Abstract
Combination antiretroviral therapy (cART), which is a lifelong therapy for people living with human immunodeficiency virus, has been associated with nephrotoxicity and hepatotoxicity leading to its discontinuation. This study aimed at investigating the ameliorative potential of naringenin and quercetin on cART-induced hepatotoxicity and nephrotoxicity. Seventy male Wistar rats (225–260 g) were divided into seven groups as control, cART, naringenin, quercetin, dimethyl sulfoxide (DMSO), naringenin/cART (CN) and quercetin/cART (CQ). cART (24 mg/kg), naringenin (50 mg/kg) and quercetin (50 mg/kg) were dissolved in 1% v/v DMSO and administered orally for 56 days. Combination of cART and bioflavonoids had significant increase in superoxide dismutase (P < 0.05), catalase (P < 0.01), reduced glutathione (P < 0.001) and decreased malondialdehyde (P < 0.001) compared to cART only. Tumor necrosis factor Alpha (TNFα) level increased significantly in cART and CQ (P < 0.01) groups, while others showed no significant changes compared to control. TNFα also significantly decreased in CQ level compared to cART (P < 0.001). In addition, significant increase in creatinine level in cART only indicated progressive renal toxicity. Also, progressive pathological changes including congested blood vessels and hepatocellular necrosis were found in the liver, while the kidney had glomerular atrophy, and tubular distortion in cART-only group. Control, naringenin- and quercetin-treated groups showed normal renal and hepatic cytoarchitecture. These findings elucidate that progressive renal and hepatic toxicity is associated with the continuous use of cART; however, a combination of quercetin and naringenin with cART showed possible potential of ameliorating the damages posed by cART.
Keywords: cART, quercetin, naringenin, liver, kidney
Introduction
The advent of combination antiretroviral therapy (cART), made up of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) combined with either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTI) has been very instrumental to the well-being of human immunodeficiency virus (HIV)-infected patients. It has prolonged their lifespan and reduced susceptibility to opportunistic infections [1]. In spite of these benefits, cART has been associated with several adverse events including gonadotoxicity [2], neuronotoxicity [3, 4], hepatotoxicity [5] and nephrotoxicity [6], which unfortunately has led to a third of the therapy discontinuation reported [7]. Another staring drawback of cART in the treatment of HIV-infected persons is that it is a lifelong therapy. Consequently, people living with HIV/acquired immunodeficiency syndrome (AIDS) (PLWHAs) suffer the drug’s adverse effects for the rest of their lives. There are reports on nephrotoxicity associated with chronic use of tenofovir disoproxil fumarate (TDF), a nucleoside reverse transcriptase inhibitor [8, 9], likewise an irreversible renal failure due to an acute overdose of cART [10]. Similarly, hepatotoxicity has been reported in both experimental [11, 12] and clinical [13] applications of antiretrovirals. It is therefore important to understand the underpinning mechanisms triggered by cART to induce hepatotoxicity and nephrotoxicity in order to proffer possible remedies. Hence the search for alternatives that can significantly minimize these adverse effects is gathering great momentum.
Naringenin and quercetin are nutraceuticals that have taken the center stage and have been hypothesized as lead compounds that may play significant roles in the management of liver and kidney injuries [14], hence the use of these bioflavonoids in the present study.
Naringin is a flavanone-7-O-glycoside naturally found in grape fruits. In humans it is metabolized to the aglycone—naringenin by naringinase present in the gut [15]. It has been reported to display strong antioxidant and anti-inflammatory activity [15]. Likewise, quercetin a plant phenolic glycoside characterized as a flavonol is metabolized by the small intestine, liver, kidney and large intestine, and commonly found in fruits (especially apples) and vegetables including onions, kale, French beans, broccoli, lettuce and tomatoes [16]. It is reported to have strong antioxidant property due to the presence of two antioxidant pharmacophores within its molecule configuring it for free radical scavenging [17]. In light of this, the present study thrives on the hypothesis that naringenin and quercetin may mitigate the adverse effects of the long-term therapy of cART on the liver and kidney.
Materials and Methods
Animals
Seventy (70) adult male Wistar rats weighing between 225 and 260 grams were allowed to acclimatize for 3 weeks in the Animal House, Department of Anatomy, College of Medicine of the University of Lagos under standard animal housing condition of 24 ± 2°C and 12/12-hour light/dark cycle. All the rats were housed in plastic cages (5 rats/cage) with soft wood shavings 30 cm long, 20 cm wide and 13 cm high employed as bedding in the cages. The rats had access to standard rat chow and water ad libitum.
All procedures were carried out in accordance with the principles of laboratory animal care of the National Medical Research Council and the Guide for the Care and Use of Laboratory Animals [18]. This research was approved by the Health Research Ethics Committee of the College of Medicine of the University of Lagos with protocol number CMUL/HREC/03/17/113.
Chemicals, reagents and drugs
Quercetin (Cat No: Q4951-100G), naringenin (Cat No: W530098-500G) and dimethyl sulfoxide (DMSO) (Cat No: 317275-500ML) were purchased from Sigma-Aldrich South Africa while cART, a combination of efavirenz 600 mg, lamivudine 300 mg and tenofovir disoproxil fumarate 300 mg, manufactured in India by Macleods Pharmaceuticals Limited was provided by the AIDS Prevention Initiative in Nigeria (APIN) center at Lagos University Teaching Hospital (LUTH).
Experimental design
The animals were randomly divided into seven (7) groups of 10 each viz control group: 1 ml of distilled water, cART group: 24 mg/kg combination antiretroviral therapy (300 mg tenofovir, 300 mg lamivudine, 600 mg efavirenz) [19], DMSO group: 1% v/v dimethyl sulfoxide, N group: 50 mg/kg naringenin, which was dissolved in 1% v/v DMSO, Q group; 50 mg/kg quercetin, which was dissolved in 1% v/v DMSO, CN group: 24 mg/kg cART +50 mg/kg naringenin, CQ group: 24 mg/kg cART +50 mg/kg quercetin. All treatments were applied daily by oro-gastric gavage and at the end of 56 days all animals were euthanized with excess urethane 24 hours after the last treatment.
Assessments of superoxide dismutase, catalase, reduced Glutathione, and tumor necrosis factor Alpha Analysis
For the preparation of oxidative stress markers and TNFα, Using tissue homogenizer, 0.5 g of the liver was homogenized in 4.5 ml of 0.4 M sodium phosphate buffer (pH 7.0), centrifuged at 3500 rpm for 10 min and the supernatant removed for the assay. Oxidative stress markers including catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH) and malonidialdehyde (MDA) were determined by modified methods by Sinha, Kakkar et al., Moron et al., Niehaus and Samuelsson, respectively [20–23]. Rat TNFα assay was done on liver homogenate using Elabscience ELISA kit with a catalog No: E-EL-R0019 in accordance with manufacturer’s instructions.
Kidney function tests
The blood sample was collected via cardiac puncture into lithium heparin bottles and centrifuged at 3000 rpm for 10 min at 4°C in a refrigerated centrifuge. The resulting supernatant (plasma) was decanted into Eppendorf tubes and prepared for biochemical analyses of blood urea and creatinine (CRT) were determined by the method reported in Pandya et al. [24].
Mean weight difference
This was determined by subtracting the final body weight of each the animals at the end of the experiment from the initial body weights.
Histomorphological studies
The tissue samples of liver and kidney were fixed in 10% neutral buffered formalin and processed for hematoxylin and eosin (H&E) via paraffin embedding after which they were sectioned at 4 μm using a Thermo Scientific HM 325 rotatory microtome (CE Bright Company Ltd. Huntington England), cleared in xylene and hydrated in decreasing alcohols, stained with hematoxylin and eosin (H&E) and mounted with DPX [25]. The histological specimen was captured and examined using the Leica Slidepath Gateway LAN software.
Statistical analysis
The values for the results were expressed as mean ± standard deviation (SD). Statistical level of significance was determined using one-way analysis of variance (ANOVA) with Tukey’s post hoc test to compare difference between groups. The mean difference was significant at 95% confidence interval (P < 0.05). Graphpad prism 5 (Graphpad software) was used.
Results
Oxidative stress markers and TNFα assessment
Animals that received cART had significantly decreased (P < 0.05) SOD level compared to control (Fig. 1). Animals that received a combination of cART and bioflavonoids (CN and CQ) had significant increase (P < 0.01) in SOD and CAT compared to those that received cART only. The animals that received only quercetin had a significant increase in CAT compared to both DMSO (P < 0.05) and control (P < 0.01), while GSH level increased (P < 0.05) compared to DMSO. Whereas, those that received only DMSO had significantly decreased (P < 0.05) GSH compared to control (Fig. 1).
Figure 1.
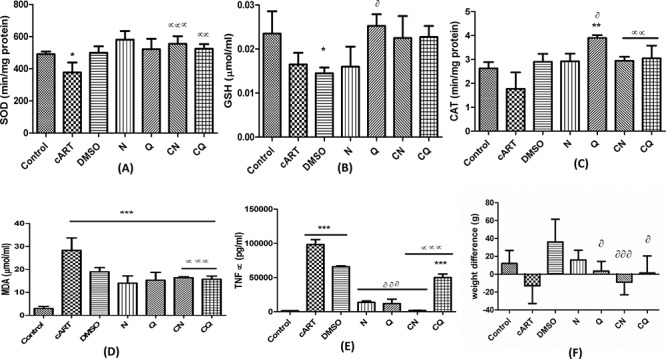
Showing oxidative stress markers (A-D), tumor necrosis factor alpha (TNFα) (E) and weight difference (F). *P < 0.05, **P < 0.01, ***P < 0.001 compared to control; αP < 0.05, ααP < 0.01, αααP < 0.001 compared to cART. ðP < 0.05, ððP < 0.01, ðððP < 0.001 compared to DMSO.
There was a significant increase (P < 0.001) in MDA across the entire groups compared to control. There was also a concomitant decrease (P < 0.001) in MDA level in animals that received a combination of both cART and bioflavonoids (CN and CQ) compared to cART-only group. TNFα increased significantly in cART, DMSO and CQ groups compared to control. Whereas, those that received a combination of cART and bioflavonoids (CN and CQ) had significantly decreased (P < 0.001) TNFα levels compared to cART-only group. Animals in groups N, Q and CN also had significantly decreased (P < 0.001) in TNFα levels compared to DMSO (Fig. 1).
Kidney function tests
There was no statistically significant difference in urea level across the groups compared to the control group. Creatinine level was statistically significantly increased in cART (P < 0.01) compared to control group. Combination of the individual bioflavonoid and cART (CN and CQ) showed a statistically significant decrease (P < 0.05) in creatinine level when compared to cART-treated group (Fig. 2).
Figure 2.
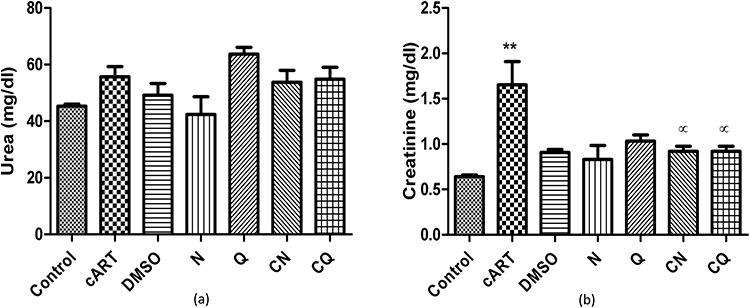
Showing kidney function tests. **P < 0.01 compared to control; αP < 0.05 compared to cART.
Mean weight difference
There was no significant difference between the treated groups and control but there was marked decrease in body weights of animals that received cART and cART and naringenin in comparison with control. Those that received quercetin, cART combined with naringenin, and cART combined with quercetin had significantly decreased weights (P < 0.05, 0.001 and 0.05 respectively) compared with DMSO (Fig. 1F).
Histomorphological changes on the liver
The histology of the liver for animals that received distilled water and those that received a combination of cART and naringenin (CN) showed normal cellular architecture of sinusoids (s), hepatocytes (H) and central vein (CV). On the contrary, animals that received cART, DMSO, N, Q and CQ showed different levels of hepatocellular necrosis including dilated sinusoids, congestion of blood vessels and presence of inflammatory cells. Groups treated with N and Q showed the least while those treated with cART presented the most severity of the hepatic cytoarchitecture (Table 1) (Fig. 3).
Table 1.
Hepatic histopathological incidence and severity scores under light microscopy assessment (+: mild severity, ++: moderate severity, +++: high severity)
| Groups | Dilated sinusoid | Damaged blood vessel wall | Inflammatory cells | Congestion of blood vessel |
|---|---|---|---|---|
| Control | +++ | |||
| Naringenin | + | + | + | |
| Quercetin | + | |||
| DMSO | ++ | ++ | + | + |
| cART | +++ | ++ | +++ | ++ |
| H/N | + | + | ||
| H/Q | + | ++ |
Figure 3.
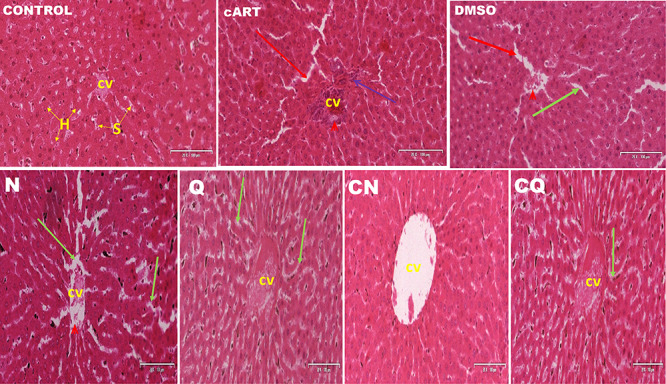
Photomicrograph of transverse section of liver (H&E 20x). CV: central vein, green arrow: dilated sinusoid, S: sinusoid, purple arrow: inflammatory cells, H: hepatocytes, red arrow: degenerating hepatocytes, red head: congested central vein.
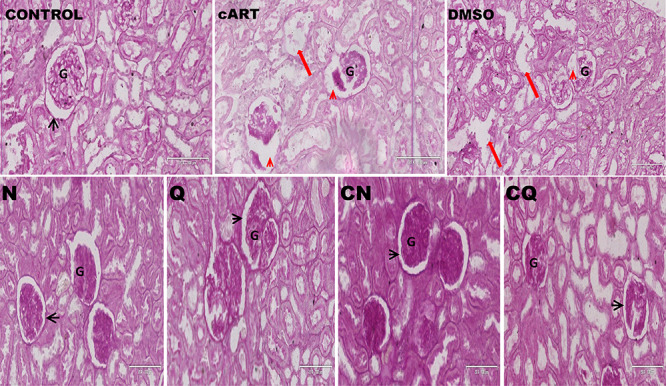
Histomorphological changes on the kidney
Normal renal glomerular and tubular morphology was observed in control and Q group. There was loss of brush borders in proximal convoluted tubules and macula densa of the DCT, congestion of blood vessels and excessive infiltration of mesangial cells in the Bowman’s capsule of cART (Fig. 4). N, Q and CQ showed improved nephritic cytoarchitecture, while persistent mesangial cells, loss of macula densa and congestion of blood vessels persisted in DMSO and CN (Table 2) (Fig. 4).
Figure 4.
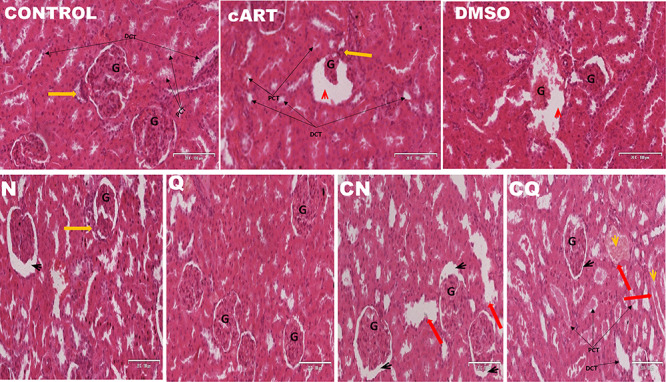
Photomicrograph of the cytoarchitecture of the kidney (H&E 20x). Yellow arrow: vascular pole, G: glomerulus, red head: widened Bowman’s capsule with degenerating wall and glomerulus, PCT: proximal convoluted tubule, DCT: distal convoluted tubule, red arrow: dilated renal tubule with flattened tubular cells, yellow head: intratubular cast formation black head: normal renal corpuscle.
Table 2.
Nephritic pathological incidence and severity scores under light microscopy assessment (+: mild severity, ++: moderate severity, +++: high severity)
| Atrophied glomerulus | Damaged basement membrane | Dilated urinary space | Fluid filled space | |
|---|---|---|---|---|
| Control | +++ | |||
| Naringenin | + | + | ||
| Quercetin | + | + | ||
| DMSO | + | + | ++ | |
| cART | ++ | ++ | +++ | |
| H/N | + | + | ||
| H/Q | + | ++ |
Control group tested positive to PAS reagent. Well-alienated basement membrane and glomerular were observed. Loss of tubular cast depositions of glycogen and atrophied glomerulus expanding the urinary space occurred in cART. Extensive tubular cast, mineralization, alienated basement membrane and normal glomerulus were observed in CQ, CN, N and Q, while, damaged basement membrane and mild mineralization were observed in DMSO (Fig. 5).
Figure 5.
Photomicrograph of the histomophorlogy of the kidney (PAS 20x). G: glomerulus, red head: widened Bowman’s capsule with degenerating wall and glomerulus, red arrow: dilated renal tubule with flattened tubular cells, black head: normal renal corpuscle.
Discussion
The kidneys are known to be major target sites for drugs interaction (including antiretroviral drugs) due to metabolic detoxification and excretion of metabolite function [26–29]. Therefore, drugs capable of significantly impairing its functions are often considered to be less effective as continuous usage of these drugs could lead to various kidney diseases. Constituents of cART (Tenofovir, efavirenz and lamivudine) have been linked with kidney perturbations and liver damages [29] leading to debates on their removal from first-line [29] treatment and management of HIV.
Non-enzymatic and enzymatic antioxidant activity level has been documented to contribute effectively to normal functioning of the liver and kidney. Antioxidant activity levels from this study showed that the use of cART significantly increased lipid peroxidation level and altered both enzymatic (SOD) and non-enzymatic antioxidant (GSH) activity levels. Reduction in GSH and SOD levels in this study, which normally protect the cells against several ROS generated by converting hydrogen peroxidase (H₂O₂) to water, was insufficient to neutralize H₂O₂ resulting in the increased lipid peroxidation observed. This correlates with reports that the use of cART has a detrimental effect on the antioxidant level of various organs [4, 30–32]. This mechanism of action might be the basis of major complications including liver damages and kidney perturbation. On the contrary, the use of quercetin and naringenin as adjuvants significantly improved the reduced enzymatic antioxidant level induced by cART activity. This improvement might be due to the potency of these antioxidants in inhibiting the functioning of ROS-forming enzymes (NADPH-oxidase and myeloperoxidase) and enhancing ROS scavengers through its ability to produce sufficient hydroxyl (-OH) substitute [33]. This toxicity of cART is implicated in the weight loss observed. Antiretrovirals drugs involving a combination with efavirenz have been reported to cause lipoatrophy on HIV-infected persons [34].
Incidence of oxidative stress and elevations in creatinine and urea level have been strongly linked with onset of renal pathology. Increase in creatinine level in cART is due to alterations to the macula densa of the kidney, impairing the contribution of the cells in the regulation of glomerular filtration, hence, encouraging retention of creatinine in the blood. This is in consonance with the report by Soto et al., that the use of cART relatively altered plasma creatinine levels. Progressive deteriorating kidney function test of cART-treated group can be due to drug–drug interaction among the constituents of cART [35].
Efavirenz has been discussed to be a potent inhibitor of organic cation transporters and toxin extrusion proteins transporters. This might be the mechanism behind it preventing the excretion of lamivudine, tenofovir and other toxins from the kidney, contributing to increased renal toxicity [35]. Improvement in the kidney function test especially in creatinine excretion by quercetin and naringenin is due to their ability to enhance podocytes and macula densa functioning and possibly modulation of ionic gate channels responsible for excretion of toxins. Antioxidants, which are present in both bioflavonoids, have also been discussed to have effectively depressed Ca2+-mediated priming and activation of NADPH oxidase enhancing renal excretion of toxins [36]. In addition, the hydroxy benzoic nature of these bioflavonoid similar to Egyptian Propolis, which have been reported to improve the digestive utilization of calcium, phosphorus and magnesium [37, 38], might have contributed to the functioning of the Bowman’s capsule, absorption and re-absorption activities at the proximal and distal convoluted tubules through its interactions and effective utilization of calcium ions at the ion channels of the renal system in this study.
Tumor necrosis factor-alpha (TNFα) released by macrophages (Kupffer cells) has been named as one of the main regulators of the inflammatory process. Hence, therapeutic restraints of these pro-inflammatory cytokines enhance kidney, liver and cardiovascular health [39, 40]. TNFα assessment in the present study elucidated that cART enhances the release of pro-inflammatory cytokines by the Mesangial cells and Kupffer cells of the kidney and liver respectively. This is possibly due to its ability to stimulate increased ROS release, mitochondrial autophagic stress triggering inflammatory pathway and the release of pro-inflammatory cytokines. [7]. This will eventually enhance lipid peroxidation within the tissue, hence, lead to necrosis and alteration in cytoarchitecture of the organs [40, 41]. This hypothesis is in tandem with several studies that the use of cART leads to a marked increase in MDA level [4, 31, 42]. Flavonoid (quercetin and naringenin) metabolites have been reported to be more active than their precursor structures (naringin—its glycone precursor) through targeting of the pro-inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [39]. Metabolites of these bioflavonoids released to the kidney and liver might have accounted for the reduction in the release of pro-inflammatory cytokines (TNFα) by their ability to suppress inflammatory processes.
Pro-inflammatory cytokines levels including TNFα regulate cellular reactions, apoptotic and necrotic cascade. Increase in this level triggers the onset of several pathological changes on the cytoarchitecture. Histomorphological findings of this study agreed with TNFα analysis. cART-treated groups showed progressive hepatocellular necrosis in the liver and tubular distortion, necrosis and glomerular atrophy in the kidney. This agrees with findings of Truter et al., that the use of cART containing both efavirenz and tenofovir triggered similar pathological changes in the kidney as observed in this study [43].
Alterations on the podocytes, brush borders, convoluted tubules of the kidney and the hepatocytes, sinusoids and blood vessels of the liver involved with the detoxification and excretion of toxins might have significantly contributed to the detrimental effects of cART on various biochemical analysis carried out in this study. Naringenin-treated groups showed similar pathological features as cART while quercetin-treated groups showed improved cytoarchitecture. Activities of quercetin on oxidative stress and pro-inflammatory cytokines have contributed to the improved well-being of the hepatic and nephritic cytoarchitecture involved with removal of toxins induced by the use of cART, hence, ameliorating the severity of pathological effects posed by the use of cART.
Similarly, PAS analysis demonstrated severe tubular vacuolization with glycogen retention and severe atrophy of the glomerulus in cART-treated group. This correlates with findings by Offor et al., that the use of highly active antiretroviral therapy containing lamivudine induces tubular vacuolization with high proportion of glycogen [44]; by Adjene et al., that chronic administration of efavirenz altered the microanatomy arrangement of the kidney [45] and by Elias et al., that the use of 600mh/kg of tenofovir for 2–8 weeks induced glomeruli damage and tubular necrosis [46]. On the contrary, the use of quercetin as an adjuvant showed improvement of the nephritic cytoarchitecture. However, the naringenin-treated group showed mild glycogen retention in the basement membrane of the kidney. The mechanism behind the hindrance to the antioxidant activities of these bioflavonoids on the cytoarchitecture of the kidney and liver is yet unknown. Although, the use of 1% v/v DMSO as a solvent as opposed to the use of distilled water in our previous study for dissolving the bioflavonoids (especially naringenin) [4] might have reduced optimal antioxidant functioning of naringenin in this study.
Conclusion
Continuous use of cART containing efavirenz, lamivudine and tenofovir has the potential of inducing progressive hepatotoxicity and nephrotoxicity due to oxidative damages on liver and kidney respectively. However, its combination with bioflavonoids (quercetin or naringenin) inhibited oxidative stress and inflammatory process, hence, improving the cytoarchitecture and biochemical activities of the organs.
Recommendation
The authors recommend that further analysis be carried out on ameliorative activities of naringenin and quercetin on liver and kidney upon longer administration periods.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgement
The authors wish to appreciate the Aids Prevention Initiative of Nigeria (APIN) center of Lagos University Teaching Hospital (LUTH) for the provision of antiretroviral drugs.
Contributor Information
Edidiong Nnamso Akang, Department of Anatomy, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria.
Olufunke O Dosumu, Department of Anatomy, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria.
Ini-ibehe Essien Okoko, Department of Anatomy, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria.
Oluwatomisin Faniyan, Department of Anatomy, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria.
Ademola A Oremosu, Department of Anatomy, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria.
Alani Sulaimon Akanmu, Department of Haematology and Blood Transfusion, College of Medicine, University of Lagos, Idi-Araba, Lagos, Nigeria.
Funding
Fogarty International Center of the National Institutes of Health under Award Number D43TW010134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the following co-funding partners: Fogarty International Center (FIC), NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH), Office of AIDS Research, Office of the Director (OAR/NIH), Office of Research on Women's Health, Office of the Director (ORWH/NIH), National Institute on Minority Health and Health Disparities (NIMHD/NIH), National Institute of Neurological Disorders and Stroke (NINDS/NIH).
References
- 1. Powell MK, Benková K, Selinge P et al. Opportunistic infections in HIV-infected patients differ strongly in frequencies and spectra between patients with low CD4+ cell counts examined postmortem and compensated patients examined Antemortem irrespective of the HAART era. PLoS One 2016;11:e0162704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogedengbe O, Naidu EC, Akang E et al. Virgin coconut oil extract mitigates testicular-induced toxicity of alcohol use in antiretroviral therapy. Andrology 2018;6:616–26. [DOI] [PubMed] [Google Scholar]
- 3. Singer EJ, Nemanim NM. The Persistence of HIV-Associated Neurocognitive Disorder (HAND) in the Era of Combined Antiretroviral Therapy (cART), in Global Virology II-HIV and NeuroAIDS. New York: Springer, 2017, 375–403. [Google Scholar]
- 4. Akang EN. Combination antiretroviral therapy (cART)-induced hippocampal disorders: highlights on therapeutic potential of Naringenin and quercetin. IBRO reports 2019;6:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baynes HW, Tegene B, Gebremichael M et al. Assessment of the Effect of Antiretroviral Therapy on Renal and Liver Functions Among HIV-Infected Patients: A Retrospective Study, Vol. 9 Auckland, NZ: HIV/AIDS, 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan L, Asriel B, Eaton EF et al. Potential kidney toxicity from the antiviral drug tenofovir: new indications, new formulations, and a new prodrug. Curr Opin Nephrol Hypertens 2018;27:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apostolova N, Gomez-Sucerquia LJ, Gortat A et al. Autophagy as a rescue mechanism in efavirenz-induced mitochondrial dysfunction: a lesson from hepatic cells. Autophagy 2011;7:1402–4. [DOI] [PubMed] [Google Scholar]
- 8. Karras A, Lafaurie M, Furco A et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 2003;36:1070–3. [DOI] [PubMed] [Google Scholar]
- 9. Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol 2013;24:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ensor J, Burger D, Visschers MJ et al. Irreversible acute kidney injury following efavirenz/tenofovir disoproxil fumarate/emtricitabine overdose. Afr J Nephrol 2018;21:12–5. [Google Scholar]
- 11. Azu OO, Jegede AI, Ugochukwu O et al. Hepatic histomorphological and biochemical changes following highly active antiretroviral therapy in an experimental animal model: does Hypoxis hemerocallidea exacerbate hepatic injury? Toxicol Rep 2016;3:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peter AI, Naidu EC, Akang E et al. Investigating organ toxicity profile of Tenofovir and Tenofovir nanoparticle on the liver and kidney: experimental animal study. Toxicol Res 2018;34:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petros Z, Lee MTM, Takahashi A et al. Genome-wide association and replication study of hepatotoxicity induced by antiretrovirals alone or with concomitant anti-tuberculosis drugs. Omics: J Integr Biol 2017;21:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahu N, Mishra G, Chandra HK et al. Naringenin mitigates antituberculosis drugs induced hepatic and renal injury in rats. J Tradit Complement Med 2020;10:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alam MA, Subhan N, Rahman MM et al. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr 2014;5:404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Formica J, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 1995;33:1061–80. [DOI] [PubMed] [Google Scholar]
- 17. D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 2015;106:256–71. [DOI] [PubMed] [Google Scholar]
- 18. National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 2010. [Google Scholar]
- 19. WHO , Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organisation, 2016. [PubMed] [Google Scholar]
- 20. Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389–94. [DOI] [PubMed] [Google Scholar]
- 21. Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21:130–32. [PubMed] [Google Scholar]
- 22. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta, Gen Subj 1979;582:67–78. [DOI] [PubMed] [Google Scholar]
- 23. Niehaus W Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6:126–30. [DOI] [PubMed] [Google Scholar]
- 24. Pandya D, Nagrajappa AK, Ravi K. Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension–a research study. J Clin Diagn Res 2016;10:ZC58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Djidja M-C, Claude E, Scriven P et al. Antigen retrieval prior to on-tissue digestion of formalin-fixed paraffin-embedded tumour tissue sections yields oxidation of proline residues. Biochim Biophys Acta, Proteins Proteomics 2017;1865:901–6. [DOI] [PubMed] [Google Scholar]
- 26. Reisler RB, Han C, Burman WJ et al. Grade 4 events are as important as AIDS events in the era of HAART. JAIDS, J Acquired Immune Defic Syndr 2003;34:379–86. [DOI] [PubMed] [Google Scholar]
- 27. Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother 2007;59:342–6. [DOI] [PubMed] [Google Scholar]
- 28. Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol 2009;5:563. [DOI] [PubMed] [Google Scholar]
- 29. Ezhilarasan D, Srilekha M, Raghu R. HAART and hepatotoxicity. J App Pharm Sci 2017;7:220–6. [Google Scholar]
- 30. Oyeyipo IP, Skosana BT, Everson FP et al. Highly active antiretroviral therapy alters sperm parameters and testicular antioxidant status in diet-induced obese rats. Toxicol Res 2018;34:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adana MY, Akang EN, Pete AI et al. Naringenin attenuates highly active antiretroviral therapy-induced sperm DNA fragmentations and testicular toxicity in Sprague-Dawley rats. Andrology 2018;6:166–75. [DOI] [PubMed] [Google Scholar]
- 32. Thamrongwonglert P, Chetchotisakd P, Anunnatsiri S et al. Improvement of lipid profiles when switching from efavirenz to rilpivirine in HIV-infected patients with dyslipidemia. HIV Clin Trials 2016;17:12–6. [DOI] [PubMed] [Google Scholar]
- 33. Nishimura FdCY, Almeida AC, Ratti BA et al. Antioxidant effects of quercetin and naringenin are associated with impaired neutrophil microbicidal activity. Evid Based Complement Alternat Med 2013;795916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Variava E, Sigauke FR, Norman J et al. Late efavirenz-induced ataxia and encephalopathy: a case series. J Acquir Immune Defic Syndr 1999;2017, 75:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ceckova M, Reznicek J, Deutsch B et al. Efavirenz reduces renal excretion of lamivudine in rats by inhibiting organic cation transporters (OCT, Oct) and multidrug and toxin extrusion proteins (MATE, Mate). PLoS One 2018;13:e0202706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mak I, Chmielinska JJ, Spurney CF et al. Combination ART-induced oxidative/Nitrosative stress, neurogenic inflammation and cardiac dysfunction in HIV-1 transgenic (Tg) rats: protection by mg. Int J Mol Sci 2018;19:2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khalil FA, El-Sheikh NM. The effects of dietary Egyptian propolis and bee pollen supplementation against toxicity if sodium fluoride in rats. J Am Sci 2010;11:310–6. [Google Scholar]
- 38. Imam TS, Khalifa HA, Hussein MA et al. Aluminum–induced oxidative stress and hepato-renal impairment in male albino rats: possible protective trial with naringenin. Life Sci J 2016;13: 93–104. [Google Scholar]
- 39. Gesso JL, Kerr JS, Zhang Q et al. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol Nutr Food Res 2015;59:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czechowska G, Celinski K, Korolczuk A et al. Protective effects of melatonin against thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol 2015;66:567–79. [PubMed] [Google Scholar]
- 41. Tanaka Y, Kaibori M, Miki H et al. Alpha-lipoic acid exerts a liver-protective effect in acute liver injury rats. J Surg Res 2015;193:675–83. [DOI] [PubMed] [Google Scholar]
- 42. Oremosu AA, Dosumu O, Owolabi O et al. Cerebellar perturbations of combination antiretroviral therapy (cART): can bioflavonoids help? Nigerian J Neurosci 2018;9:53–9. [Google Scholar]
- 43. Truter D, Chellan N, Strijdom H et al. Histomorphological changes in the pancreas and kidney and histopathological changes in the liver in male Wistar rats on antiretroviral therapy and melatonin treatment. Acta Histochem 2018;120:347–55. [DOI] [PubMed] [Google Scholar]
- 44. Offor U, Naidu EC, Ogedengbe OO et al. Nephrotoxicity and highly active antiretroviral therapy: mitigating action of Momordica charantia. Toxicol Rep 2018;5:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adjene JO, Ajakaye IO, Nosakhare PO. Morphological effects of chronic efavirenz administration on the kidney of adult Wistar rats. Genomic Med, Biomarkers, Health Sci 2011;3:76–80. [Google Scholar]
- 46. Elias A, Obele IR, Nelson B. Toxicological impact of co-treatment with rifampicin and Tenofovir on the renal function of male albino rats. Adv Pharmacol Pharm 2016;4:1–7. [Google Scholar]


