Abstract
The Tepary bean (Phaseolus acutifolius) lectin fraction (TBLF) exhibits differential cytotoxicity on colon cancer cells and inhibition of early tumorigenesis in the colon (50 mg/kg, three times per week, for 6 weeks). TBLF showed low toxicity with the ability to activate the immune system; however, some adverse effects are the loss in body weight gain, intestinal atrophy, and pancreatic hyperplasia. After a recovery period of 2 weeks after treatment, reversion of pancreatic hyperplasia but no recovery of intestinal atrophy was observed. As TBLF has shown anticancer effects on the colon, it is important to characterize the adverse effects and how they can be reversed. Sprague Dawley rats were administered with TBLF (50 mg/kg) for 6 weeks, three times per week, and then allowed to recover for 6 weeks post-treatment. After TBLF administration, small intestine atrophy, villus atrophy, and cryptic hyperplasia were confirmed, as well as increased intestinal mucus production, increased permeability and a decrease in the apparent ileal digestibility of crude proteins. The colon showed damage in the simple prismatic tissue and decreased crypt depth, and changes in microbiota and a decrease in the apparent fecal digestibility of crude protein were determined. Our results show that the adverse effects provoked by TBLF were partially reversed after 6 weeks of recovery post-treatment, suggesting that increasing the recovery period it could be possible to reverse all adverse effects observed.
Keywords: adverse effects, lectins, tepary bean, intestinal integrity, intestinal health, protein digestibility
Introduction
Lectins are mostly glycoproteins of nonimmune origin with the ability to bind specifically and reversibly to membrane carbohydrates [1]. Plant lectins exhibit wide potential in the treatment of digestive system cancer [2]; however, when considering the use of lectins as anticancer therapies, it is necessary to consider that they are antinutritional factors [2–4]. After they transit through the gastrointestinal tract, lectins can bind to intestinal cell membrane carbohydrates, mainly in the duodenum and jejunum, without losing their biological and immune functions. These interactions are considered to be the main antinutritional effect of lectins, since they can provoke damage to the intestinal tissue [5, 6]. Some lectins, such as ricin, abrin, or the lectin from leaves of Myracrodruon urundeuva, can affect the brush border, decrease cell proliferation, and provoke apoptosis and oxidative stress when tested on insects after 48 h of treatment [7]. Similarly, Coprinopsis cinerea lectin 2 (CCL2) causes loss and invaginations or holes in the intestinal membrane in Caenorhabditis elegans (C. elegans) [8]. Soy lectins provoke epithelial hyperplasia but, in high doses, the hyperplasia is replaced by dysplasia [9]. Lectins extracted from beans show reduction of the villi height and the brush edge, increase of crypt depth, and increase of jejunum crypt cell proliferation [10–12]. All of these adverse effects are dose and time dependent [13]. Binding of lectins to the brush border affects cell development, enzyme production, and transport mechanisms, which leads to malabsorption [9, 14], hyperplasia in intestinal mucus-producing cells [9, 15], and increased intestinal permeability [11, 16].
Previous studies have demonstrated the anticancer potential of a lectin fraction from the Tepary bean lectin fraction (TBLF), which exhibits a differential cytotoxic effect [17], as well as inhibition of early malignant lesions in rats [18] when 50 mg/kg of weight is administered every third day for 6 weeks. Good tolerability without systemic toxic effects were observed, and immune system activation was determined after TBLF treatment. Adverse effects include a 10% loss in body weight gain, intestinal villi atrophy, cryptic hypertrophy, and pancreatic acini hyperplasia [10, 19].
Digestive tract damage caused by lectins is widely characterized; however, studies on whether these damages are reversed are based on short administration periods with a fast recovery time, which suggests that recovery time is proportional to exposure [20, 21]. Reversal of pancreatic hyperplasia was observed with 2 weeks of recovery after treatment with TBLF 50 mg/kg administered every third day for 6 weeks [10] but without changes in the intestinal damage. Therefore, this study evaluated the effects of TBLF on intestinal health parameters after 6 weeks of administration every third day and 6 weeks of recovery post-treatment.
Materials and Methods
Extraction of TBLF
Tepary bean was purchased in Hermosillo, Sonora, Mexico and a sample was deposited and identified in the Dr Jerzy Rzedowski herbarium of the Faculty of Natural Sciences, Autonomous University of Querétaro. TBLF extraction was carried out according to the method described by García-Gasca et al. [17].
Food preparation: Standard feed for rats (Rodent Lab Chow 5001, Saint Louis, MO, USA) was heat treated for 15 min at 105°C and 6 psi in order to inactivate food lectins. After cooling to room temperature and grinding in a 2 mm sieve industrial mill, food was subjected to a single screw extruder (CINVESTAV-IPN, Queretaro). Extrusion was done under the following conditions: screw of a compression ratio of 3: 1, with an output matrix 20 × 1.0 × 100 mm; constant flow of 15 g/min; speed of constant screw at 75 rpm and residence time of 20 s inside the extruder; constant temperature in the heating zones of 45, 79, and 104°C, in the area of feeding, mixing/cooking, and output matrix, respectively. The pellets obtained were dried at 40°C for 24 h.
For the digestibility assay, food was cooled to room temperature, ground in a 2 mm sieve industrial mill, pulverized, and 5 g of titanium dioxide per kg was added as a digestibility marker. Two days prior to sacrifice, animals were fed with the titanium dioxide-added food.
In vivo assay: Five-week-old Sprague Dawley rats were purchased at the Institute of Neurobiology, National Autonomous University of Mexico. The animals were placed in individual boxes with water and food ad libitum (Rodent Laboratory Chow 5001, Saint Louis, MO, USA), with a circadian cycle adjusted to 12 h of light/12 h of darkness, at 21–23°C and under 80% relative humidity. The animals were left for 2 weeks in acclimatization before beginning the experiments. The experimental protocols were based on official Mexican standards (NOM-062) and were approved by the Bioethics Committee of the Faculty of Natural Sciences, Autonomous University of Querétaro, México.
After acclimatization, rats were randomized into two groups (n = 12 per group). The control group received 0.5 ml of saline solution and the treated group received 50 mg/kg of TBLF (4000 agglutination units/mg of protein) in 0.5 ml of saline solution by intragastric administration three times per week (Monday, Wednesday, and Friday) for 6 weeks. At the end of the treatment, six rats from each group were sacrificed by decapitation and the other six continued for six more weeks without treatment (recovery period). Feed intake was determined by weighing the food twice a week and the daily average intake was calculated. Rat weights were determined using a three-bar rat scale (Ohaus triple beam balance, NY, USA). To assess protein digestibility, 2 days prior to slaughter, food with digestibility marker was provided and on the day of slaughter, feces and ileal content were collected and frozen at −80°C. Intestines were dissected and weighed using an analytical balance (300 g ± 0.0001 g) (Precisa, XB220A, Dietikon, Switzerland), and small intestine and colon length were determined using a fiberglass tape measure (Seca 201, CDMX, México). The weight and length of the intestines were adjusted to body weight individually and normalized with respect to the control group. A 45 cm portion of the jejunum was placed on a cold base and cut to expose the intestinal mucosa. The mucus was scraped with a metal spatula and placed in plastic tubes where its volume was quantified; the weight was obtained by an analytical balance (300 g ± 0.0001 g) (Precisa, XB220A, Dietikon, Switzerland).
Histopathological and electronic microscopy analyses
A section of each portion of the intestines was fixed in 10% formalin and three different cuts of 0.3 cm were obtained and processed (Histoquinete Leica TP1020, Buffalo Grove, IL, USA). Briefly, cuts of 5 μm were made using a microtome (Leica RM 21, Buffalo Grove, IL, USA) and stained with hematoxylin–eosin for histological analysis by optical microscopy (VE-BC3PLUS Velab, McAllen, TX, USA). IScapture software was used for the analysis, where three cuts were analyzed for each section of the intestine, placed in one lamella, in total six lamellae were analyzed per rat (36 lamellae per group); 40 villi and 40 crypts were analyzed in each section of small intestine and 40 crypts in the colon, using ×10 magnification.
Another section of each portion of the small intestine was fixed in glutaraldehyde 2% and cuts of 0.3 cm were obtained. Samples were dehydrated using an ethanol gradient, drying at the critical point (EM CPD300, Leica; Buffalo Grove, IL, USA), mounted on a pedestal and finely coated with gold (EM ACE200, Leica; Buffalo Grove, IL, USA). For image analysis, the electronic scanning microscope’s own software (EVO-50 Carl Zeiss, Oberkochen, Germany) was used, where four different samples (two samples per pedestal) were analyzed for each section of small intestine (12 samples per group), using ×100 magnification.
Occludin determination by immunohistochemistry
The analyses were performed with 10% formalin fixed jejunum fragments. Cuts of 0.3 cm were processed (Histoquinete Leica TP1020, Buffalo Grove, IL, USA). Cuts of 5 μm were obtained and, to remove the paraffin, the samples were incubated at 60°C for 20 min, hydrated in xylol, 100% alcohol, 96% alcohol, 70% alcohol, 50% alcohol, and finally placed in water. Subsequently, the samples were boiled in citric acid with tween 20 for 20 min, endogenous peroxidases were blocked for 30 min with 3% H2O2 (30%), and then nonspecific sites were blocked using degreased milk for 45 min at room temperature. Tissues were incubated for 24 h at 4°C with the polyclonal antioccludin antibody (Invitrogen, Thermo Fisher Sci. Inc. Waltham, MA, USA) diluted to 1:20. The tissues were placed for 4 h at room temperature with the biotinated goat antirabbit secondary antibody (Santa Cruz, CA, USA), then incubated with the avidin peroxidase complex for 5 min at room temperature. The reaction was performed using 0.01 g of diaminobenzidine in 50 ml of 0.05 M Tris-HCl, pH 7.8, and 100 μl of 30% H2O2 for 10 min. This reaction produced a sepia precipitate in the immunoreactive cells. The samples were contrasted with Harris hematoxylin, dehydrated with 70% alcohol, 96% alcohol, absolute alcohol, absolute xylol, and mounted with resin. Three different sections of the jejunum were analyzed, placed on the same lamella; in total, six lamellae were analyzed per group (one per rat). Samples were photographed using a microscope (VE-BC3PLUS Velab, McAllen, Texas, US) with a 40× augmentation.
Determination of protein digestibility coefficient
Feces and ileal contents were lyophilized and subsequently ground with a 1 mm sieve (Labconco, Kansas City, MO, USA). The protein content and dry matter of feces and food were determined by the AOAC methods 920-152 and 934-01, respectively [20]. To determine the titanium content, the method described by Short et al. [21] was used.
Analysis of microbiota changes
DNA extraction from feces was carried out by a commercial extraction kit (QIAamp DNA mini kit, Qiagen, Hilden, Germany) with silica columns, with some modifications. Briefly, 200 mg of sample was weighed in an Eppendorf tube containing feces from two rats. Homogenization and cell lysis of the samples was performed by adding 1 ml of inhibitEX buffer, vortexed until the sample was homogenized, and incubated at 70°C for 5 min. Subsequently, it was centrifuged for 1 min at 11 200 × g at room temperature and 600 μl of the supernatant was taken and poured into an Eppendorf tube containing 15 μl of proteinase K, 200 μl of AL buffer, and vortexed for 15 s, then the sample was incubated at 70°C for 10 min. At this step, DNA was attached to the silica membrane and 200 μl of ethanol was added, 600 μl of sample was taken, and double centrifugation was performed for 1 min at 11 200 × g at room temperature. Then, two washes were carried out with 500 μl of buffer AW1 and AW2. The sample was centrifuged 1 min with the AW1 buffer and with the AW2 buffer double centrifugation of 1 and 3 min was performed. Finally, 50 μl of AE buffer (elution solution) was added. It was centrifuged 1 min at 11 200 × g. The DNA sample was eluted, purity and integrity were determined by using a Nanodrop 2000c spectrophotometer (Thermo Fisher, Waltham, MA, USA) and by electrophoresis in 0.7% agarose gels at a constant voltage of 70 V for 45 min where 2 μl of each sample was placed with 1 μl of loading buffer (Red gel, Biotium brand). Visualization of the gels was carried out with UV light by means of a photo documenter (Gel doc XR System, BioRad, Singapore).
Figure 5.
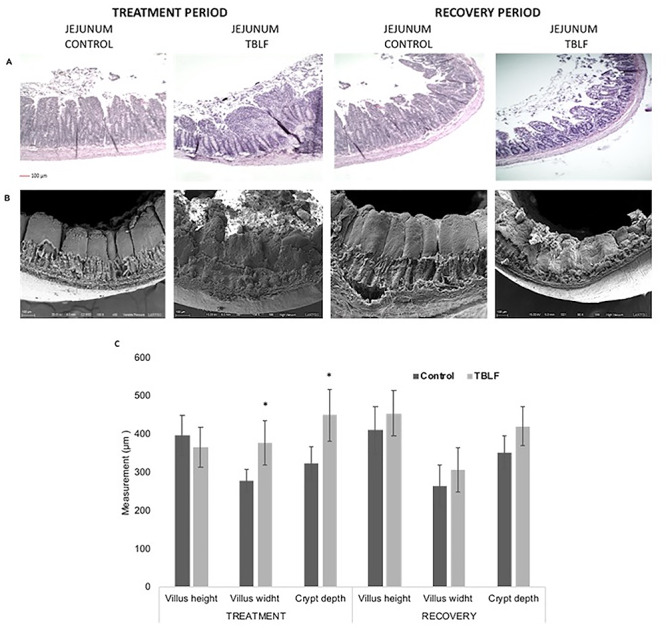
TBLF effect on jejunum. Rats were treated with TBLF (50 mg/kg) for 6 weeks, three times per week and without treatment for a further 6 weeks (recovery). (A) Histological analyses, (B) analyses by scanning electron microscope, (C) histomorphometric test. *Significant difference between control vs treatment (Student’s t-test, P ≤ 0.05).
Identification of microbial diversity was carried out by a modification of the molecular technique of RISA-PCR (Ribosomal InterSpace Analysis). Each reaction contained 10 μl composed of the following elements: 4.38 μl of water, 1 μl of buffer (10×), 1 μl of Bovine serum albumin at 0.2% w/v, 0.8 μl of 2' deoxynucleotides 5' triphosphate (dNTPs), 0.4 μl of sense internal transcriber spacer (ITS) oligonucleotides (5′-GTCGTAACAAGGTAGCCGTA-3), 0.4 μl of antisense ITS (5′-GCCAAGGCATCCACC-3), 0.06 μl of Taq enzyme (TAKARA), and 2 μl of DNA previously extracted from each sample, at a concentration of 5 ng/μl. The amplification cycle was performed in an end point thermocycler (BioRad c1000, Singapore) as described by Cardinale et al. [22]. The initial denaturation temperature was 94°C for 3 min, with 35 cycles of 94°C for 45 s, alignment of 55°C for 1 min, and extension of 72°C for 2 min, the final extension was 72°C for 7 min. Polymerase chain reaction product visualization was performed on 3% agarose gels that were run in a horizontal electrophoresis chamber at a constant voltage of 70 V for 90 min. Five μl of each sample was placed with 2 μl of loading buffer (Red gel, Biotium brand) and visualization was carried out with UV light using a photo documenter (Gel doc XR System, BioRad, Singapore). The analysis of the gel bands was conducted with the Quantity One program, in which a band profile and a binary matrix were obtained and analyzed in Phylip version 3.6 software to make the comparison between treatments. The phylogenetic analysis was performed by the UPGMA algorithm (Unweighted Pair Group Method using Arithmetic averages).
Statistical analysis
Data is expressed as the mean ± standard deviation. The Statistical Package for Social Sciences (SPSS) version 18 software was used. Multilevel regressions were performed for variables grouped or nested per subject over time. Student’s t-tests were performed for independent samples in order to compare control rats with treated rats, and treated rats against the remainder. The analysis for microbiota changes was made by phylogenetic tree and Quantity One and Phylip version 3.6 software was used. A value of P ≤ 0.05 was considered statistically significant.
Results and Discussion
Effect of TBLF on growth
Previous studies showed that 2 weeks is not sufficient for the recovery of the intestinal damage after TBLF treatment [10]. Some studies [23, 24] suggest that the damage reversal is proportional to the treatment time, so it was decided to increase the recovery time to 6 weeks. TBLF administration did not affect the growth (multilevel regression P = 0.508) or feed consumption (multilevel regression P = 0.593) of the treated rats with respect to control rats (Fig. 1). These results are inconsistent with those reported for other lectins, where treated rats reported loss in weight gain and growth retardation [5, 15, 25–29]. Our previous studies showed that TBLF exhibited a 10% loss in weight gain with respect to control in adult rats [19]. However, in young rats there were no significant differences in weight gain [10], which is consistent with our results, suggesting that the TBLF effect is dependent on age.
Figure 1.

Tepary bean (Phaseolus acutifolius) lectin fraction (TBLF) effect on corporal weight and feed intake. After 2 weeks of acclimatization, rats were treated with TBLF (50 mg/kg) for 6 weeks every third day (treatment) and without treatment for a further 4 weeks (recovery). (A) TBLF effect on rat weight, (B) TBLF administration effect on food consumption. Black arrows indicate the beginning of the TBLF administration, blue arrows indicate sacrifices at 6 weeks after the beginning of the treatment and 6 weeks of recovery after the treatment.
Effect on intestinal mucus production
After treatment, mucus production was increased in TBLF-treated rats, where intestinal mucus of control rats was of 0.9 ± 0.14 g and for the TBLF-treated group was 1.46 ± 0.32 g, which represents a significant increase (P = 0.003) of 62% (Fig. 2). The intestinal mucus of the group treated with TBLF presented a viscous consistency to the touch, as well as a coloration between strong yellow and brown, whereas in the control group the consistency of the intestinal mucus was less viscous with a slight yellow coloration. This increase is considered a defense mechanism against lectins, since epithelial cells are the key piece in the immune system. Given their ubication and contact with intestinal lumen, it allows them to initially recognize foreign molecules and generate a signal that is transmitted to the immune cells [30, 31]. Several studies conducted in murine models have reported a proinflammatory response after administration of some plant lectins [32, 33].
Figure 2.

TBLF effect on intestinal mucus. Rats were treated with TBLF (50 mg/kg) for 6 weeks three times per week and without treatment for a further 6 weeks (recovery period). (A) Intestinal mucus production. (B) Intestinal mucus color changes between control and treated groups. *Significant difference between control and treatment groups. &Significant difference between groups during and after TBLF treatment (Student’s t-test, P ≤ 0.05).
When the administration of TBLF stopped, the stimulus disappeared and the mucus production became normalized, as can be seen after the recovery period of 6 weeks, where there were no differences (P = 0.517) between the control and the treated groups, 0.56 ± 0.08 g and 0.6 ± 0.12 g, respectively. The physical changes were not perceived, suggesting reversion of the damage caused by TBLF administration.
Effect of TBLF administration on intestinal integrity
No differences in small intestine and colon weight and length were observed between treated and untreated rats (data not shown). Macroscopically, intestine thinning was observed in treated rats; however, after the recovery period, differences were imperceptible (Fig. 3).
Figure 3.

TBLF effect on duodenum. Rats were treated with TBLF (50 mg/kg) for 6 weeks, three times per week and without treatment for a further 6 weeks (recovery). C, control group; T, treated group.
Figures 4–6 show the histomorphometric tests of the three sections of the small intestine. A significant decrease (P = 0.001) in the duodenal villi height of 24% in TBLF-treated rats (376.69 ± 34.9 μm) with respect to control rats (495.9 ± 59 μm) was observed; for the jejunum and the ileum, no significant differences were observed (P = 0.202 and P = 0.214, respectively). Regarding the villi width, we observed a significant increase of 63% (P < 0.001) in the duodenum for the TBLF-treated group (389.3 ± 37.5 μm) with respect to the control group (237.5 ± 49.3 μm). A significant increase of 35% was observed for the jejunum (P = 0.005) and the ileum (P = 0.001). Our results suggest a villi fusion, with a triangular aspect (wide at the base and thin at the apical part). After the 6-week recovery period, a partial recuperation was observed where no significant differences were observed for villi height with respect to control rats.
Figure 4.

TBLF effect on duodenum. Rats were treated with TBLF (50 mg/kg) for 6 weeks, three times per week and without treatment for a further 6 weeks (recovery period). (A) Histological analyses, (B) analyses by scanning electron microscope, (C) histomorphometric test. *Significant difference between control vs treatment, and &significant difference between TBLF treatment and the recovery period (Student’s t-test, P ≤ 0.05).
Figure 6.

TBLF effect on ileum. Rats were treated with TBLF (50 mg/kg) for 6 weeks, three times per week and without treatment for a further 6 weeks (recovery). (A) Histological analyses, (B) analyses by scanning electron microscope, (C) histomorphometric test. *Significant difference between control vs treatment (Student’s t-test, P ≤ 0.05).
These results agree with data reported for other lectins, such as red bean lectin, that decreased the villi height considerably with respect to the control, whereas the crypt depth was increased [11]. Soy agglutinin provoked epithelial hyperplasia evidenced by the discontinuity of the epithelial villi and the agglomeration of cells, when moderate doses were given, whereas in high doses dysplasia replaced hyperplasia, given by a continuous epithelium, but with disordered layers of immature cells [9]. Lectins extracted from beans caused the reduction of brush border cells, and increased the population of villi enterocytes and proliferation of cells in the jejunum crypts [12]; small bowel hyperplasia was dose and time dependent, resulting in tissue elongation and thickening of the intestinal walls [13]. However, there are lectins that provoke serious effects, such as Myracrodruon urundeuva leaf lectin, which causes the brush edge to be swept in insects 48 h after administration [7] or CCL2, that provokes loss of villi, and invaginations or orifices after some hours in C. elegans [8].
After the 6-week treatment with TBLF, the duodenum showed a marked Lieberkühn crypt hypertrophy between TBLF-treated rats (356.7 ± 66.8 μm) and control rats (219.7 ± 49.2 μm), with a significant increase of crypt depth of 62% (P = 0.008). The jejunum showed an increase of 38% (P = 0.003) with crypt depth of 450 ± 67 μm for the TBLF treated rats and 324.83 ± 42.4 μm for the control rats; ileum crypt depths were 404 ± 45 μm for TBLF-treated rats compared to 300 ± 49.1 μm for control rats, with an increase of 34% (P = 0.004). These results suggest moderate damage and it was even possible to observe functional crypts; however, a negative crypt–villus relationship was observed during the treatment.
After the 6-week recovery period, only the depth of the duodenal crypts of the treated group remained increased (349.9 ± 59.6 μm) with respect to the control group 217.1 9 ± 37.9 μm, showing an increase of 47% (P = 0.42). The results suggest that, given the duodenum length and the villi atrophy, the crypts increased their depth as a compensatory mechanism to increase cell proliferation, and thus be able to carry out a rapid exchange of the atrophied villi. This has been previously reported in the case of peanut agglutinin and phytohematoglutinin, that caused increases in gastric fund cell proliferation of 90% and 166%, respectively [34, 35].
When comparing the results of TBLF administration and the postadministration recovery period, a decrease of 28% (P = 0.001) of the duodenal villi width was observed. This result suggests a recovery of the damage provoked by TBLF, unlike that observed by Alatorre-Cruz et al. [10], where it was not possible to observe changes after 2 weeks of recovery. The results suggest that the recovery time depends on the level of damage caused since, when the effect has been studied after the single administration of lectins, recovery is achieved in a few hours [23, 24].
In the case of the colon (Fig. 7), damage to the simple prismatic epithelium after TBLF treatment was observed, which is related to the decrease in the Lieberkühn crypt depth throughout the entire colon. In the ascending colon, the treated group presented a depth of 436 ± 34 μm, whereas the control group presented a depth of 526.2 ± 37 μm, which represents a decrease of 18% (P = 0.001). For the transverse section, the depth was 529.1 ± 33.6 μm and 619.3 ± 37.3 μm for the treated and control groups, respectively. In the descending colon, depths were 503.8 ± 35 μm for the treated group and 588.7 ± 34.5 μm for the control group, representing a decrease of 17% for both the transverse colon (P = 0.002) and the descending colon (P = 0.004). After the recovery period, slight damages were observed in the simple prismatic epithelium; however, no significant changes were observed regarding the depth of the crypts, suggesting the recovery of the damage caused by TBLF, which was not possible to observe with 2 weeks of recovery [10].
Figure 7.

TBLF effect on colon. Rats were treated with TBLF (50 mg/kg) for 6 weeks, three time per week and without treatment for a further 6 weeks (recovery). (A) Histological analyses, (B) histomorphometric test. *Significant difference between control vs treatment (Student’s t-test, P ≤ 0.05).
Membrane permeability was evaluated by occludin determination. The narrow junction is given by transmembrane proteins such as occludin or claudins, and peripheral membrane proteins such as occlusion zones. When evaluating the presence of occludin, a decrease was observed in the TBLF-treated group for 6 weeks with respect to the control, observed as a loss of continuity of the membrane occludin (Fig. 8).
Figure 8.

TBLF effect on intestinal permeability. Rats were treated with TBLF (50 mg/kg) for 6 weeks three times per week and without treatment for a further 6 weeks (recovery). Occludin was determined by immunohistochemistry. Red arrows show membrane continuity of occludin. Images were taken at ×40 augmentation.
These results agree with observations for other lectins where it has been observed that after binding of lectins to epithelial cells, the expression of occludin and ZO-1 is reduced, which decreases the cross-linking of proteins and increases the permeability of the membrane, allowing the absorption of lectins, peptides, and bacteria, and provoking detrimental effects on the immune system and some other organs [16, 36]. However, after the recovery period, the TBLF administered group presented partial recovery of the occludin location, suggesting recovery of membrane permeability.
Effect of TBLF administration on protein digestibility
With regard to the apparent digestibility of proteins, apparent ileal (AIDCP) and fecal (AFDCP) crude protein digestibility was determined. It was possible to observe a decrease of 4.9% (P = 0.007) and 10.9% (P = 0.001), respectively, during the 6-week treatment with respect to the control group (Fig. 9). Such results are related with intestinal atrophy since the absorption surface is reduced and the increase in Lieberkühn crypt depth causes an increase in cell proliferation and, as a consequence, some cells do not reach the necessary maturity to carry out the right absorption of nutrients. These results are similar to those previously reported after administration of soy lectin where an increase in nitrogen loss was observed [29], in addition to a decrease in the digestibility of proteins [37, 38].
Figure 9.
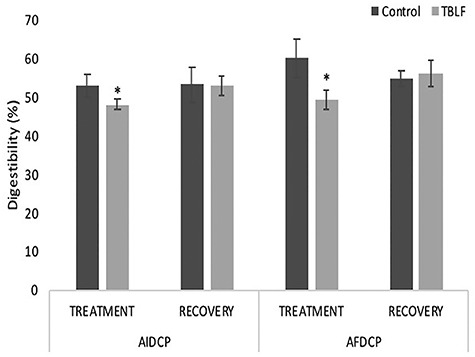
TBLF effect on digestibility percentage. Rats were treated with TBLF (50 mg/kg) for 6 weeks three times per week and without treatment for a further 6 weeks (recovery). *Significant difference between groups (Student’s t-test, P ≤ 0.05).
AFDCP corresponds to the fermentation of nitrogen compounds in the chyme by the colonic microbiota which contributes to digestibility. The decrease in AFDCP is perhaps related to the atrophy of the Lieberkühn crypts, the decrease in some parts of the simple prismatic epithelium, and the changes in the intestinal microbiota. After recovery, no differences were observed for AIDCP and AFDCP between groups. These results suggest functional recovery that is related to intestinal structures and to the decrease in the production of intestinal mucus, so nutrient absorption is normalized.
Effect of TBLF administration on microbiota
The microbiota can be modified by different factors, including age. This change was observed in the control group between the different weeks of experimentation. Treatment with TBLF changed the microbiota profile and a different cluster was formed (Fig. 10). Two well-differentiated groups were observed for nontreated rats and TBLF-treated rats. It has been observed that common bean lectins induce Escherichia coli growth, that was suppressed by administering isolated lectins of mannose specific monocotyledons; however, the adverse effects on the small intestine epithelium were not modified [35]. Some lactic bacteria, considered probiotics, can bind to legume lectins, reducing their adverse effects [39]. Another mechanism that can explain the changes in the microbiota is the production of short-chain fatty acids. Diets supplemented with legume lectins increase the content of volatile acids in the intestine, indicated by increased intestinal microbial activity [40].
Figure 10.
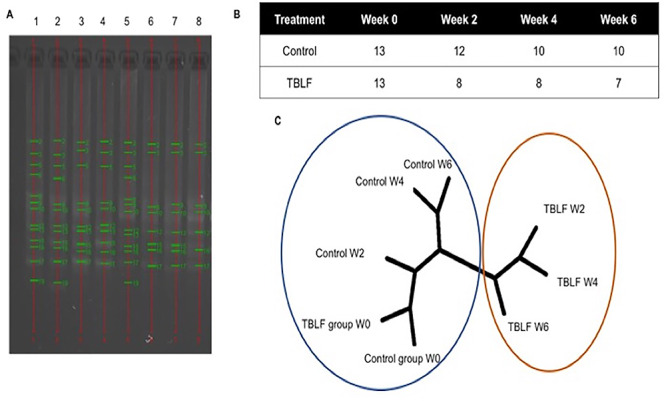
Effect of TBLF on fecal microbiota. (A) Bacterial DNA electrophoretic profile of feces pools per week. (1) Control rats at Week 0; (2) control rats at Week 2; (3) control rats at Week 4; (4) control rats at Week 6; (5) TBLF at Week 0; (6) TBLF at Week 2; (7) TBLF at Week 4; (8) TBLF at Week 6. (B) Number of bands observed per treatment and per week. (C) Dendogram (UPGMA algorithm) of the bacterial band profiles by weeks. The numbers followed by the group name indicate the week evaluated based on treatment with TBLF (PHYLIP 3.6). Each cluster is marked by color circles.
In summary, TBLF interaction with the intestinal epithelium causes an increase in intestinal mucus production, suggesting an inflammatory process. The small intestine exhibited villi atrophy and crypt hyperplasia, whereas the duodenum was the most affected. The colon showed decrease in crypt depth, damage to the simple prismatic epithelium, and changes in microbiota, which contributes to the decrease of the apparent ileal and fecal digestibility of crude proteins. After a period of 6 weeks of recovery, normal intestinal structures were largely observed, the intestinal permeability was partially recovered, and the production of intestinal mucus was normalized, as well as the apparent fecal and ileal digestibility of crude protein. These results suggest a recovery of intestinal health, which indicates that the adverse effects of TBLF on the intestinal tract are time-dependent reversible.
Conclusions
Previous studies have shown that TBLF has an inhibitory effect in precancerous lesions in rats. In addition, typical antinutritional side effects for lectins have been observed, such as weight loss, intestinal villi atrophy, Lieberkühn crypt hyperplasia, and increased lymphoid follicles of Peyer’s patches. Intragastric administration of TBLF 50 mg/kg provoked increase in mucus production and intestinal damage, particularly in the duodenum, with a partial recovery after a recovery of 6 weeks that were not observed before, where only 2 weeks of recovery period was tested. In addition, intestinal permeability, where a considerable increase after TBLF treatment was determined, showed a partial recovery. Apparent ileal and fecal digestibility of raw proteins fully recovered after the 6-week recovery period. General microbiota changes were observed after TBLF treatment, which could be related with the intestinal alterations. Our results show that the adverse effects of TBLF on the intestinal tract were temporary and normal conditions were partially or fully recovered after 6 weeks, suggesting that full recovery is a function of the recovery period after treatment. It will be necessary in further studies to try longer recovery periods in order to achieve total reversion of the adverse effects, as part of the study of the potential of Tepary bean lectins against colon cancer.
Acknowledgments
We thank to Veronica Andrade Portillo for the technical support. This work received financial support from Consejo Nacional de Ciencia y Tecnología (CONACYT) Ciencia Básica (CB-2014-01-241181), Fondo de Fomento a la Investigación de la UAQ (FOFI-UAQ) y Fondo de Proyectos Especiales de Rectoría (FOPER). We also thank CONACYT for the fellowship to WPL.
Contributor Information
Wendoline Pita-López, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Mery Gomez-Garay, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Alejandro Blanco-Labra, Laboratory of Plant Defense Mechanisms, CINVESTAV-Irapuato, 36824, Irapuato, Mexico.
Araceli Aguilera-Barreyro, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Tércia C Reis-de Souza, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Andrea Olvera-Ramírez, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Roberto Ferriz-Martinez, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Teresa García-Gasca, Faculty of Natural Sciences, Autonomous University of Querétaro, 76010, Querétaro, Mexico.
Conflicts of interest statement
The authors declare no conflict of interest.
References
- 1. Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 2004;14:53–62. [DOI] [PubMed] [Google Scholar]
- 2. Estrada-Martínez LE, Moreno-Celis U, Cervantes-Jiménez R et al. Plant Lectins as medical tools against digestive system cancers. Int J Mol Sci 2017;18:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. González de Mejía E, Prisecaru V. Lectins as bioactive plant proteins: a potencial in cancer treatment. Crit Rev Food Sci Nutr 2005;45:425–45. [DOI] [PubMed] [Google Scholar]
- 4. Castillo-Villanueva A, Abdullaev F. Lectinas vegetales y sus efectos en el cancer. Rev Invest Clin 2005;57:55–64. [PubMed] [Google Scholar]
- 5. Lajolo F, Genovese M. Nutricional significance of lectins and enzyme inhibitors from legumes. J Agric Food Chem 2002;50:6592–8. [DOI] [PubMed] [Google Scholar]
- 6. Rhodes JM. Beans means lectins. Gut 1999;44:593–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lima TA, Fernandes KM, Oliveira APS et al. Termiticidal lectins from Myracrodruon urundeuva (Anacardiaceae) cause midgut damage when ingested by Nasutitermes corniger (Isoptera: Termitidae) workers. Pest Manag Sci 2017;73:991–8. [DOI] [PubMed] [Google Scholar]
- 8. Stutz K, Kaech A, Aebi M et al. Disruption of the C. elegans intestinal brush border by the fungal Lectin CCL2 Phenocopies dietary Lectin toxicity in mammals. PLoS ONE 2015. doi: 10.1371/journal.pone.0129381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casaubon-Huguenn MA, Villa-Gonzalez EA, Vazquez-Pelaez C et al. The effect of raw full-fat soybean and its Lectin on the nutrition and pigmentation of broilers. J Agric Food Chem 2004;52:5702–8. [DOI] [PubMed] [Google Scholar]
- 10. Alatorre-Cruz JM, Pita-López W, López-Reyes RG et al. Effects of intragastrically-administered Tepary bean lectins on digestive and immune organs: preclinical evaluation. Toxicol Rep 2018;5:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radberg K, Biernat M, Linderoth A et al. Enteral exposure to crude red kidney bean lectin induces maturation of the gut in suckling pigs. J Anim Sci 2001;79:2669–78. [DOI] [PubMed] [Google Scholar]
- 12. Zucoloto S, Scaramello AC, Lajolo FM, et al. Effect of oral phytohemagglutinin intake on cell adaptation in the epithelium of the small intestine of the rat. Int J Exp Pathol 1991;72:41–5. [PMC free article] [PubMed] [Google Scholar]
- 13. Bardocz S, Grant G, Ewen SWB et al. Reversible effect of phytohemagglutinin on the growth and metabolism of rat gastrointestinal tract. Gut 1995;37:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meanz D, Irish G, Classen H. Carbohydrates-binding and agglutinating lectins in raw and processed soybean meals. Anim Feed Sci Technol 1999;76:335–43. [Google Scholar]
- 15. Pusztai A, Bardocz S. Biological effects of plant lectins on the gastrointestinal tract: metabolic consequences and applications. Trends Glycosci Glyc 1996;8:149–65. [Google Scholar]
- 16. Zhao Y, Qin G, Sun Z et al. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int J Mol Sci 2011;12:8502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García-Gasca T, García-Cruz M, Hernandez-Rivera E et al. Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and Semipure Lectin fractions on cancer cells. Nut and Cancer 2012;64:1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreno-Celis U, López-Martínez J, Blanco-Labra A et al. Phaseolus acutifolius Lectin fractions exhibit apoptotic effects on colon cancer: preclinical studies using Dimethilhydrazine or Azoxi-methane as cancer induction agents. Molecules 2017;22. doi: 10.3390/molecules22101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferriz-Martinez R, Garcia-Garcia K, Torres-Arteaga I et al. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol Rep 2015;2:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AOAC Official methods of analysis, 15th edn. Arlington, VA, USA: Association of Official Analytical Chemists, 1990. [Google Scholar]
- 21. Short FJ, Gorton P, Wiseman J, et al. Determination of titanium dioxide as an inert marker in chicken digestibility studies. Anim Feed Sci Tech 1996;59:215–21. [Google Scholar]
- 22. Cardinale B, Inchausti P. Effects of species diversity on the primary productivity of ecosystems: extending our spatial and temporal scales of inference. Oikos 2004;104:437–50. [Google Scholar]
- 23. King TP, Pusztai A, Clark EMW. Kidney bean (Phaseolus vulgaris) lectin induced lesions in rat small intestine: light microscope studies. J Comp Path 1980;90:585–95. [DOI] [PubMed] [Google Scholar]
- 24. Weinman MD, Allan CH, Trier JS, et al. Repair of microvilli in the rat small intestine after damage with lectins contained in the red kidney bean. Gastroenterol 1989;97:1193–204. [DOI] [PubMed] [Google Scholar]
- 25. Le Gall M, Quillien L, Guéguen J et al. Identification of dietary and endogenous ileal protein losses in pigs by immunoblotting and mass spectrometry. J Nutr 2005;135:1215–22. [DOI] [PubMed] [Google Scholar]
- 26. Czerwinski JH, Leontowicz M, Gralak MA. Response of rats to a moderate intake of soybean lectin. J Anim Feed Sci 2005;14:537–40. [Google Scholar]
- 27. De Dios A, Porrilla YP, Chaparro DC. Factores antinutricionales en Semillas. Bio Agro 2009;7:45–54. [Google Scholar]
- 28. Qin G, Elst ER, Bosch MW, Van der Poe AFB. Thermal processing of whole soy beans: studies on the inactivation of antinutritional factors and effects on ileal digestibility in piglets. Anim Feed Sci Technol 1996;57:313–24. [Google Scholar]
- 29. Li Z, Li D, Qiao S et al. Anti-nutritional effects of a moderate dose of soybean agglutinin in the rat. Arch Anim Nut 2003;57:267–77. [DOI] [PubMed] [Google Scholar]
- 30. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–7. [DOI] [PubMed] [Google Scholar]
- 31. Guarner F. Papel de la flora intestinal en la salud y en la enfermedad. Nutr Hosp 2007;22:14–9. [PubMed] [Google Scholar]
- 32. Rougé P, Culerrier R, Granier C et al. Characterization of IgE-binding epitopes of peanut (Arachis hypogaea) PNA lectin allergen cross-reacting with other structurally related legume lectins. Mol Immunol 2010;47:2359–66. [DOI] [PubMed] [Google Scholar]
- 33. Kumar S, Verma AK, Sharma A et al. Clinical complications of kidney bean (Phaseolus vulgaris L.) consumption. Proteomics 2013;93:50–64. [DOI] [PubMed] [Google Scholar]
- 34. Jordinson M, Goodland R, Rbrynes A et al. Gastrointestinal responses to a panel of lectins in rats maintained on total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 1999;246:1253–42. [DOI] [PubMed] [Google Scholar]
- 35. Pusztai A, Grant G, Spencer RJ et al. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J Appl Bacteriol 1993;75:360–8. [DOI] [PubMed] [Google Scholar]
- 36. Schulze H, Saini HS, Huisman J et al. Increased nitrogen secretion by inclusion of soya lectin in the diets of pigs. J Sci Food Agric 1995;69:501–10. [Google Scholar]
- 37. Qin GX, Poult P. The anti-nutritional factors in the feed and their eliminating methods. Anim Feed Sci Technol 2003;23:10–3. [Google Scholar]
- 38. Gu C, Pan H, Sun Z, et al. Effect of soybean variety on anti-nutritional factors content, and growth performance and nutrients metabolism in rat. Int J Mol Sci 2010;11:1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Badot JD, Argañaraz-Martínez E, Lorenzo-Pisarello MJ et al. Cytotoxic damage of soybean agglutinin on intestinal epithelial cell of broiler chicks: in vitro protection by Bifidobacterium infantis CRL 1395. FEMS Microbiol Lett 2016;363:1–7. [DOI] [PubMed] [Google Scholar]
- 40. Pellegrina CD, Perbellini O, Scupoli MT et al. Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol Appl Pharmacol 2009;237:146–53. [DOI] [PubMed] [Google Scholar]


