AHF among the study groups. Also, multivariate logistic regression analyses failed to show any significant differences in these variables across our study groups (Table S2; see supplementary materials associated with this article online).
Table 3.
Patients’ clinical outcomes according to metformin subgroups.
| CON | N-MFOM | MFOM | P | |
|---|---|---|---|---|
| Acute kidney injury | 9 (0.7) | 3 (3.3) | 8 (1.7) | 0.021 |
| Inotrope use | 73 (5.6) | 9 (9.5) | 39 (8.3) | 0.060 |
| Conventional oxygen therapy | 302 (23.2) | 36 (37.9) | 136 (29.0) | <0.001 |
| High-flow nasal cannula | 69 (5.3) | 5 (5.3) | 40 (8.5) | 0.041 |
| Mechanical ventilation | 47 (3.6) | 7 (7.4) | 31 (6.6) | 0.012 |
| Extracorporeal membrane oxygenation | 6 (0.5) | 2 (2.1) | 5 (1.1) | 0.096 |
| Cardiac arrest | 3 (0.2) | 1 (1.1) | 3 (0.6) | 0.250 |
| Myocardial infarction | 3 (0.2) | 1 (1.1) | 2 (0.4) | 0.354 |
| Acute heart failure | 5 (0.4) | 0 | 0 | 0.337 |
Data are expressed as n (%); CON, patients not taking diabetic drugs; N-MFOM, patients taking diabetic medications other than metformin; MFOM, patients taking metformin.
Discussion
In our study, DM patients in the N-MFOM (participants taking DM medications other than metformin) group had a greater prevalence of comorbidities than those in the other two groups. Multivariate Cox regression analyses revealed more favourable survival in the CON (participants not taking DM medication) group than in the N-MFOM group, and there was no statistically significant difference between the MFOM (participants taking metformin for DM) group and the other groups. In addition, there were no significant differences in rates of inotrope use, conventional oxygen therapy use, HFNC use, MV use, ECMO use, AKI, cardiac arrest, MI and AHF across the three study groups.
Previous human and experimental studies of infectious and chronic diseases, such as bacterial infections, tuberculosis and inflammatory disorders, had shown more favourable outcomes in patients treated with metformin than in those who were not [17], [18], [19], [20], [21], [22]. This led many researchers to assume there would be a positive association between metformin use and clinical outcomes in DM patients with COVID-19. Indeed, in a large retrospective study by Bramante et al. [7] in the US, women taking metformin had better survival rates than those not taking metformin. Such gender-specific findings may be related to differences in mast cell activation and in expression of angiotensin-converting enzyme 2 (ACE2). On the other hand, a French multicentre study evaluating various factors of poor clinical outcomes found no association between metformin use and death or composite outcomes, including MV and death within 7 days of admission [9], while two Chinese studies showed different results for the association between metformin use and clinical outcomes in DM patients with COVID-19 [8], [10]. Thus, although a meta-analysis involving four previous studies showed favourable outcomes in patients taking metformin, further investigations are still needed to determine the effects of metformin in DM patients with COVID-19 [6].
In the present study, patients were divided into three groups according to DM medication use. Clinical outcomes were different between the CON and N-MFOM groups. DM patients in the CON group had the best outcomes, which might be explained by their capacity to maintain blood glucose levels without the need for medication, and differences in age. The N-MFOM group included those who could maintain blood glucose levels with DM medications other than metformin. However, clinical practice guidelines in both the US and South Korea recommend that metformin be the preferred initial pharmacological agent for DM and, once initiated, that it be maintained as long as it is tolerated [23], [24]. Therefore, patients in the N-MFOM group were not using metformin as maintenance therapy, and it may have been withdrawn due to adverse side-effects or other contraindications (comorbidities such as CKD or heart problems). Thus, patients in the CON group would be expected to have the best clinical outcomes, whereas those in the N-MFOM would be expected to have the worst clinical outcomes.
Indeed, in our study, patients in the CON and N-MFOM groups had the best and worst survival rates, respectively, although the statistical significance was weak owing to being statistically underpowered due to a limited cohort. In addition, rates of inotrope use, conventional oxygen therapy use, MV use, ECMO use, AKI, cardiac arrest and MI were higher in the N-MFOM group than in the MFOM group by univariate analysis, although statistical significance was not reached. An important problem that may arise when comparing metformin non-users with users involves inherent differences in their underlying comorbidities. Favourable outcomes in metformin users may be due to their having fewer comorbidities than metformin non-users. Unfortunately, our study did not include patients’ laboratory findings or accurate past medical histories based on medical charts. Therefore, blood glucose levels were not compared among our three groups, and there was no evaluation of the prescription/changes of antidiabetic drugs for reasons of metformin non-use despite recommendations for metformin as initial therapy or no prescription of medication despite the presence of DM.
Furthermore, the younger age of patients in the CON group could have been associated with better outcomes compared with the other groups’ outcomes. Age is one of the most important prognostic factors in COVID-19 and could be a confounding factor in our study. For that reason, multivariate and subgroup analyses were performed to attenuate the impact of age: the results showed similar trends as for univariate analyses.
In our study, the trend of Kaplan–Meier curves showed the best survival in the CON group and poorest survival in the N-MFOM group (P < 0.001 for trend), although the survival rate difference between the MFOM and N-MFOM groups was not statistically significant (P = 0.577). However, Cox regression analyses also showed that the CON group had better survival than the two other groups on univariate analysis, whereas it had better survival than only the N-MFOM group on multivariate analysis. In contrast, there were no significant differences in survival between the N-MFOM and MFOM groups on either univariate or multivariate analyses, although the trend was towards best survival in the CON group and poorest survival in the N-MFOM group. Such differences in the results of univariate and multivariate analyses, and discrepancies between trends and statistical significance, could be related to our study’s low statistical power owing to the disproportionate patient numbers in the three groups and small number of clinical outcomes such as death.
Our study also evaluated the concomitant use of thiazolidinedione, DPP-4 inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists and RAAS blockers, which could have associations with the prognosis of DM patients with COVID-19. However, there were no significant differences in the use of these medications (except for DPP-4 inhibitors) between the N-MFOM and MFOM groups, nor was there any significant difference in survival according to use of DPP-4 inhibitors. This suggests that the concomitant medications, antidiabetic drugs and RAAS blockers had no influence on patients’ survival.
This study has several limitations. First, it was based on health insurance claims data using procedural and diagnostic codes from HIRA instead of laboratory and clinical data. The possibility of over- or undercoding in the dataset may therefore have led to discrepancies between the relevant codes and the actual disease. Second, other than the insurance data, our study did not consider the biological/physiological effects of metformin, such as serum glucose levels, inflammatory markers and body mass index. Third, metformin use might have been underestimated by setting an adherence ratio ≥80% to definitively identify its effects. Also, some patients in the CON and N-MFOM groups may have been prescribed metformin, but may have taken it irregularly, and categorizing them in the MFOM group could be associated with yet another bias. Fourth, the number of clinical events was small: in our cohort, the total mortality rate was only 8%, and the number of outcomes was also limited, including AKI, inotrope use, HFNC use, MV use, ECMO use, cardiac arrest, MI and AHF. An insufficient number of events would tend towards statistical non-significance and statistical errors, whereas large sample sizes, including more clinical events, would help to better identify the association between metformin use and clinical outcomes in DM patients with COVID-19. Fifth, the number of patients in our N-MFOM group was considerably smaller than in the other groups. For type 2 DM patients, the guidelines in both the US and South Korea recommend metformin as the first-line therapy [23], [24]: in South Korea, metformin prescriptions constitute 80.4% of the total antidiabetic prescriptions [25] and, in the US, metformin is the most commonly used antidiabetic drug [26]. Our study enrolled adult patients with type 2 DM diagnosed with COVID-19. Larger numbers of patients taking metformin are an inherent limitation of our study, considering that this was a limited cohort and that metformin is a first-line therapy, whereas the relatively small number of patients not taking metformin could be associated with statistical non-significance and being statistically underpowered. Sixth, our study used claims data with no laboratory findings and did not evaluate whether patients had proper glycaemic control. Yet, glucose control at admission or during COVID-19 may be more important than the class of antidiabetic drug use per se. Further studies with large sample sizes and including assessments of serum glucose levels are now needed to assess the impact of antidiabetic drugs regardless of glucose control. Moreover, studies that can overcome the statistically underpowered results of our study and, thus, lead to statistical significance for similar analyses, are also required.
Conclusion
Our study failed to show any clear association between metformin use and clinical outcomes, including survival, in COVID-19 patients. However, in light of the disproportionate patient numbers in our study groups and limited number of events, further studies are now needed to determine whether the use of metformin may have favourable or unfavourable effects in DM patients with COVID-19.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Yeungnam University Medical Centre, which waived the need for obtaining informed consent (IRB No: YUMC 2020-07-024). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Authors’ contributions
S.H.K. and K.H.C. conceived and planned the study. S.H.K. and S.W.K. identified and obtained the data for this analysis. S.H.K., S.W.K. and J.Y.D. extracted and processed the data. S.W.K. and J.W.P. carried out the statistical analyses. S.H.K. wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Availability of data and materials
Data-sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Competing interests
The authors have no competing interests to declare.
Funding
This work was supported by the 2020 Yeungnam University Medical Centre COVID-19 Research Grant. The funders played no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgements
The authors are grateful to the healthcare professionals dedicated to treating COVID-19 patients in South Korea, the Ministry of Health and Welfare, and the Health Insurance Review and Assessment Service (HIRA) of South Korea for sharing its invaluable national health insurance claims data in a prompt manner.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.diabet.2020.10.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. [Accessed August 2020].
- 2.Korea Centers for Disease Control and Prevention. The updates on COVID-19 in Korea as of 6 August. Available at: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030. [Accessed August 2020].
- 3.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335–337. doi: 10.1016/j.diabet.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;1:S1262–S3636. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J., et al. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. medRxiv. 2020 doi: 10.1101/2020.06.19.20135095v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., et al. Metformin treatment was associated with decreased mortality in covid-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and covid-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 11.#opendata4covid19. Available at: https://covid19data.hira.or.kr. [Accessed August 2020].
- 12.Granger B.B., Swedberg K., Ekman I., Granger C.B., Olofsson B., McMurray J.J., et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 13.Bramley T.J., Gerbino P.P., Nightengale B.S., Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho P.M., Bryson C.L., Rumsfeld J.S. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 17.Gras V., Bouffandeau B., Montravers P.H., Lalau J.D. Effect of metformin on survival rate in experimental sepsis. Diabetes Metab. 2006;32:147–150. doi: 10.1016/s1262-3636(07)70261-6. [DOI] [PubMed] [Google Scholar]
- 18.Malik F., Mehdi S.F., Ali H., Patel P., Basharat A., Kumar A., et al. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34:e2975. doi: 10.1002/dmrr.2975. [DOI] [PubMed] [Google Scholar]
- 19.Mendy A., Gopal R., Alcorn J.F., Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology. 2019;24:646–651. doi: 10.1111/resp.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho T.W., Huang C.T., Tsai Y.J., Lien A.S., Lai F., Yu C.J. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir Res. 2019;20:69. doi: 10.1186/s12931-019-1035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M., He J.Q. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:149–159. doi: 10.1007/s00228-019-02786-y. [DOI] [PubMed] [Google Scholar]
- 22.Pernicova I., Kelly S., Ajodha S., Sahdev A., Bestwick J.P., Gabrovska P., et al. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020;8:278–291. doi: 10.1016/S2213-8587(20)30021-8. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl. (1)):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 24.Korean Diabetes Association. Treatment Guideline for Diabetes. Available at: https://www.diabetes.or.kr/pro/publish/guide.php?code=guide&number=735&mode=view. [Accessed August 2020].
- 25.Ko S.H., Kim D.J., Park J.H., Park C.Y., Jung C.H., Kwon H.S., et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: Nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e4018. doi: 10.1097/MD.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ClinCalc.com Metformin hydrochloride-drug usage statistics, United States, 2007-2010. Available from: https://clincalc.com/DrugStats/Drugs/MetforminHydrochloride. [Accessed September 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data-sharing is not applicable to this article as no datasets were generated or analyzed during the study.