Abstract
Aims
The purpose of our study was to determine whether mesenchymal stem cells (MSCs) are an effective and safe therapeutic agent for the treatment of knee osteoarthritis (OA), owing to their cartilage regeneration potential.
Methods
We searched PubMed, Embase, and the Cochrane Library, with keywords including “knee osteoarthritis” and “mesenchymal stem cells”, up to June 2019. We selected randomized controlled trials (RCTs) that explored the use of MSCs to treat knee OA. The visual analogue scale (VAS), Western Ontario and McMaster University Osteoarthritis Index (WOMAC), adverse events, and the whole-organ MRI score (WORMS) were used as the primary evaluation tools in the studies. Our meta-analysis included a subgroup analysis of cell dose and cell source.
Results
Seven trials evaluating 256 patients were included in the meta-analysis. MSC treatment significantly improved the VAS (mean difference (MD), –13.24; 95% confidence intervals (CIs) –23.28 to –3.20, p = 0.010) and WOMAC (MD, –7.22; 95% CI –12.97 to –1.47, p = 0.010). The low-dose group with less than 30 million cells showed lower p-values for both the VAS and WOMAC. Adipose and umbilical cord–derived stem cells also had lower p-values for pain scores than those derived from bone marrow.
Conclusion
Overall, MSC-based cell therapy is a relatively safe treatment that holds great potential for OA, evidenced by a positive effect on pain and knee function. Using low-dose (25 million) and adipose-derived stem cells is likely to achieve better results, but further research is needed.
Cite this article: Bone Joint Res 2020;9(10):719–728.
Keywords: Knee osteoarthritis, Mesenchymal stem cells, Cell dose, Cell source
Article focus
To evaluate the efficacy of mesenchymal stem cell (MSC) application in the treatment of patients with knee osteoarthritis (OA), as well as the most effective source and number of stem cells.
Key messages
The treatment of knee OA presents a major challenge.
MSC-based stem cell therapy can achieve substantial benefits and reduce treatment costs, but many problems still need to be addressed.
Strengths and limitations
Unlike other meta-analyses, this article summarized the effect of MSC injection on knee arthritis in the elderly, clinically accounting for the largest and most difficult-to-treat group.
Our study focused on randomized controlled trials (RCTs) only, and all patients received MSC injections with no other treatments during the same period, avoiding the influence of other factors; we also evaluated the effects of cell dose and cell source on the treatment outcomes, which, to our knowledge, is an area that has not been previously explored.
The preparation method varied between studies, which may have affected the analysis results.
Introduction
Knee osteoarthritis (OA) is a common disease, causing pain and limited mobility in patients, which seriously affects patients' daily lives.1,2 It is characterized in particular by the degeneration of the joint, causing loss of cartilage. The synovial membrane or other joint components may also be damaged.3 Depending on the severity of knee OA, various medications - such as glucosamine, nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular injection of hyaluronic acid, or platelet-rich plasma (PRP) - are applied.4 These treatments have been proven to have modest clinical benefits, but the long-term results of these treatments are poor, and most patients eventually choose knee arthroplasty.5,6
At present, the irreversible damage of cartilage is the most difficult problem in the treatment of OA and tissue engineering is considered to be an innovative and promising therapy for OA.7,8 Among various cell therapies, mesenchymal stem cell (MSC) therapy appears to hold promise.9–12 MSCs have the ability to differentiate into different cell types, including bone, cartilage, and adipose cells. Additionally, MSCs are immunoprivileged owing to their low immunogenicity. They can improve the local microenvironment of the joint cavity by regulating the inflammatory response, and producing cell growth factors, which in turn facilitates tissue repair and induces cartilage regeneration.13,14
Many clinical studies have been conducted on MSC therapy for OA. Adjunct therapy after high tibial osteotomy and partial meniscus resection have also achieved encouraging results.15,16 However, adjuvant surgery and the addition of PRP may lead to ambiguous conclusions about the efficacy of knee OA with MSCs, so we excluded these from this study.17 Furthermore, the source and dosage of MSCs for knee OA remain unclear, so they need to be standardized. Bone marrow is the most common source of MSCs and has a high potential for cartilage formation. However, adipose tissue is easier to obtain, and researchers are increasingly interested in adipose-derived MSCs.18,19 Although the use of stem cells for knee OA is becoming increasingly prevalent, the number of cells delivered has not yet been identified. This article focuses on comparing cell dosages and the most effective cell source for treating knee OA, with the aim of providing guidance for future clinical treatment.
Methods
Search strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews. Relevant studies were identified by searching PubMed, Embase, and the Cochrane Library (to June 2019). The following search terms were used, alone or in combination: “knee Osteoarthritides” or “knee Osteoarthritis” and “Mesenchymal Stromal Cell” or “Mesenchymal Stem Cells” or “Mesenchymal Progenitor Cells” or “Wharton Jelly Cells.” We did not impose any language restrictions on our search.
Eligibility criteria
We reviewed all the retrieved abstracts and full texts. The inclusion criteria for this analysis were as follows: 1) RCTs of MSCs for knee OA; 2) detailed information of patients reported before and after treatment; 3) transplanted stem cells limited to MSCs without PRP; and 4) knee OA patients with no recent surgery conducted.
Quality assessment
The data we extracted included the first author's name, year of publication and country, sample size per group, mean patient age, OA grade, follow-up time and dose administered by MSCs, the control compositions, and the study results. We followed the guidance established by the Cochrane Collaboration to conduct a risk assessment of bias to measure the risk of systematic errors. To assess risk of bias and provide a summary chart of risk assessments, we used: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective reporting.20,21
Outcome measures
The primary goal of this study was to determine whether pain and joint function could be improved after MSC injection therapy and whether damaged cartilage could heal. Pain improvement was defined as mean change in visual analogue scale (VAS) from baseline and mean change in Western Ontario and McMaster University Osteoarthritis Index (WOMAC)22 pain from baseline.23 The mean change in WOMAC was the primary criterion for functional change, and the secondary outcome measure was the change in some other scoring indicator. We also performed a subgroup analysis of cell dose and source, with low doses of less than 30 million, high doses of 50 million or more, and medium doses. The bone marrow-derived stem cells comprised one group and the stem cells from other sources comprised the other. Autologous and allogeneic stem cells were divided into two groups.https://en.wikipedia.org/wiki/Magnetic_resonance_imagingMRI was found to be the best means of observing cartilage in the study. The whole-organ MRI score (WORMS) scale,24 area of cartilage, and cartilage signal changes were all taken into account. Although other scoring indicators such as the 36-ItemShort-Form Health Survey questionnaire (SF-36)25 were used, few articles included such indicators, so they were not used in our evaluation.
Statistical analysis
In this meta-analysis, RevMan 5.3 software (Nordic Cochran Centre, Copenhagen, Denmark) was used to compare MSC-treated groups with their respective control groups. We used the inverse variance method for continuous data collection and the Mantel–Haenszel method for binary data. The effects of MSC treatment were reflected by the mean difference (MD) and 95% confidence interval (CI). The chi-squared Q test (p-value less than 0.10 for high heterogeneity) and the I2 statistic (value 50% or higher for high heterogeneity) were used to assess heterogeneity in the assay. The results were summarized with random-effect models and all p-values < 0.05 were considered statistically significant.
Results
In total, we identified 410 articles through the search of the databases, and then removed any duplicates. After review of the abstracts and full texts, seven articles remained, involving 256 patients (Figure 1).26–32
Fig. 1.
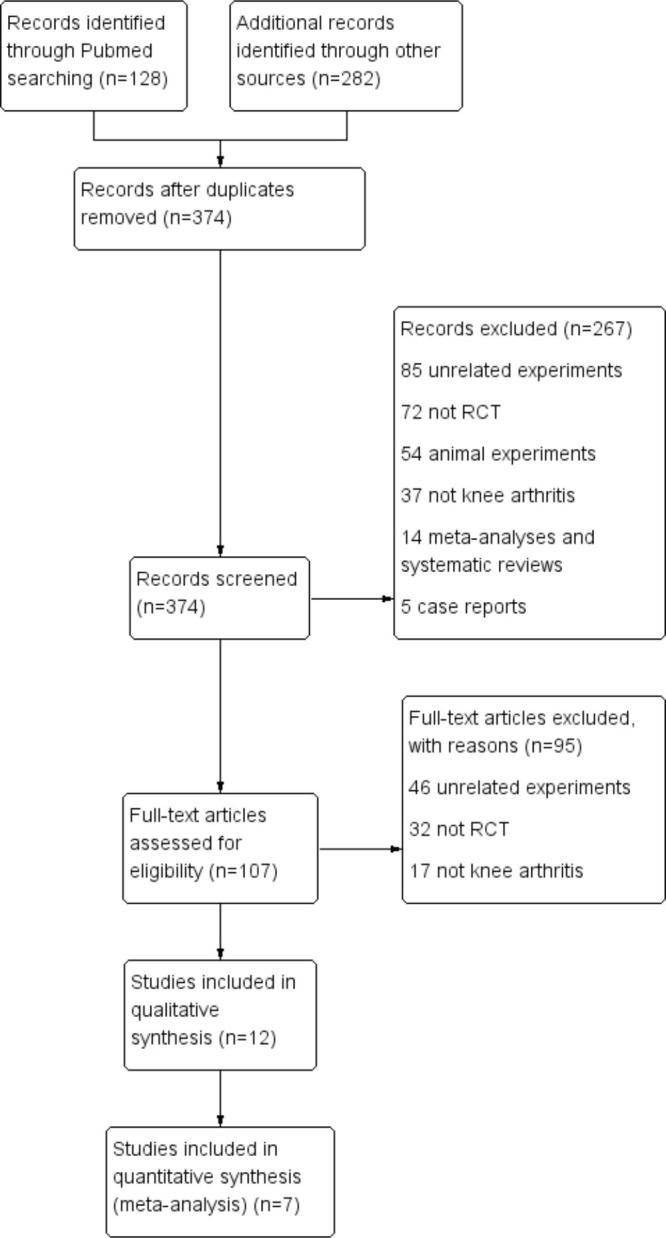
Flow diagram showing the process of inclusion and exclusion. RCT, randomized controlled trial.
The seven studies were published between 2015 and 2019, comprising a total of 151 patients in the trial group and 105 patients in the control group. The mean age range of the enrolled patients was 53 to 61 years. The majority of patients in the trials were grade 2 to 3 of knee OA. Three studies compared MSCs with placebo,26–28 and the remaining studies compared an MSC group with a control group receiving hyaluronic acid.29–32 MSCs were mainly derived from bone marrow, except for two studies derived from adipose tissue28,31 and one study derived from umbilical cord.32 Three trials used autologous cells,26,29,31 and another four trials used allogeneic cells.27,28,30,32 The patients received cell infusions ranging from 3.9 × 106 to 1.5 × 108 cells. All experiments were direct injections and followed up for at least six months (Table I).
Table I.
Trials included in the meta-analysis.
| Source (country) |
Publication year | Mean age, yrs (SD) | Mean BMI, kg/m2 (SD) | Male sex, n | Patients, n (control) | Control arm (stem cell arm) |
Regimen dose, number of cells | Follow-up, mths | OA K-L grade33 | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|---|
| Emadedin et al (Iran)26 | 2018 | 53 (7) | 31 (5) | 27 | 43 (24) | Placebo (BMSCs) (autologous) | 40 million | 3,6 | 2 to 4 | VAS, WOMAC22 |
| Gupta et al (India)27 |
2016 | 56 (7) | 28 (4) | 15 | 60 (20) | Placebo (BMSCs + 2 ml hyaluronic acid) (allogenic) |
25, 50, 75, or 150 million | 1,3,6,12 | 2 to 3 | VAS, WOMAC, WORMS (MRI), AE |
| Kuah et al (Australia)28 |
2018 | 53 (7) | 27 (3) | 12 | 20 (4) | Placebo (ADSCs) (allogenic) |
3.9, 6.7 million | 12 | 1 to 3 | VAS, WOMAC, MRI, AE |
| Lamo-Espinosa et al (Spain)29 |
2016 | 61 (4) | 28 (2) | 19 | 30 (10) | Hyaluronic acid (BMSCs + 4 ml hyaluronic acid) (autologous) | 10, 100 million | 3,6,12 | 2 to 4 | VAS, WOMAC, WORMS (MRI) |
| Vega et al (Spain)30 |
2015 | 57 (9) | N/A | 11 | 30 (15) | Hyaluronic acid (BMSCs) (allogenic) |
40 million | 12 | 2 to 4 | VAS, WOMAC, MRI, AE |
| Lu et al (China)31 |
2019 | 57 (8) | 24 (3) | 6 | 47 (24) | Hyaluronic acid (ADSCs) (autologous) |
50 million | 6,12 | 1 to 3 | VAS, WOMAC, MRI, AE |
| Matas et al (Chile)32 |
2018 | 56 (5) | 27 (3) | 10 | 26 (8) | Hyaluronic acid (UCMSCs) (allogenic) |
20 million or 20 million twice |
6,12 | 2 to 3 | VAS, WOMAC, WORMS (MRI), AE |
ADSC, adipose-derived stem cell; AE, adverse events; BMI, body mass index; BMSC, bone marrow-derived stem cell; K-L, Kellgren-Lawrence grade; N/A, not available; OA, osteoarthritis; UCMSC, umbilical cord tissue-derived mesenchymal stem cell; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index ; WORMS, whole-organ MRI score
Assessment for risk of bias
Most trials generated random sequence but did not explain the method of allocation concealment. Only one trial had a high risk of reporting bias as it did not report all clinical outcomes, specifically WOMAC scores. The risk of attrition bias and performance bias for most of the trials was low. In general, the risk of bias in all the trials was relatively low (Figure 2).
Fig. 2.
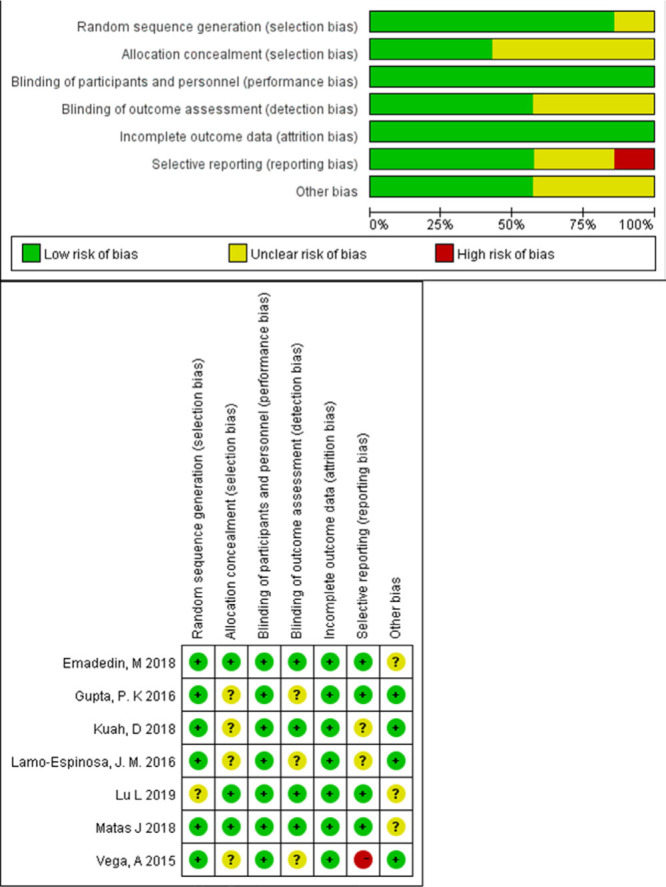
Quality assessment of included trials, risk of bias graph, and summary.
Safety
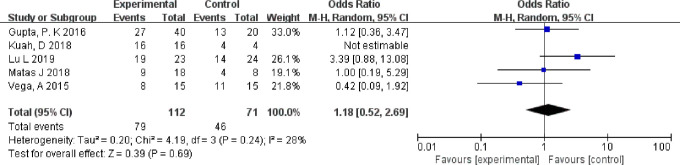
Firstly, we assessed the level of safety of injecting MSCs into the knee. Only two trials of five patients reported serious adverse events: three cases from the experimental group and two cases from the control group.27,31 We counted the number of patients who reported having had mild-to-moderate adverse events in all studies.27,28,30–32 No significant difference was found between the experimental group and the control group, although almost every patient experienced adverse events (p = 0.690, chi-squared test, Figure 3). The most common adverse events were joint pain and swelling, which were controlled without special treatment. The four studies that used allogeneic bone marrow MSCs did not show an increased number of adverse events.27,28,30–32
Fig. 3.
Forest plot of mesenchymal stem cell injection on adverse events. Random-effect models were used. CI, confidence interval; M-H, Mantel-Haenszel method.
Pain
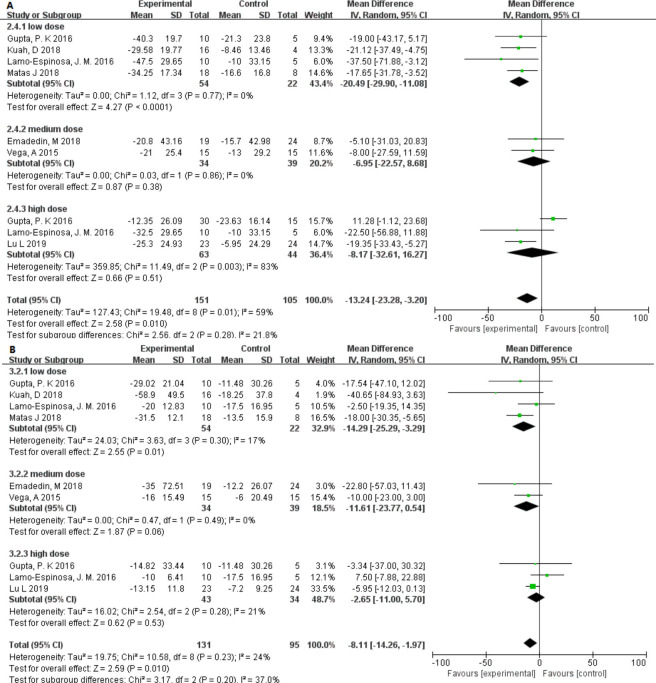
Pain was another clinical parameter evaluated in these studies. All studies used VAS to assess the effect of MSC injection on pain.26–32 A total of 151 patients received MSC treatment and 105 received either placebo or hyaluronic acid. The mean difference (MD) in VAS changes in patients treated with MSCs significantly decreased by 13.24 (95% CI –23.28 to –3.20; p = 0.010) compared with that of the controls. Heterogeneity was found in one high-dose group, but did not affect the final results.27
In addition, all studies also used the WOMAC pain scale to assess the effect of MSC injection on pain relief.26–32 The test group comprised 131 subjects and the control group comprised 95 subjects. Because the WOMAC scales differed between studies, we have made some conversions. The MD of the changes in WOMAC pain was statistically significant at –8.11 (95% CI –14.26 to –1.97; p = 0.010) and there was only slight heterogeneity (I2 = 24%).
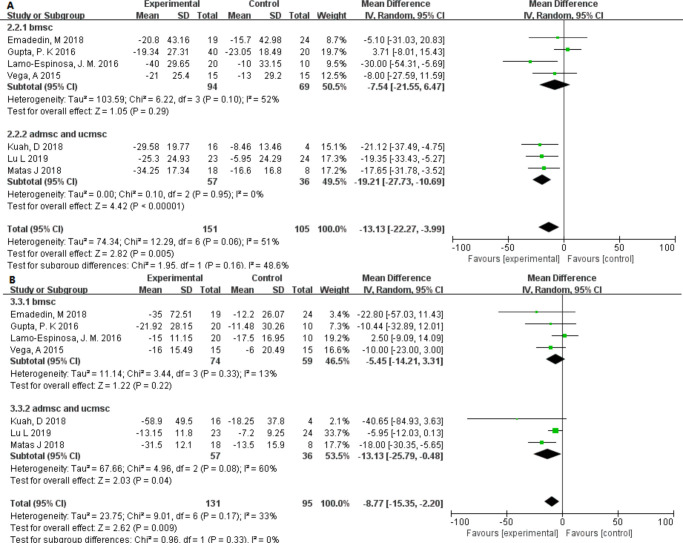
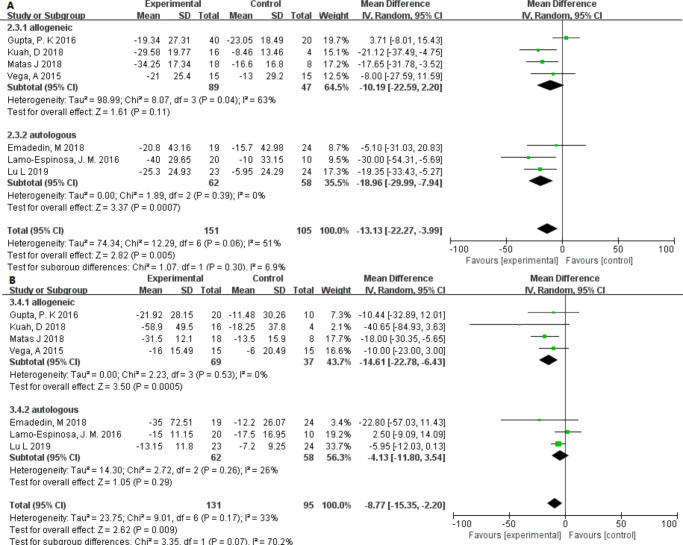
The VAS and WOMAC pain scales showed slight heterogeneity (I2 = 21.8% and I2 = 37.0%) among the different dose groups. It is worth noting that only in the low-dose group was the p-value found to be lower and statistically significant (Figure 4). Interestingly, in different source groups, the p-value of adipose- or umbilical cord–derived cells was smaller than that of bone marrow, and the VAS score had moderate heterogeneity (I2 = 48.6%) (Figure 5). In addition, after excluding studies showing heterogeneity, allogeneic and autologous stem cells all showed good therapeutic effects (p < 0.050) (Figure 6).
Fig. 4.
Forest plots of mean difference (MD) on pain according to dose groups. a) MDs of pain according to visual analogue scale and b) MDs of pain according to Western Ontario and McMaster Universities Osteoarthritis Index.22 CI, confidence interval; IV, inverse variance method.
Fig. 5.
Forest plots of mean difference (MD) on function according to dose groups: a) MDs of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)22 stiffness, b) MDs of WOMAC physical function, and c) MDs of WOMAC total. CI, confidence interval; I, inverse variance method.
Fig. 6.
Forest plots of mean difference (MD) on cartilage: a) MDs of whole-organ MRI score (WORMS) at six months and b) MDs of WORMS at 12 months. CI, confidence interval; IV, inverse variance method.
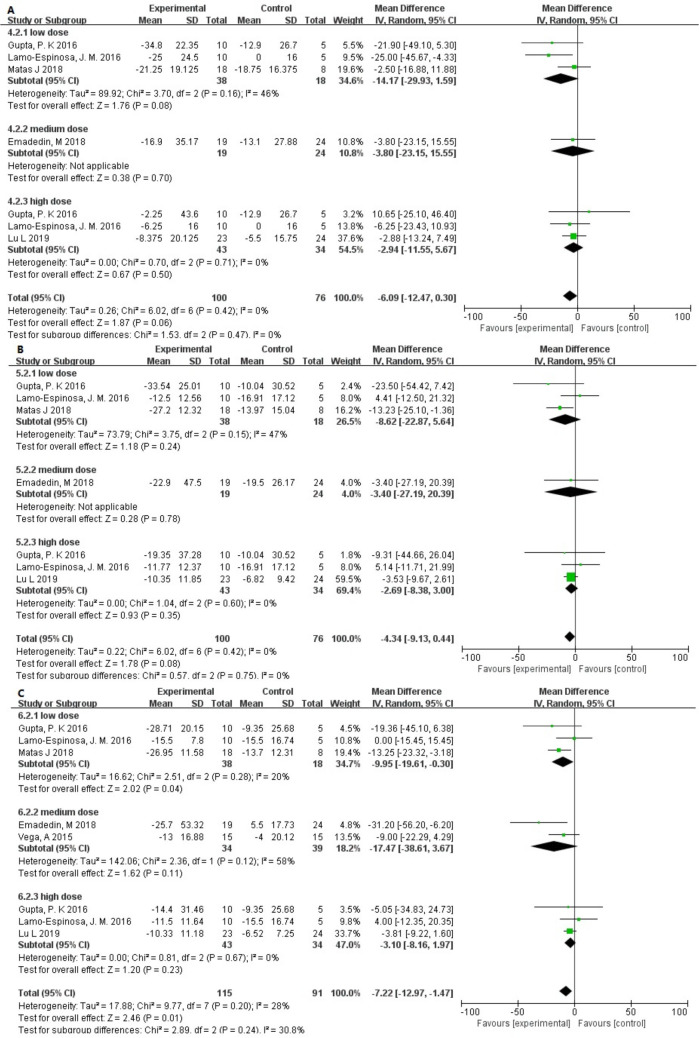
Function
To assess the improvement in knee function in the patients, we extracted changes in WOMAC, except for one study that did not provide specific data.30 We observed that the p-values of WOMAC stiffness and WOMAC physical function were very close to 0.05. The MD in changes was –6.09 (95% CI –12.47 to 0.30; p = 0.060) and –4.34 (95% CI –9.13 to 0.44; p = 0.080), and no heterogeneity was found between the different dose groups (I2 = 0%). However, in the WOMAC total score, an improvement in function was found (p = 0.01). The low-dose group also showed a lower p-value (Figure 7).
Fig. 7.
Forest plots of mean difference (MD) on pain according to cell source (bone marrow-, adipose-, or umbilical cord tissue-derived): a) MDs of pain according to visual analogue scale and b) MDs of pain according to Western Ontario and McMaster Universities Osteoarthritis Index.22 ADMSC, adipose-derived mesenchymal stem cell; BMSC, bone marrow-derived stem cell; CI, confidence interval; IV, inverse variance method; UCMSC, umbilical cord tissue-derived mesenchymal stem cell.
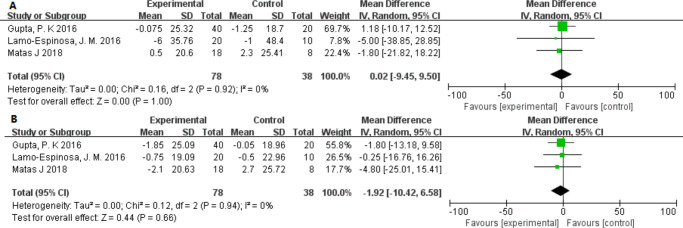
Cartilage
The problem of greatest concern is the role MSCs play in regenerating cartilage during the treatment. However, in only three studies could we compare the data relating to the WORMS scoring for MRI of the knee.27,29,32 The WORMS scores for MRI of the knee showed no significant difference (MD: 95% CI 0.02 (–9.45 to 9.50); p = 1.000) between the scores at six months (MD: 95% CI –1.92 (–10.42 to 6.58); p = 0.660) and at 12 months after treatment (Figure 8).
Fig. 8.
Forest plots of mean difference (MD) on pain according to cell source (allogeneic or autologous): a) MDs of pain according to visual analogue scale and b) MDs of pain according to Western Ontario and McMaster Universities Osteoarthritis Index22 pain. CI, confidence interval; IV, inverse variance method.
Discussion
MSCs are multipotent stem cells that share all the commonalities of stem cells—namely, self-renewal and multidirectional differentiation. MSCs are found not only in the bone marrow but also in the synovium and umbilical cord tissues, placental tissues, and adipose tissues.34,35 Because they can differentiate into bone, cartilage, muscle, or tendon, MSCs provide a source of cells for the clinical treatment of various wounds and injuries, so their clinical application is of great value.36 To achieve the successful treatment of OA, we need to consider cell source, cell dose, and appropriate MSC implantation methods.
The data of the research included in this meta-analysis are detailed and meet the minimum reporting requirements agreed by experts.37 We found that MSC-based therapy is safe. Joint pain or swelling after injection may diminish over time or following oral medication. Severe complications are rare and there is no firm evidence linking such complications with MSC injections. Moreover, the most important finding is that MSC-based interventions can significantly reduce VAS and WOMAC pain, as shown by several studies.26,28,29 Therefore, we conclude that MSCs can relieve pain and improve the quality of life in patients with knee OA.
However, there was no evidence in this meta-analysis that cartilage was repaired, and in three particular studies27,29,32 no changes were observed in the WORMS score of the knee MRI. While some studies have shown no progressive loss of cartilage and others have found an increase in cartilage area, following MSC injection, cartilage repair remains controversial.28,30,31 Cell types, cell numbers, cell culture methods, and implantation methods may influence the therapeutic effect. We performed a subgroup analysis of cell doses and concluded that < 30 million cells improved pain and function best. Our conclusion is similar to that of Gupta et al,27 who suggested that knee joint space is limited and that injecting too much solution and too many cells could cause adverse effects such as joint pain and swelling. Although the volume of the solution varies greatly between studies and no quantitative analysis has been performed, it is well-demonstrated that for injection purposes, the optimum number of cells is 25 million. Doyle et al38 also consider that the use of large numbers of cells carries increased risk, as shown by their systematic review. However, Lamo-Espinosa et al29 reported that ten million or 100 million cells were effective, and found that the clinical effect was maintained for longer in the high-dose group. In terms of research into repeated injections of hyaluronic acid, Matas et al32 compared single and repeated injections of MSCs and observed better results with repeated dosing. Two studies in particular have shown that multiple injections may reduce adverse reactions and enhance the effect compared with a single injection of high numbers of cells; however, the literature is limited in this area, which may be a direction for future research.32,39
In recent years, in addition to bone marrow-derived stem cells, adipose, umbilical cord, and synovium-derived cells have attracted an increasing amount of attention.40 In our study, the adipose and umbilical cord stem cell groups had lower p-values, which were statistically significant and showed advantages in treating knee OA. Migliorini et al41 reviewed 18 studies of fat- and bone marrow-derived MSCs used in the treatment of arthritis and found that cells from both sources improved pain and functional scores. However, Shariatzadeh et al42 found that the collection/isolation, culture conditions, and characterization criteria of stem cells vary. The optimal source of MSCs remains speculative, and a more consistent method is needed to standardize the assessment of cell source. Interestingly, our subgroup analysis revealed that allogeneic stem cells have a greater pain-relieving effect than autologous stem cells, and fewer adverse events are observed with allogeneic stem cells than with autologous stem cells.
There were several limitations to our study. First, the number of studies we included was too small. The minimum number of participants in the trial group was less than ten, and the dose difference between the studies was also large. The methods for MSC preparation varied, which may have caused greater biases. All evaluation indicators were subjective evaluations of patients, yet differences among individuals were still apparent. We also need more objective evidence to assess the specific effects of MSC treatment on cartilage damage.43 The number of injections, the interval between injections, and the sustainability of injections should be the focus of future research. Two studies included in this review used hyaluronic acid in the experiment group and the control group.27,29 Considering that both groups used hyaluronic acid, we believe that the difference in clinical results between the two groups can be considered the effect of MSCs.44 In addition, MSCs in combination with PRP, stents, growth factors, and even gene therapy also need to be explored to achieve optimal results.
In conclusion, without surgical assistance, intra-articular injection of MSCs can improve pain and function in patients with knee OA, but may not improve cartilage status. Adipose tissue may be a good source of MSCs because of its easy accessibility. High-dose stem cell (100 million) injections may induce more adverse reactions, and low-dose (20 million) multiple injections may be a good choice.
Author contributions
J. Wang: Edited the manuscript.
L. Zhou: Edited the manuscript.
Y. Zhang: Reviewed the manuscript.
L. Huang: Drafted the manuscript
Q. Shi: Provided ideas for the study.
J. Wang and L. Zhou contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical review statement
This study was approved by the local research ethics committee.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Nyvang J, Hedström M, Gleissman SA. It's not just a knee, but a whole life: a qualitative descriptive study on patients' experiences of living with knee osteoarthritis and their expectations for knee arthroplasty. Int J Qual Stud Health Well-being. 2016;11:30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. [DOI] [PubMed] [Google Scholar]
- 3.Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS One. 2017;12(4):e0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttgereit F, Burmester G-R, Bijlsma JWJ. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open. 2015;1(1):e000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusad A, Pedro S, Mikuls TR, et al. Impact of total knee arthroplasty as assessed using patient-reported pain and health-related quality of life indices: rheumatoid arthritis versus osteoarthritis. Arthritis Rheumatol. 2015;67(9):2503–2511. [DOI] [PubMed] [Google Scholar]
- 6.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musumeci G, Castrogiovanni P, Leonardi R, et al. New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J Orthop. 2014;5(2):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeo SA. Cell therapy in orthopaedics: where are we in 2019? Bone Joint J. 2019;101-B(4):361–364. [DOI] [PubMed] [Google Scholar]
- 9.Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: macroscopic and histological assessments. Bone Joint Res. 2017;6(2):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T, Akagi R, Akatsu Y, et al. The effect of systemic administration of G-CSF on a full-thickness cartilage defect in a rabbit model MSC proliferation as presumed mechanism: G-CSF for cartilage repair. Bone Joint Res. 2017;6(3):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh Y-G, Jo S-B, Kwon O-R, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748–755. [DOI] [PubMed] [Google Scholar]
- 12.Jo CH, Lee YG, Shin WH, et al. Intra-Articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. [DOI] [PubMed] [Google Scholar]
- 13.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-Derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136(3):978–989. [DOI] [PubMed] [Google Scholar]
- 14.Mazor M, Lespessailles E, Coursier R, Daniellou R, Best TM Toumi H. Mesenchymal stem-cell potential in cartilage repair: an update. J Cell Mol Med. 2014;18(12):2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh Y-G, Kwon O-R, Kim Y-S, Choi Y-J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30(11):1453–1460. [DOI] [PubMed] [Google Scholar]
- 16.Vangsness CT, Farr J, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96-A(2):90–98. [DOI] [PubMed] [Google Scholar]
- 17.Mifune Y, Matsumoto T, Takayama K, et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage. 2013;21(1):175–185. [DOI] [PubMed] [Google Scholar]
- 18.Koga H, Muneta T, Nagase T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333(2):207–215. [DOI] [PubMed] [Google Scholar]
- 19.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–129. [DOI] [PubMed] [Google Scholar]
- 20.ten Broek RPG, Stommel MWJ, Strik C, van Laarhoven CJHM, Keus F, van Goor H. Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. The Lancet. 2014;383(9911):e59:48–59. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester, UK: Wiley, 2011. [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ magnetic resonance imaging score (worms) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. 1992;30(6):473–556. [PubMed] [Google Scholar]
- 26.Emadedin M, Labibzadeh N, Liastani MG, et al. Intra-Articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–1246. [DOI] [PubMed] [Google Scholar]
- 27.Gupta PK, Chullikana A, Rengasamy M, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuah D, Sivell S, Longworth T, et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-Articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matas J, Orrego M, Amenabar D, et al. Umbilical cord-derived mesenchymal stromal cells (MscS) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culvenor AG, Engen CN, Øiestad BE, Engebretsen L, Risberg MA. Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3532–3539. [DOI] [PubMed] [Google Scholar]
- 34.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. [DOI] [PubMed] [Google Scholar]
- 35.Herrero C, Perez-Simon JA. Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res. 2010;425:e30:43. [DOI] [PubMed] [Google Scholar]
- 36.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78(3):188–198. [DOI] [PubMed] [Google Scholar]
- 37.Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020;10:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoud EE, Adachi N, Mawas AS, Deie M, Ochi M. Multiple intra-articular injections of allogeneic bone marrow-derived stem cells potentially improve knee lesions resulting from surgically induced osteoarthritis: an animal study. Bone Joint J. 2019;101-B(7):824–831. [DOI] [PubMed] [Google Scholar]
- 39.Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16(2):523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Migliorini F, Rath B, Colarossi G, et al. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch Orthop Trauma Surg. 2020;140(7):1007–868. [DOI] [PubMed] [Google Scholar]
- 41.Shariatzadeh M, Song J, Wilson SL. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378(3):399–410. [DOI] [PubMed] [Google Scholar]
- 42.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. [DOI] [PubMed] [Google Scholar]
- 43.Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363–2372. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Djaja YP, Park Y-B, Park JG, Ko YB, Ha CW. Intra-Articular injection of culture-expanded mesenchymal stem cells without adjuvant surgery in knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2019:036354651989227. [DOI] [PubMed] [Google Scholar]