Abstract
Diethylhexyl phthalate (DEHP) is known as a persistent environmental pollutant. However, the possible effects of DEHP on human neural tube defects (NTDs) remain elusive. We set out to investigate the exposure of DEHP in human and explore the association of DEHP and NTDs. The level of DEHP in maternal urine was measured and analyzed by GC-MS. To further validate the results in human NTDs, chick embryos were used as animal models. Viability, reactive oxygen species (ROS) level, oxidative stress indicators and apoptosis were detected in DEHP-treated chick embryos. Our research revealed that the detection ratio of positive DEHP and its metabolites in maternal urine were observed dramatically higher in NTDs population than that in normal controls (P < 0.01, P < 0.05, respectively). Moreover, DEHP treatment (10−6 M) led to developmental toxicity in chick embryos via accelerating oxidative stress response and cell apoptosis, and changing the level of oxidative stress-related indicators. Moreover, high dose choline (100 μg/μl) could partially restrain the toxicity effects induced by DEHP. Our data collectively imply that the incidence of NTDs may closely associate with DEHP exposure, which disturbs the development of neural tubes by enhancing oxidative stress.
Keywords: DEHP, human NTDs, oxidative stress, apoptosis, chick embryo
Introduction
Neural tube defects (NTDs) are among the most common birth defects worldwide with a prevalence that varies from 0.5 to >10 per 1000 pregnancies [1]. This variance likely reflects differing contributions from risk factors such as nutritional status, prevalence of obesity and diabetes, usage of folic acid (FA) supplementation and/or fortification, the presence of environmental toxicants and differing genetic predisposition among ethnic groups [2]. There are three common types of NTDs, including encephalocele, anencephaly and spina bifida cystica (open spina bifida), which are considered to be resulted from the failure of normal neural tube closure between the third and fourth week of embryonic development [3,4]. NTDs are multifactorial in origin, with contributions from both genetic and environmental factors [5].
Phthalic acid esters (PAEs) are prevalent industrial chemicals and mainly used as the plasticizer of plastics that have been found in a variety of environmental samples [6]. Diethylhexyl phthalate (DEHP), dibutyl phthalate (DBP) and benzyl butyl phthalate (BBP) are the dominant phthalate and cause a constellation of reproductive defects [7]. Studies indicated that the incidence of embryonic anterior NTDs, including anencephaly and exencephaly, were sharply increased with the addition of DEHP into diet of the pregnant ICR mice [8]. While mice were treated with the combination of DEHP and DBP, the external malformations of embryos increased significantly and major external malformations were NTDs (exencephaly and myeloschisis) [9]. Exposure to 250 and 500 mg/kg DEHP was teratogenic and induced exencephaly and limb malformations such as polydactyly in the B6:126S4 mouse embryos [10]. These results implied that DEHP may affect neural tube closure in developing embryos in animals. However, up to now, there were no reports about the exposure level of DEHP in maternal body liquids in NTDs population. Moreover, it’s still unclear that the molecular mechanisms in which DEHP induced NTDs during embryo development.
In this study, we detected the level of DEHP in urine from mothers containing harbored NTDs fetuses and age-matched controls. To further validate the results in human NTDs, chick embryos were used as animal models. Viability, reactive oxygen species (ROS) level and oxidative stress indicators were detected in DEHP-treated chick embryos, respectively. Moreover, recovery experiments by nutritional supplements were also performed to seek the nutrients which can antagonize toxicity caused by DEHP. Our research may provide useful clues for prevention and diagnosis of NTDs at early stage of pregnancy.
Method and materials
Sample collections
A population-based embryo development defects survey was performed in Family Planning Technique Service Station in Qian’xi, Hebei Province. A total of 44 mothers containing harbored NTDs fetuses and age-matched controls were surveyed by questionnaire which contained the general condition. The control groups were obtained from allowable therapeutic abortions or pregnancy test. In case group, induced abortions were performed when fetus was found to be spina bifida or anencephaly by B-mode ultrasound. In our study case and control groups were strictly matched in age, FA usage, diabetes, BMI, gestational week, no genetic or family history, same residential area. A total of 43 pairs of urine were collected and frozen in −20°C refrigerator after approval of the patient consent, which was written informed. This study was approved by the Ethics Committee of National Research Institute for Family Planning (2011 No.9). The collection of fetal tissues followed the procedures that are in accordance with the ethical standards as formulated in the Helsinki Declaration.
The detection of DEHP in maternal urine by GC-MS
The urine samples (100 μl) were pretreated according to the study from Wang et al. [11]. After the pretreatment of urine, the detection of DEHP and its metabolites monoethylhexyl phthalate (MEHP) were performed by gas chromatography/mass spectrometry (GC-MS) assay. Recovery (%), characteristic ion, retention time (RT), limits of quantification (LOQs) of DEHP and MEHP was shown in Supplementary Table S1, respectively.
Embryo treatment
According to our previous experiments, chick embryos were treated with DEHP (10−6 M) with/without 10 μg/μl FA, 20% ferrous sulfate (FeSO4) or 100 μg/μl choline (CHO) or 0.9% saline vehicle alone at Hamburger-Hamilton (HH) stages 6, 8 and 12 [12]. DEHP, FA, FeSO4 or CHO were directly injected into the center of the egg yolk via a small hole at the blunt end of the egg using an established protocol at Hhstage 6, 8, 12, respectively [13]. Embryos were harvested for analysis after incubation for 72 h (Hhstage 20).
Embryo viability
Embryo viability was indirectly analyzed by measuring embryo body weight, embryo survival and embryonic malformation at Day 3 of incubation (theoretical HH stage 19–20). Those that showed grossly visible defects, including smaller body size, delayed development of hindbrain, cardiac dysplasia NTDs and small head etc. were considered as malformation. In our experiment, 120 chick embryos were used to examine embryo viability; the embryos were divided into eight groups, each group containing 15 embryos. The experiment was repeated at least three times. Live embryos were defined as those possessing a beating heart. Each surviving chick embryo was photographed using a SONY Cyber-shot camera. The images were scaled to 1 cm in the plane of the embryo, and scanned into Adobe Photoshop (Microtek, Scanmaker III, Redondo Beach, CA) for quantification of relative vascular area of yolk sac vascellum, using ImagePro software 5.1. Embryos were examined and recorded using a 20× objective under the bright field of a Zeiss lumar V12 fluorescence stereomicroscope (Zeiss, Jena, Germany). The body weight of each embryo was recorded to do statistical analysis.
The detection of ROS in chick embryos
A total of 120 live chick embryos, harvested after 72 h, were taken into the 24-well cell culture plate, and 10 μM DCFH-DA 500 μl was added into the plate at 37°C for 30 min. Samples without the addition of DCFH-DA was used as the negative control. The whole mount images were visualized using 20× objective under Zeiss lumar V12 fluorescence stereomicroscope (Zeiss, Jena, Germany). Fluorescence intensity was analyzed using Axiovision Rel.4.8 software. Quantification of fluorescence in each treatment group was done after subtracting the background from the negative control values.
The analyzation of oxidative stress-related indicators in chick embryos
The level of malondialdehyde (MDA) was analyzed by Lipid Peroxidation MDA Assay Kit (Beyotime, China). The level of total superoxide dismutase (SOD) and CuZn/Mn-SOD were performed by Cu/Zn-SOD and Mn-SOD Assay Kit with WST-1 (Beyotime, China). The detection of glutathione peroxidase (GPX) was analyzed by Cellular Glutathione Peroxidase Assay Kit (Beyotime, China). The calculation of level of these oxidative stress-related indicators was performed according to the study from Wang et al. [11].
Terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL) staining
In whole-mount TUNEL staining, 120 chicken embryos were treated according to the study from Wang et al. [11]. Embryos were examined and recorded using 20× objective under bright field of a Zeiss lumar V12 fluorescence stereomicroscope (Zeiss, Jena, Germany). At least three embryos were analyzed in each treatment group in whole-mount TUNEL staining. The mean optical densities (MOD) of apoptotic signals were analyzed using NIS-Elements Br 3.0 software (Nikon, Japan) in five different optical fields selected in a random manner for each sample. The percentage of MOD of apoptotic signals were expressed as ratio of MOD of apoptotic signals vs total signals of five different optical fields selected in a random manner.
Data analysis
The analyses were performed using the SPSS 25.0 statistical software package. All data were analyzed using analysis of variance (ANOVA), in which results were presented as mean values (±SD) at least three independent experiments. A value of P < 0.05 was considered statistically significant.
Results
The distribution of DEHP in urine in NTDs population
To investigate whether NTDs are associated with DEHP, the level of DEHP and MEHP in urine from pregnant women containing harbored NTDs fetuses and age-matched controls was analyzed by GC-MS. Our results indicated that the detection ratio of positive DEHP and MEHP in the NTDs population was about 29.27 and 7.32%, respectively (Table 1 and Supplementary Fig. S1). However, DEHP and MEHP were not detected in control group. These results implied that the occurrence of NTDs in human may be related with the distribution of DEHP.
Table 1.
Detection of DEHP and its metabolities in urine
| Metabolities | Controls | Cases | ||||
|---|---|---|---|---|---|---|
| N | N of positive samples | Positive ratio | N | N of positive samples | Positive ratio | |
| DEHP | 40 | 0 | 0% | 41 | 12 | 29.27%** |
| MEHP | 40 | 0 | 0% | 41 | 3 | 7.32%* |
**Compared with controls, P < 0.01.
*Compared with controls, P < 0.05.
DEHP affects viability of chick embryos
Since the results from NTDs population indicated that high positive ratio of DEHP was observed in women with NTD affected fetuses, a chick embryo model was used to further investigate the effects of DEHP exposure on embryo development. As shown in Table 2, the chick embryo body weight was significantly decreased in DEHP-treated samples compared with the controls (P < 0.01). The survival rate showed no obvious difference compared with the controls following DEHP treatment (Table 2). However, the incidence of teratogenicity was remarkably increased in DEHP-treated chick embryos compared with the control (Fig. 1,Table 2, P < 0.01). These results suggest that DEHP may have adverse effects on the chick embryo viability and development.
Table 2.
The effects of DEHP with or without nutrients on chick embryo viability
| Exogenous treatment | Embryo body weight (mg) (n = 15) | Survival rate of embryos (%) (n = 15) | Teratogenic rate of embryos (%) (n = 15) |
|---|---|---|---|
| CON | 12.6 ± 0.6 | 96.7 ± 4.7 | 6.7 ± 1.2 |
| FA (10) | 12.1 ± 1.2 | 90.2 ± 3.1 | 8.3 ± 1.3 |
| CHO100 | 12.9 ± 1.4 | 91.2 ± 2.3 | 8.8 ± 1.1 |
| Fe (20%) | 10.1 ± 1.3 | 89.8 ± 2.3 | 7.1 ± 1.6 |
| DEHP | 3.9 ± 2.4** | 83.2 ± 2.4 | 68.7 ± 3.3** |
| DEHP+FA (10) | 3.3 ± 2.0** | 89.1 ± 2.2 | 83.7 ± 1.7** |
| DEHP+CHO100 | 9.7 ± 3.8# | 88.5 ± 2.2 | 20.1 ± 1.1**/# |
| DEHP+Fe (20%) | 3.2 ± 2.0** | 6.7 ± 2.3** | 86.7 ± 2.5** |
CON: control; FA(10): 10 μg/μl folic acid; CHO100: 100 μg/μl choline; Fe(20%): 20% FeSO4. Data presented as mean ± SD. **P < 0.01, compared with the control [including CON, FA(10), CHO100 and Fe (20%)]; #P < 0.01, compared with corresponding DEHP.
Figure 1.

DEHP induces malformation in chick embryos. The morphology of chick embryos was analyzed by DEHP (10–6 M) or saline treatment.
DEHP injuries the development of yolk sac vascellum
The effect of DEHP on the development of yolk sac vascellum was analyzed by vascular area (Fig. 2). The vascular area was significantly decreased in DEHP-treated chick embryos, compared with control (Fig. 2, P < 0.01), implying that yolk sac vascellum development was injured by DEHP exposure.
Figure 2.

Effects of DEHP on the vascular area of yolk sac vascellum in chick embryos. (A)The yolk sac vascellum was observed in chick embryos treated with DEHP (10–6 M) and/or nutrients, respectively. (B) The histogram represents the relative percent of the vascular area of yolk sac vascellum in chick embryos treated with DEHP and/or nutrients. *P < 0.05, compared with control (administration of saline, FA, Fe, CHO or DEHP alone), or **P < 0.01, compared with control (administration of saline).
DEHP induces ROS and alters the level of oxidative stress indicators in chick embryos
Studies reveal that NTDs may associate with oxidative stress [14–16]. Therefore, ROS level and oxidative stress indicators were detected in DEHP-treated chick embryos, respectively. The results revealed that the fluorescence signals of ROS was increased 2.8 fold after DEHP treatment in chick embryos compared with the control (Fig. 3), implying that DEHP could lead to the production of oxidative stress in chick embryos. The result was similar from the analysis of fluorescence signals of ROS in brain, heart and spine of chick embryos, respectively (Fig. 3 B2−B4).
Figure 3.

Effects of DEHP on ROS in chick embryos. (A) The ROS activity was analyzed in chick embryos treated with DEHP (10–6 M) and/or nutrients, including 10 μg/μl FA, 20% FeSO4 or 100 μg/μl CHO. Scale bar = 500 μm. The red arrow indicated positive signals. (B1–B4) The histogram represents the MOD of positively signals of ROS in whole embryos, brain, heart and spine, respectively. *P < 0.05, or **P < 0.01, compared with controls (administration of saline, FA, Fe or CHO alone); #P < 0.05, or ##P < 0.01, compared with administration of DEHP alone.
Oxidative stress indicators, including SOD, MDA and GPX, were also altered following DEHP treatment. The total SOD activity was dramatically reduced in chick embryos treated by DEHP (P < 0.05), as compared with the control (Fig. 4). Cu-Zn SOD activity was also obviously decreased when DEHP (P < 0.01) was administered to chick embryos. The level of MDA was also significantly reduced in DEHP-treated chick embryos (P < 0.01). However, the GPX activity had no evident changes. All these results indicated that abnormal embryonic development induced by DEHP was possibly related with oxidative stress response.
Figure 4.
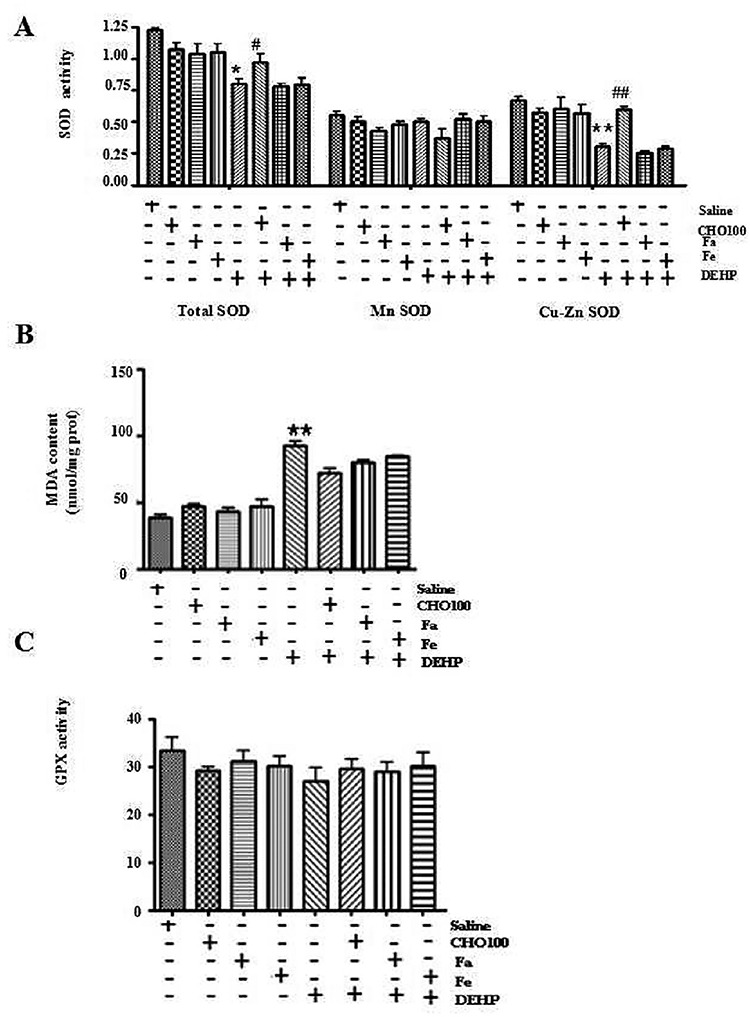
Effects of DEHP on the oxidative stress-related indicators in chick embryos. (A)The activity of total SOD, Mn SOD, Cu-Zn SOD was analyzed in chick embryos treated with DEHP (10–6 M) and/or nutrients, including 10 μg/μl FA, 20% FeSO4 or 100 μg/μl CHO. (B and C) The MDA content and the GPX activity were detected in chick embryos treated with DEHP and/or nutrients. All data correspond to mean ± S.D (n = 3). *P < 0.05, or **P < 0.01, compared with control (administration of saline, FA, Fe or CHO alone); #P < 0.05, or ##P < 0.01, compared with administration of DEHP alone.
DEHP affects the apoptosis in chick embryos
Studies show that oxidative stress can result in cellular apoptotic responses [15]. Therefore, cell apoptosis was analyzed in chick embryos after DEHP treatment by Whole-mount and TUNEL assay. As shown in Fig. 5, TUNEL-positive signals were obviously elevated in whole embryo after DEHP treatment, compared with the control (P < 0.01; Fig. 5), which implied that DEHP may affect apoptosis. The result was similar from the analysis of TUNEL-positive signals in brain, heart and spine of chick embryos, respectively (Fig. 5 B2−B4).
Figure 5.

Effects of DEHP on the apoptosis in chick embryos. (A) Apoptosis signals were detected by whole-mount TUNEL staining. Scale bar = 500 μm. (B1–B4) The histogram represents the relative MOD of apoptotic signals in whole embryos, brain, heart and spine, respectively. The percentage of MOD of apoptotic signals were expressed as ratio of MOD of apoptotic signals vs total signals of five different optical fields selected in a random manner. *P < 0.05, or **P < 0.01, compared with control (administration of saline, FA, Fe or CHO alone); #P < 0.05, or ##P < 0.01, compared with administration of DEHP alone.
High dose of CHO protects chick embryos from DEHP-induced malformation
In order to seek the nutrients which can antagonize embryotoxicity induced by DEHP, recovery experiments by nutritional supplements were performed. As shown in Table 3, the supplement of Fe (20%), FA (10 μg/μl) or CHO (100 μg/μl) had no remarkable reverse effect on the embryo survival, embryo body weight and embryo malformation in DEHP-treated chick embryos (Table 3). CHO (100 μg/μl) could partially reverse these effects (Table 3). Moreover, the vascular area of yolk sac vascellum was obviously increased in DEHP-treated chick embryos by CHO (100 μg/μl) supplement compared with control (Fig. 2, P < 0.01). However, no remarkable reverse effect was observed with the supplement of Fe (20%) and FA (10 μg/μl) on the vascular area of DEHP-treated chick embryos (Fig. 2).
Table 3.
The effect of supplement of nutritive elements on DEHP-induced teratogenic effects in chick embryos
| Risk factor | DEHP | ||
|---|---|---|---|
| Nutritive element | Fa | CHO | Fe |
| Concentration | 10 μg/μl | 100 μg/μl | 20% |
| Apoptosis | No | Yes | No |
| ROS | No | Yes | No |
| Total SOD | No | Yes | No |
| Cu-zn SOD | No | Yes | No |
| Mn SOD | No | No | No |
| GPX | No | No | No |
| MDA | No | Yes | No |
Fe: FeSO4. Yes means that the supplement of nutritive element can partially restore teratogenic effects in chick embryos. No means that this kind of nutritive element has little effect.
As shown in Fig. 3, ROS level was significantly restrained in DEHP-treated chick embryos by CHO (100 μg/μl), especially in heart and brain (Fig. 3, B2 and B3; Table 3). CHO (100 μg/μl) supplement significantly increased total SOD (Fig. 4A, P < 0.05; Table 3) and Cu-Zn SOD activity in DEHP-treated chick embryos (Fig. 4A, P < 0.01; Table 3). However, CHO (100 μg/μl) supplement had no obvious effects on MDA content and GPX activity in DEHP-treated chick embryos. Other nutrients, such as Fe (20%), FA (10 μg/μl) had almost no obvious effects on the level of oxidative stress indicators (Fig. 4, Table 3).
In addition, CHO (100 μg/μl) supplement evidently attenuated the sensitivity of chick embryos to DEHP-induced apoptosis (Fig. 5A, B1, P < 0.01). The result was similar from the analysis of TUNEL-positive signals in brain (P < 0.01), heart (P < 0.01) and spine (P < 0.01) of chick embryos, respectively (Fig. 5 B2−B4). However, it seems that Fe or FA also had little effects on DEHP-induced apoptosis in chick embryos. All these results implied that CHO (100 μg/μl) might protect chick embryos from DEHP-induced abnormal changes of related indicators.
Discussion
The study firstly reported the exposure conditions of DEHP in urine of pregnant women containing harbored NTDs fetuses, indicating that DEHP may have adverse effects on human pregnancy and early embryo development.
Lei et al. [9] reported that DEHP could induce splenic toxicity in quail via disturbing Nrf2-mediated defense response. Du et al. [17] found that DEHP could induce cerebellar toxicity in quails. DEHP could induce the abnormal development of nerve system in embryo [8,9]. Maternal exposure to DEHP has a lasting effect on physiological functions of the vascular system, adipose tissue and nerve system in offspring of mice [18]. Higher levels of DEHP may have adverse effects on neurobehavioral parameters in mice [8,9]. However, there was no report of the distribution of DEHP in NTD population. The study found that the detection ratio of DEHP and its metabolites in maternal urine in NTD population was higher than that in controls, which indicated that DEHP might associate with the occurrence of NTDs (P < 0.01, P < 0.05, respectively).
To further investigate the effects DEHP has on early embryo development and explore the possible pathogenic mechanism, chick embryos were selected as an animal model for further research. Humans and birds have an overlap region in the posterior neuropore during the process of primary and secondary neurogenesis. Moreover, the chick embryo has been extensively used as a model for the study of neural tube development and the effects of many drugs on the early embryo neurogenesis [19,20]. Therefore, central nervous system (CNS) of the chick embryo is an excellent model to explore the mechanisms of the patterning of CNS in early embryo development, which also can provide clues for our research. Our results indicated that the body weight of chick embryo was significantly decreased, while teratogenic rate was obviously increased in DEHP-treated samples compared with the controls (Pÿ0.01, respectively), implying that DEHP may have adverse effect on the chick embryo viability.
Reports indicated that the occurrence of NTDs may be related with oxidative stress [21–23]. Our results revealed that DEHP elevated the level of ROS, enhanced lipid peroxidation and inhibited the antioxidation in chick embryos. These results suggested that DEHP may induce abnormal development of neural by enhancing the response of oxidative stress.
Studies indicated that oxidative stress closely associates with programmed cell death (PCD) [15,24,25]. It reported that oxidative stress-mediated cell apoptosis play an important part in the pathogenesis of several neurogenerative diseases [26]. Our research revealed that DEHP can obviously induce apoptosis in chick embryos, which was consistent with the results of oxidative stress response in DEHP-treated chick embryos, indicating that oxidative stress was closely associated with DEHP-induced abnormal embryo development via promoting the process of cell apoptosis.
To further investigate the effects of DEHP on embryo development, recovery experiments by nutritional supplements were performed to seek the nutrients which can reverse embryos toxicity of DEHP. Our research indicated that Fe or FA supplementation had almost no effects on ROS-related indicators in DEHP-treated chick embryos. CHO (100 μg/μl) supplement could partially reduce the incidence of teratogenicity, increase the vascular area of chick embryos, reduce embryo apoptosis, restrain the level of ROS and oxidative stress indicators. Studies imply that CHO closely associates with adverse health outcomes, including birth defects, neurodevelopment and cognition alterations, cancer and cardiovascular disease (CVD) [27]. In rodents, DNA and histone methylation were altered in the offspring with treatment of maternal CHO-deficient diets during the perinatal period [28,29]. In humans, low maternal CHO intake during pregnancy can alter DNA methylation in the placenta and cord blood [30]. Wiedeman et al. [27] report that the function of CHO in neurodevelopment and cognition involves the synthesis of components of cellular membranes and acetylcholine as well as gene expression. It seems that high dose CHO may inhibit oxidative stress response by enhancing the SOD activity and attenuating MDA content to antagonize the damage of embryotoxicity induced by DEHP.
FA is an essential nutrient for the development, function and regeneration of nervous systems [31,32]. The growing awareness of the pathogenesis associated with folate deficiency has drastically increased the public demand for FA supplementation. Besides folate fortification, ample amounts of FA are often ingested by pregnant mother and general population as daily supplement [33]. However, FA supplement, which surprisingly showed relatively minimal effect in our study. The similar report from Rui-Rong Tong also indicated that FA had little protective effect on hyperglycemia-induced NTDs [33]. It may attribute to its little effect on oxidative stress response. These findings may provide clues for the prevention of NTDs.
However, the research still has limitations. In humans, teratogens that have been associated with NTDs include the anticonvulsant drug valproic acid and the fungal product fumonisin [34]. Other teratogens should also be detected to further explore the effects of environmental factors on the occurrence of NTDs. Moreover, the alteration of genes with NTDs should be investigated in the future to illustrate the mechanism underlying NTDs.
Conclusion
The research established the possible relation between DEHP and the occurrence of human NTDs by analyzing the detection rate of positive DEHP in NTD population. The results were then further confirmed by experiments from chick embryos. These results may provide useful clues for prevention and diagnosis of NTDs.
Supplementary Material
Acknowledgements
This work was supported by grants from The National Key Research and Development Program of China (2016YFC1000307) and CAMS Innovation Fund for Medical Sciences (CIFMS, 2018-I2M-1-004).
Contributor Information
Ge Song, Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China; Graduate School, Peking Union Medical College, Dong Dan San Tiao, Beijing 100730, China.
Rui Wang, Department of Blood Transfusion, First medical center, Chinese People’s Liberation Army General Hospital, Fu Xing Road, Beijing 100853, China; Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China.
Yi Cui, Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China; Graduate School, Peking Union Medical College, Dong Dan San Tiao, Beijing 100730, China.
Chan Juan Hao, Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China; Graduate School, Peking Union Medical College, Dong Dan San Tiao, Beijing 100730, China.
Hong-Fei Xia, Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China; Graduate School, Peking Union Medical College, Dong Dan San Tiao, Beijing 100730, China.
Xu Ma, Reproductive and Genetic Center of National Research Institute for Family Planning, Da Hui Si Road, Beijing 100081, China; Graduate School, Peking Union Medical College, Dong Dan San Tiao, Beijing 100730, China.
Conflict of Interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of National Research Institute for Family Planning. The study protocol was clarified for all participants and a signed written informed consent was obtained from them.
References
- 1. Greene ND, Copp AJ. Neural tube defects. Annu Rev Neurosci 2014;37:221–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Racine E, Bell E, Yan A et al. Ethics challenges of transition from paediatric to adult health care services for young adults with neurodevelopmental disabilities. J Paediatr Child Health 2014;19:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren A, Qiu X, Jin L et al. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A 2011;108:12770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol 2010;88:653–69. [DOI] [PubMed] [Google Scholar]
- 5. D'Antoine H, Bower C. Folate status and neural tube defects in aboriginal Australians: the success of mandatory fortification in reducing a health disparity. Current Development Nutrition 2019;3:nzz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee W, Cho JH, Lee Y et al. Dibutyl phthalate impairs neural progenitor cell proliferation and hippocampal neurogenesis. Food Chem Toxicol 2019;129:239–48. [DOI] [PubMed] [Google Scholar]
- 7. Main KM. Phthalate monoesters and infant reproductive health. Gesundheitswesen 2008;70:S46–8. [DOI] [PubMed] [Google Scholar]
- 8. Shiota K, Mima S. Assessment of the teratogenicity of di(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in mice. Arch Toxicol 1985;56:263–6. [DOI] [PubMed] [Google Scholar]
- 9. Lei Y, Hui-Xin L, Jian-Ying G et al. Di (2-ethyl hexyl) phthalate (DEHP)-induced splenic toxicity in quail (Coturnix japonica) via disturbing Nrf2-mediated defense response. Environ Pollut 2019;251:984–9. [DOI] [PubMed] [Google Scholar]
- 10. Erica U, Emmi R, Tanika B et al. From the cover: teratogenic effects of in utero exposure to Di-(2-Ethylhexyl)-phthalate (DEHP) in B6:129S4 mice. Toxicol Sci 2017;157:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang R, Sun DG, Song G et al. Choline, not folate, can attenuate the teratogenic effects ofdibutyl phthalate (DBP) during early chick embryo development. Environ Sci Pollut Res 2019;26:29763–79. [DOI] [PubMed] [Google Scholar]
- 12. Rosenquist TH, Ratashak SA, Selhub J. Homocysteine induces congenital defects of the heart and neural tube: effect of folic acid. Proc Natl Acad Sci U S A 1996;93:15227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 1992, 195:231–72. [DOI] [PubMed] [Google Scholar]
- 14. Lin S, Ren A, Wang L et al. Oxidative stress and apoptosis in benzo[a]pyrene-induced neural tube defects. Free Radic Biol Med 2018;116:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coll TA, Chaufan G, Pérez-Tito L et al. Oxidative stress and cellular and tissue damage in organogenic outbred mouse embryos after moderate perigestational alcohol intake. Mol Reprod Dev 2017;84:1086–99. [DOI] [PubMed] [Google Scholar]
- 16. Du ZH, Xia J, Sun XC et al. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ Pollut 2017;226:435–43. [DOI] [PubMed] [Google Scholar]
- 17. Lee KI, Chiang CW, Lin HC et al. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol 2016;90:1211–24. [DOI] [PubMed] [Google Scholar]
- 18. Ertekin T, Bilir A, Aslan E et al. The effect of diclofenac sodium on neural tube development in the early stage of chick embryos. Send to Folia Morphol (Warsz) 2019;8:307–13. [DOI] [PubMed] [Google Scholar]
- 19. Temiz C, Temiz P, Demirel A et al. Effect of sodium phenytoin concentration on neural tube development in the early stages of chicken embryo development. J Clin Neurosci 2009;16:307–11. [DOI] [PubMed] [Google Scholar]
- 20. Cetinkal A, Colak A, Topuz K et al. The effects of meloxicam on neural tube development in the early stage of chick embryos. Turk Neurosurg 2010;20:111–6. [DOI] [PubMed] [Google Scholar]
- 21. Yuan Y, Zhang L, Jin L et al. Markers of macromolecular oxidative damage in maternal serum and risk of neural tube defects in offspring. Free Radic Biol Med 2015;80:27–32. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Ren A, Wang L et al. Oxidative stress and apoptosis in benzo[a]pyrene-induced neural tube defects. Free Radic Biol Med 2018;116:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang G.X., Tu H.C, Dong Y., et al. ΔNp63 inhibits oxidative stress-induced cell death, including Ferroptosis, and cooperates with the BCL-2 family to promote Clonogenic survival. Cell Rep, 2017,1, 2926–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amri F, Ghouili I, Amri M et al. Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J Neurochem 2017;140:151–69. [DOI] [PubMed] [Google Scholar]
- 25. Russell JW, Golovoy D, Vincent AM et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 2002;16:1738–48. [DOI] [PubMed] [Google Scholar]
- 26. Wiedeman Alejandra M, Barr Susan I, Green Timothy J. Dietary choline intake: current state of knowledge across the life cycle. Nutrients 2018;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J 2006;20:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kovacheva VP, Mellott TJ, Davison JM et al. Gestational choline deficiency causes global and igf2 gene DNA hypermethylation by up-regulation of dnmt1 expression. J Biol Chem 2007;282:31777–88. [DOI] [PubMed] [Google Scholar]
- 29. Jiang X, Yan J, West AA et al. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 2012;26:3563–74. [DOI] [PubMed] [Google Scholar]
- 30. Kao TT, Chu CY, Lee GH et al. Folate deficiency-induced oxidative stress contributes to neuropathy in young and aged zebrafish — implication in neural tube defects and Alzheimer's diseases. Neurobiol Dis 2014;71:234–44. [DOI] [PubMed] [Google Scholar]
- 31. Kim GB, Chen Y, Kang W et al. The critical chemical and mechanical regulation of folic acid on neural engineering. Biomaterials 2018;178:504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan RR, Li YF, Zhang SJ et al. Abnormal O-GlcNAcylation of Pax3 occurring from hyperglycemia-induced neural tube defects is ameliorated by carnosine but not folic acid in chicken embryos. Mol Neurobiol 2017;54:281–94. [DOI] [PubMed] [Google Scholar]
- 33. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 1992;195:231–72. [DOI] [PubMed] [Google Scholar]
- 34. Nicholas DEG, Andrew JC. Neural tube defects. Annu Rev Neurosci 2014;37:221–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


