Abstract
Aims
Osteoarthritis (OA) is a disabling joint disorder and mechanical loading is an important pathogenesis. This study aims to investigate the benefits of less mechanical loading created by intermittent tail suspension for knee OA.
Methods
A post-traumatic OA model was established in 20 rats (12 weeks old, male). Ten rats were treated with less mechanical loading through intermittent tail suspension, while another ten rats were treated with normal mechanical loading. Cartilage damage was determined by gross appearance, Safranin O/Fast Green staining, and immunohistochemistry examinations. Subchondral bone changes were analyzed by micro-CT and tartrate-resistant acid phosphatase (TRAP) staining, and serum inflammatory cytokines were evaluated by enzyme-linked immunosorbent assay (ELISA).
Results
Our radiographs showed that joint space was significantly enlarged in rats with less mechanical loading. Moreover, cartilage destruction was attenuated in the less mechanical loading group with lower histological damage scores, and lower expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, matrix metalloproteinase (MMP)-3, and MMP-13. In addition, subchondral bone abnormal changes were ameliorated in OA rats with less mechanical loading, as reduced bone mineral density (BMD), bone volume/tissue volume (BV/TV), and number of osteophytes and osteoclasts in the subchondral bone were observed. Finally, the level of serum inflammatory cytokines was significantly downregulated in the less mechanical loading group compared with the normal mechanical loading group, as well as the expression of NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), caspase-1, and interleukin 1β (IL-1β) in the cartilage.
Conclusion
Less mechanical loading alleviates cartilage destruction, subchondral bone changes, and secondary inflammation in OA joints. This study provides fundamental insights into the benefit of non-weight loading rest for patients with OA.
Cite this article: Bone Joint Res 2020;9(10):731–741.
Keywords: Osteoarthritis, Cartilage destruction, Mechanical loading, NLRP3 inflammasome, Subchondral bone
Article focus
To investigate the benefits of less mechanical loading for joints of osteoarthritis (OA) animal model, since there is rare direct and in vivo evidence of protection roles of less mechanical loading for joints.
To investigate the effects of less mechanical loading on cartilage damage, subchondral bone remodelling, and secondary inflammation of joint in OA rats.
To investigate the activation of NACHT, LRR, and PYD domains-containing protein 3 (NLRP3)/caspase-1/interleukin 1β (IL-1β) cascade in cartilage of OA, and the effects of less mechanical loading on its activation.
Key messages
Less mechanical loading alleviates cartilage destruction, aberrant subchondral bone changes, and secondary inflammation of joints in OA rats.
Less mechanical loading downregulates the activation of NLRP3/caspase-1/IL-1β axis in cartilage of joints in OA rats.
Our intermittent tail suspension approach had no detrimental effect on the bone mineral density (BMD) of lower limbs in normal condition, and thus did not increase the possibility of osteoporosis.
Strengths and limitations
Strengths: in vivo comparative results; comprehensive assessment of joint damages; and the examination of NLRP3/caspase-1/IL-1β axis in the cartilage.
Limitations: animal model limited to three comparative groups, and no cellular experiments conducted to explore the mechanism.
Introduction
Osteoarthritis (OA) is a disabling joint disorder characterized by cartilage damage, subchondral bone changes, and synovial joint inflammation.1,2 Patients with OA suffer from joint pain, stiffness, and functional disabilities, which intensively affect their life quality.3,4 Reportedly, patients with OA are expected to rise with the ageing of the general population, while current pharmacological therapies for OA are ineffective in delaying the disease progression.
Mechanical loading plays an important role in the development and progression of OA,5–8 and less mechanical loading of joints is believed to be effective in attenuating the progression of OA.9,10 Therefore, activities and exercises with less mechanical loading are always recommended for OA patients. However, robust evidence is absent for the protective roles of less mechanical loading, and how to achieve it is still unclear. While clinical studies can only provide indirect measurements of the pathological changes by means of radiology or surrogate markers,5,11,12 animal studies are essential to assess tissue repair potential directly using OA model with less mechanical loading, including cartilage damage and subchondral bone remodelling in the joints.
Furthermore, in-depth knowledge about the inherent mechanism of mechanical loading is fundamental to improving the understanding of the pathogenesis of OA. It is widely accepted that secondary inflammation is the main reason accounting for pain and stiffness in patients with OA.13,14 Among the various inflammatory cytokines, interleukin 1β (IL-1β) plays a key role in amplifying and perpetuating inflammation and further damaging bone and cartilage.14,15 In addition, IL-1β is conducive to the production of prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) in synovial joint and cartilage, which are essential for pain initiation and persistence. Activation and secretion of IL-1β is mainly controlled by the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome.16 The NLRP3 inflammasome cleaves pro-caspase-1 into caspase-1, which then activates pro-IL-1β into IL-1β, the secreted form.16 Recently, activation of NLRP3 inflammasome has been proposed to be an important event in the pathogenesis of OA and other degenerative diseases.17,18 Yet, the role of NLRP3 activation in cartilage degeneration, in particular under different mechanical loading, is not clear.
Therefore, a rat OA model was established by anterior cruciate ligament (ACL) transaction plus medial meniscus resection, and intermittent tail suspension was applied to study the benefit of less mechanical loading for OA joints, including cartilage destruction, subchondral bone changes, and secondary inflammation. The NLRP3/caspase-1/IL-1β axis was also investigated to examine its role in less mechanical loading for cartilage protection in OA.
Methods
Animal surgery
In total, 30 male Sprague Dawley rats (12 weeks old, weighing 400 g to 450 g) were used in this study. A post-traumatic OA model was established in 20 rats by an ACL transaction plus medial meniscus resection.19 In brief, each rat was anaesthetized with 1% sodium pentobarbital (40 mg/kg) via intraperitoneal injection. After being shaved and disinfected, the right knee joint was exposed through a medial parapatellar approach. The patella was dislocated laterally and the knee was placed in full flexion followed by ACL transection and medial meniscus resection with micro-scissors. The surgical incisions were then sutured and analgesic medication was given after the surgical procedure. The rats were randomly divided into two groups (ten in each group) based on the mechanical loading of the knee. In the less mechanical loading group, the rats received intermittent non-weight loading by tail suspension. In brief, the hind limbs of rats were lifted off the ground with tail suspension for two hours and then returned to the ground for two hours for free and full access to all the activities. This protocol was repeated for three cycles from 8:00 to 20:00 every day for a whole month. The OA rats receiving no additional treatment acted as OA controls. Another ten rats receiving sham operation were set as sham controls. The loading protocol of the different experimental groups are presented in Supplementary Figure a.
Sample preparation
During the treatment, the status of rats under tail suspension was monitored every day. Rats were euthanized by cervical vertebra dislocation after 1% sodium pentobarbital (40 mg/kg) was administered via intraperitoneal injection. Blood samples were collected for enzyme-linked immunosorbent assay (ELISA) test. The joints of three rats in each group were dissected to perform gross evaluation of joints, and surface abrasion and fibrotic tissue were visible as surface roughness. Since dissection altered the spatial relationships among the tibia, femur, and synovium, the cartilage tissues were only used for molecular test. The joints of the other seven rats were fixed in 4% paraformaldehyde, and were then subjected to radiological and pathological examination.
Digital radiographs
The frontal and lateral knee film was obtained using digital radiography (Philips, Best, The Netherlands) under an exposure time of 3,000 ms and a voltage of 43 kV. The radiograph was finished in two hours after joint dissection from rats. The measurements of joint space were obtained from the frontal radiograph to avoid the effects of different joint flexions in the lateral radiograph. It was performed by two authors (BZ and JG) blinded to the group information and the mean of the measurements was used.
Micro-CT
The structural changes within the subchondral bone of the tibia were quantitatively assessed using micro-CT (μCT). At the end of the study, the knees of rats in each group were extracted. The specimens were then fixed in 10% formalin before examination and were then analyzed using a high-resolution (20 μm/voxel resolution, 0.48 mm field of view, and 1024 × 1024-pixel) μCT scanner (μCT 100; Scanco Medical, Wangen-Brüttisellen, Switzerland). The scanning was performed with peak voltage 80 kVP, current 200 mA, and integration time 300 ms. Longitudinal images of the tibial subchondral bone were used to construct 3D histomorphometric analysis. Trabecular bone parameters (bone volume/tissue volume (BV/TV), trabecular separation (TbSp), and number of osteophytes) were measured with the manufacturer’s program (μCT 100; Scanco Medical).20 The region of interest was designated to cover the medial compartment of tibial subchondral bone (200 μm to 400 μm below the growth plate), and was then obtained for reconstruction and analysis. Osteophytes were calculated in three sections for each rat and a mean was obtained. Bony outgrowths at the joint margins were counted as osteophytes.
Cartilage degeneration assessment
The joints were then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) after clearing out the soft tissue for about two weeks, and serial 5 μm sagittal sections were obtained for histological examination. Sectioning was started at the medial margin of the joint at about 200 mm apart from the lateral margin. Three sections from medial knee joints at 200 μm intervals were obtained to perform cartilage degeneration evaluation. Sections were first stained with 0.02% Fast Green (Sigma-Aldrich, St. Louis, Missouri, USA) for ten minutes, then 0.1% Safranin O (Sigma-Aldrich) for seven minutes. The severity of OA was evaluated according to the rat-specific Osteoarthritis Research Society International (OARSI) histopathology initiative.21 We evaluated the cartilage degeneration score (five points), calcified cartilage and subchondral bone damage score (five points), and synovial membrane inflammation score (four points), as described in the rat-specific OARSI. Both tibial plateau and femoral condyle were rated using the OARSI scoring system, and the score was presented separately with the highest OARSI score being 14.
Osteoclasts were detected by enzyme histochemistry in serial decalcified sections, using tartrate-resistant acid phosphatase (TRAP) staining. Briefly, sections were stained for acid phosphatase using naphthol phosphate as substrate in the presence of 50 mM tartrate with hexazotized pararosaline. All the TRAP-positive cells were counted in the tibial epiphysis area and the mean number was obtained from three slides of the median joints for each rat. Two independent observers (PN and YL), who were blinded to the experimental groups, rated the sections.
Immunohistochemical analysis
Dewaxed and dehydrated sections were first incubated with 3% peroxyl in methanol for 15 minutes for endogenous peroxidase blocking. After washing in phosphate-buffered saline (PBS), sections were then immersed into the boiled citrate buffer solution for ten minutes for antigen retrieval. Following blocking with 5% bovine serum albumin (BSA) for 20 minutes, sections were then covered with anti-a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5 antibody (1:200; Abcam, San Francisco, California, USA), anti-matrix metalloproteinase (MMP)-3 antibody (1:150; Abcam), anti-MMP-13 antibody (1:150; Abcam), anti-NLRP3 antibody (1:150; Abcam), anti-caspase-1 antibody (1:150; Abcam), and anti-IL-1β antibody (1:150; Abcam) overnight at 4°C in a humidified chamber. On the following day, sections were incubated with a biotinylated anti-rabbit antibody (Boster Biological Technology, Wuhan, China) for 30 minutes at room temperature and then coupled with diaminobenzidine to visualize the positive expression of the targeted proteins, respectively. After all sections were counterstained with haematoxylin, they were mounted using neutral resins.
An Olympus microscope (Olympus, Tokyo, Japan) was used to examine the finished sections. Positive cells of the targeted proteins were counted at magnification 400× in three fields of each slide. Three sections were selected from each group for counting. The positive cell numbers were normalized to the cell number per 100 total cells.
Quantitative polymerase chain reaction
The total RNA in the three rats from each group was extracted with TRIzol reagent (Takara Bio, Kusatsu, Japan). RNA (1 μg) was reversely transcribed into complementary DNA (cDNA) with the RevertAid First Strand cDNA Synthesis kit (Takara Bio) according to the manufacturer's instructions. cDNA was amplified using the primers shown in Table I (synthesized by Shanghai Sangon Biological Engineering Technology Services, Shanghai, China). Quantitative polymerase chain reaction (qPCR) was performed using ABI PRISM 7000 (Applied Biosystems; Thermo Fisher Scientific, Waltham, Massachusetts, USA) and SYBR-Green Real-Time PCR Master mix (Takara Bio). The condition was set as follows: initial denaturation at 95°C for three minutes, followed by 30 to 40 cycles at 95°C for 35 seconds, 55°C for 30 seconds, and 72°C for 40 seconds. Quantification of target genes was achieved by the relative expression levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the formula 2-ΔΔCt, where ΔΔCt = (Ct of the target gene - Ct of GAPDH) after treatment - (Ct of the target gene - Ct of GAPDH) in the control.22
Table I.
Primer sequences of quantitative polymerase chain reaction.
| Gene | Sense (5’-3’) | Antisense (5’-3’) |
|---|---|---|
| ADAMTS-5 | CCTGGGAATGGCAGACGTT | GGAGGCATCGATACTGGTGAG |
| MMP-3 | TTACACACCGGATCTGCCAA | AGCATGAGCCAAAACATTTCCA |
| MMP-13 | GCTTTCAAGGTTTGGTCTGATG | GGTCCAGGAGGAAAAGCGTG |
| NLRP3 | CAGTCCCCAAACAACCTCCA | GCCCGAGAAGCTGATCTGAG |
| Caspase-1 | AACCCGTCTCTGCACACTGG | TCAGCTCATACGTGCCAGAC |
| IL-1β | TGCCATGCTGAAAGAGTACG | GTGGCATCAAGGGAATAGGA |
| GAPDH | TGAAGGGTGGAGCCAAAAGG | GGCAGGGATGATGTTCTGGG |
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-1β, interleukin 1β; MMP, matrix metalloproteinase; NLRP3, NACHT, LRR, and PYD domains-containing protein 3.
ELISA analysis of serum iNOS, COX2, and IL-1β
We collected a 5 ml blood sample by cardiac puncture immediately after euthanizing the animals. The blood sample was then centrifuged at 1,800 g for ten minutes and resultant serum was stored at -80°C until analysis. The concentrations of iNOS, COX-2, and IL-1β were determined using ELISA kits (Biorbyt, San Francisco, California, USA). All samples were performed in triplicate.
Statistical analysis
Data were expressed as the mean and standard error of the mean (SEM) and analyzed with the statistical package from GraphPad Prism (version 6.0; GraphPad Software, San Diego, California, USA). Intergroup comparison was subject to one-way analysis of variance (ANOVA) for the cartilage destruction scores, positive cell numbers, and inflammatory cytokine level, and Dunnett’s test was used to perform post hoc analysis. Statistical significance was set at p < 0.05.
Results
Less mechanical loading ameliorated the knee joint space narrowing
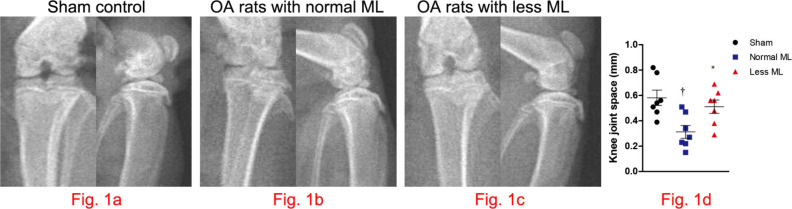
Compared with that in sham control (Figure 1a), radiograph of knee joints suggested that joint space narrowing was significant in OA rats with normal mechanical loading (Figure 1b), while lessened in OA rats with less mechanical loading (Figure 1c). Also, indistinct joint space and high bone mineral density (BMD) at the subchondral bone area can be observed in normal mechanical loading OA knee (Figure 1b). Furthermore, joint space narrowing ameliorated significantly in rats with less mechanical loading (n = 7; Figure 1d).
Fig. 1.
Less mechanical loading (ML) strategy enlarging the knee joint space in an osteoarthritis (OA) rat. a) The frontal and lateral radiographs of knee joint in sham control. As compared with the narrowed and indistinct joint space in OA rats with normal ML (arrow, b), the joint space was enlarged in OA rats with less ML (arrow, c). d) Furthermore, joint space, as measured in the frontal images, enlarged significantly in rats with less ML. One-way analysis of variance (ANOVA) was used, *p < 0.05 between OA rats with normal and less ML. †p < 0.01 between sham controls and OA rats with normal ML.
Less mechanical loading attenuated cartilage degeneration
Compared with the smooth and glassy appearance of joint in sham control (Figure 2a), macroscopic observation revealed cartilage surface abrasion and large defects in joints of normal mechanical loading OA rats (Figure 2b), which was also observed under microscopic inspection (Figure 2b). However, articular surface was relatively smooth and there was no evidence of breakdown in less mechanical loading OA rats (Figures 2a and 2b). OARSI initiative scoring suggested that cartilage destruction was more severe in the tibial terminal than that of the femoral terminal under normal loading, thus more benefits were obtained under less mechanical loading. In comparison with normal mechanical loading OA rats, both the tibial and femoral cartilage OARSI initiative scores were significantly lower in the less mechanical loading group (one-way ANOVA was used, p < 0.01, n = 7; Figure 2c).
Fig. 2.
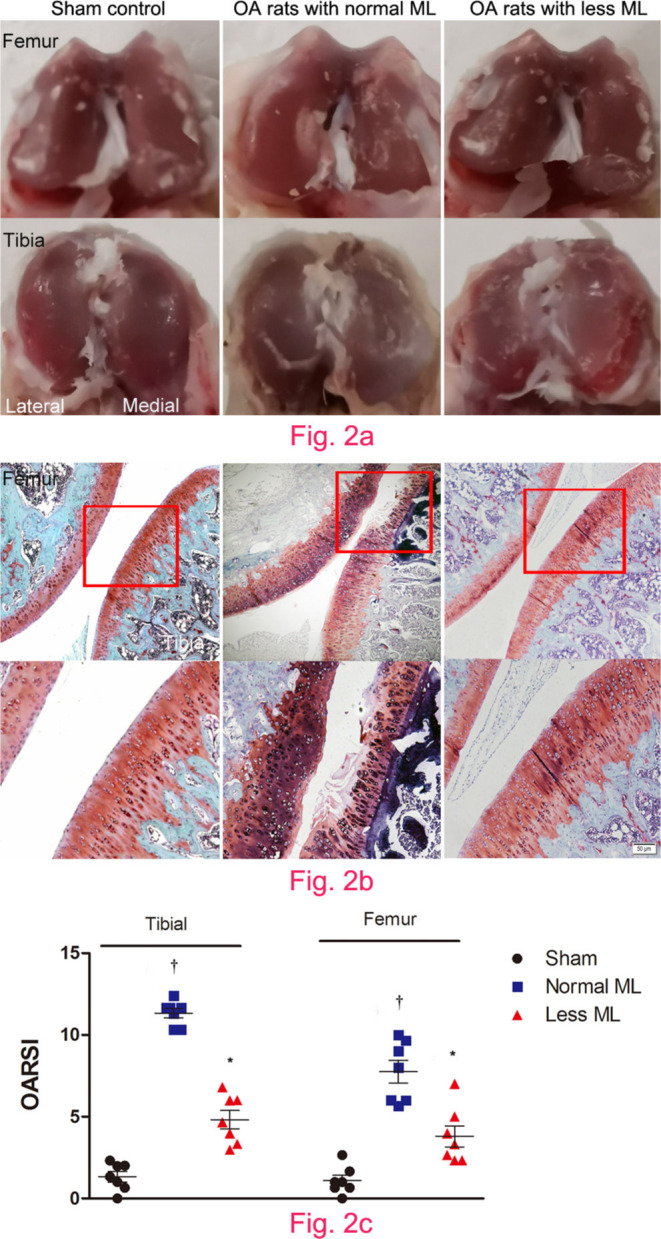
Less mechanical loading (ML) attenuated articular cartilage degeneration in an osteoarthritis (OA) rat. a) Gross appearance of the articular cartilage in the normal ML group showed that joint surface was rough with focal defects in the proximal medial tibial plateau (lower panel), while smooth joint surface was present in the less ML joint articular cartilage. b) Safranin O and Fast Green staining demonstrated that there were some clefts and breakdown in the cartilage in OA rats with normal ML. a) and b) However, articular surface was relatively smooth and there was no evidence of breakdown in OA rats in the less ML group. c) Furthermore, the Osteoarthritis Research Society International (OARSI) scores in both tibial and femur cartilage were greater in the OA rats with normal ML than that of OA rats with less ML. Scale bar: 50 μm. Values are presented as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used, *p < 0.001 between OA rats with normal and less ML. †p < 0.001 between sham controls and OA rats with normal ML. Magnification of upper panel in Fig. 2b was 100×, and lower panel was 400×.
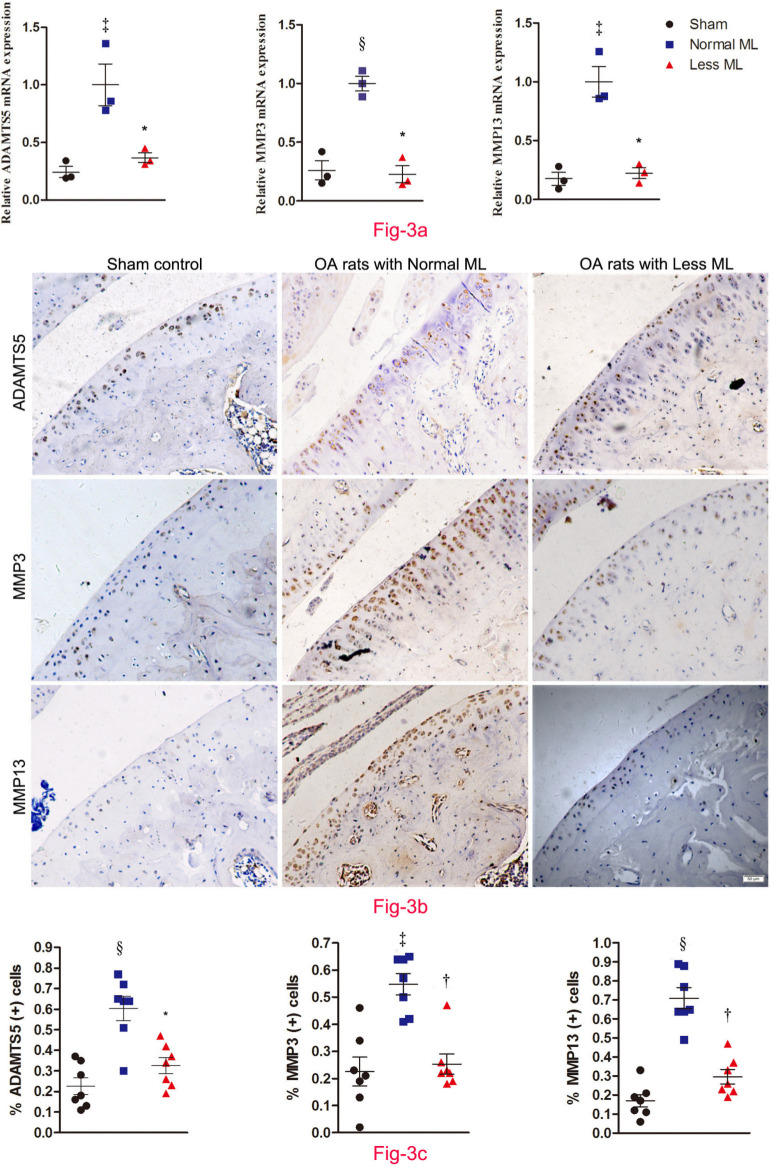
Less mechanical loading down-regulated AMAMTS-5, MMP-3, and MMP-13 expression in cartilage
Furthermore, cartilage catabolism was more pronounced in normal mechanical loading OA rats, since the messenger RNA (mRNA) expression of ADAMTS-5, MMP-3, and MMP-13 in the less mechanical loading group was statistically lower than that of the normal mechanical loading group (n = 3; Figure 3a). Likewise, the percentage of ADAMTS-5, MMP-3, and MMP-13-positive chondrocytes in the cartilage of the less mechanical loading group was statistically lower than that of the normal mechanical loading group (n = 7; Figures 3b and 3c). The negative controls are provided in Supplementary Figure b.
Fig. 3.
Less mechanical loading (ML) ameliorates joint catabolism in an osteoarthritis (OA) rat. a) Quantitative polymerase chain reaction (qPCR) revealed that the messenger RNA (mRNA) expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, matrix metalloproteinase (MMP)-3, and MMP-13 in the less ML group was lower than that of the normal ML group. b) Furthermore, immunohistochemical examination showed that there were numerous ADAMTS-5, MMP-3, and MMP-13 positive chondrocytes in both the superficial layer and deep layer of cartilage from OA rats with normal ML, while they were seldom expressed in the superficial cartilage in the less ML group. The presentative images were selected from weight-bearing area of tibial cartilage. c) In addition, quantitative analysis demonstrated that the percentage of target positive chondrocytes in articular cartilage was significantly reduced in the less ML group, compared to the normal ML group. Scale bar: 50 μm. Values are presented as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used, *p < 0.01; †p < 0.001, between OA rats with normal and less ML. ‡p < 0.01; §p < 0.001, between sham controls and OA rats with normal ML. Magnification: 200×.
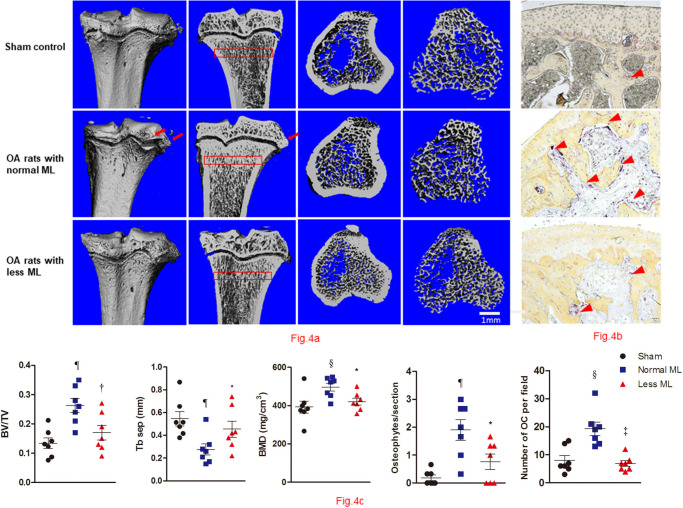
Less mechanical loading reduced abnormal bone remodelling in subchondral bone
To compare the effects of less mechanical loading on the subchondral bone remodelling, we analyzed the microarchitectural indices and bone resorption for underlying trabecular bone (Figure 4a). While normal mechanical loading increased osteophyte prevalence for the OA rats (Figure 4a), less mechanical loading tended to decrease the mean number of osteophytes per joint (1.9 (SEM 0.37) vs 0.76 (SEM 0.28); one-way ANOVA was used, p < 0.05; n = 7). Furthermore, the mean number of osteoclasts in the subchondral bone of the less mechanical loading group was also less than that of the normal group (19.3 (SEM 2.4) vs 6.9 (SEM 1.0) for the less and normal groups, respectively; one-way ANOVA was used, p < 0.05; n = 7, Figure 4b). Furthermore, quantitative analysis showed that rats with less mechanical loading had significantly lower BV/TV and BMD, and greater trabecular separation than that of the normal mechanical loading group (n = 7; Figure 4c).
Fig. 4.
Less mechanical loading (ML) reduced abnormal bone remodelling in subchondral bone in an osteoarthritis (OA) rat. a) Micro-CT of the tibia subchondral bone of rats showed that OA rats with less ML displayed decreased bone mineral density (BMD) and increased trabecular separation (TbSp), compared with OA rats with normal ML. b) The osteophytes were counted in three sections of each rat, and findings revealed that the mean number of osteophytes per joint in the less ML group was significantly lower than that of the normal ML group (red arrows). In addition, OA rats with less ML also showed a significantly lower number of osteoclasts (OCs) per bone volume, compared with the normal ML group (red arrowheads). c) Furthermore, quantitative analysis showed that OA rats with less ML had significantly lower bone volume/tissue volume (BV/TV) and BMD, and greater TbSep, which was seen in the subchondral bone 200 μm to 400 μm below the growth plate (red boxes in a). Scale bar: 1 mm. Values are presented as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used, *p < 0.05; †p < 0.01; ‡p < 0.001, between OA rats with normal and less ML. §p < 0.01; ¶p < 0.001, between sham controls and OA rats with normal ML. Magnification of Fig. 4b: 200×.
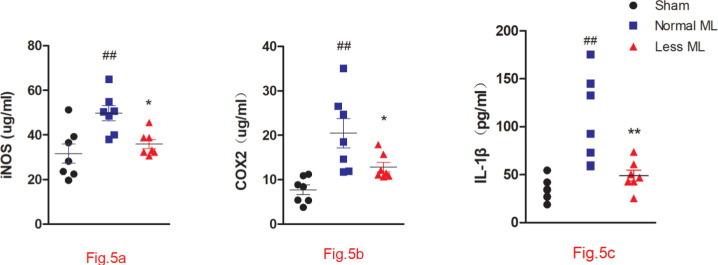
Less mechanical loading reduced inflammation in the joints
The secondary inflammatory status in the less mechanical loading group was alleviated, as indicated by the lower serum levels of iNOS (Figure 5a), COX-2 (Figure 5b), and IL-1β (Figure 5c) in the less mechanical loading group compared to those of the normal mechanical loading group (Figure 5).
Fig. 5.
Less mechanical loading (ML) ameliorates joint inflammation in an osteoarthritis (OA) rat. Enzyme-linked immunosorbent assay (ELISA) experiment revealed that the concentrations of a) cyclooxygenase-2 (COX-2), b) inducible nitric oxide synthase (iNOS), and c) interleukin 1β (IL-1β) in the less ML group were significantly lower than those in the normal ML group. Values are presented as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used, *p < 0.05; †p < 0.01, between OA rats with normal and less ML; ‡p < 0.01, between sham controls and OA rats with normal ML.
Less mechanical loading decreased the expression of NLRP3, caspase-1, and IL-1β in the cartilage of OA rats
The activation of NLRP3, caspase-1, and IL-1β was examined to explore the inflammatory cascade in OA cartilage under different mechanical loading. The transcriptional level of NLRP3, caspase-1, and IL-1β was lower in the less mechanical loading group than in the normal mechanical loading group (n = 3; Figure 6a). Furthermore, extensive immunostaining of NLRP3, caspase-1, and IL-1β in OA cartilage from rats treated with normal mechanical loading was noted, whereas fewer immunopositive cells were found in the less mechanical loading OA cartilage (Figure 6b). The percentage of immunopositive cells of NLRP3, caspase-1, and IL-1β in the less mechanical loading group was significantly lower than that of the normal mechanical loading group (59.1% (normal loading) vs 30.4% (less loading); one-way ANOVA was used, p < 0.01; 51.1% vs 24.3%, p < 0.05; and 47.4% vs 27.4%, p < 0.01, respectively, n = 7; Figure 6c). The negative controls are provided in Supplementary Figure b.
Fig. 6.
Less mechanical loading (ML) reduced the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3)/caspase 1/interleukin 1β (IL-1β) expression in the cartilage of an osteoarthritis (OA) rat. a) The transcriptional level of NLRP3, caspase-1, and IL-1β was lower in the less ML group than in the normal ML group. b) Furthermore, extensive immunostaining of NLRP3, caspase-1, and IL-1β in normal ML OA cartilage was noted, whereas fewer immune-positive cells could be found in the less ML OA cartilage. The presentative images were selected from weight-bearing area of tibial cartilage. c) The percentage of immunopositive cells of NLRP3, caspase-1, and IL-1β in the less ML group was significantly lower than that of the normal ML group. Scale bar: 50 μm. Values are presented as mean standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used, *p < 0.05; †p < 0.01; ‡p < 0.001, between OA rats with normal and less ML. §p < 0.05; ¶p < 0.01; **p < 0.001, between sham controls and OA rats with normal ML. Magnification in Fig. 6b: 200×.
Discussion
Despite great medical advancement in diagnosing degenerative joint diseases, no effective therapy has yet been found to reverse or halt the progression of OA to date. The pathogenesis of OA is multifactorial with many contributing factors, and repetitive and excessive joint loading is a well-accepted risk factor for OA.23–25 It was reported that weight loading can result in abnormal mechanical stress in joints and increase the risk of OA, while less mechanical loading was thought to be beneficial for disease progression.23–25 However, robust and detailed evidence is absent. Our study is the first to reveal that less mechanical loading can alleviate both cartilage damage and subchondral bone remodelling, and also inflammation in OA rats. In addition, this report provides insight into the benefit of non-weight loading rest for patients with OA and will help in upgrading our understanding in the mechanism of mechanical loading of joints. Also, we expect this report to remind us of the importance of easing mechanical loading and anti-inflammation in OA therapy.
Recently, researchers have suggested that mechanical stress plays an important role during OA development and progression.23–26 Accumulating data indicated that joint distraction was beneficial to patients with OA by relieving pain intensity. Furthermore, several animal studies have demonstrated the superior repair potential of joint tissues upon joint distraction, including cartilage degeneration and subchondral bone remodelling.27–29 The mechanism was supposedly attributed to the fact that joint distraction was able to reduce abnormal stress and allow cartilage tissue repair at the joint. Since joint distraction needs invasive operation and pin track infection may complicate knee joint arthroplasty surgery,29 these two considerations indicate a need for a non-invasive strategy to reduce joint mechanical loading. We found that intermittent periods of no mechanical loading of knee joint could slow down OA progression.
The integrity of articular cartilage was closely associated with the underlying subchondral bone, the impairment of which plays a pivotal role in the progression of OA.30,31 Recent studies have indicated that abnormal mechanical stress is an important factor in the development of subchondral bone changes during OA.32,33 It was demonstrated in an OA mouse model that subchondral bone resorption and bone cysts induced by aberrant stress contributed to OA development.33 The present research suggested that less mechanical loading reduced abnormal subchondral bone remodelling in OA rats. We speculated that intermittent unloading can, to some extent, decrease the aberrant stress at the subchondral bone.
On the other hand, inflammatory cytokines brought about by diet or injuries are also intensively involved in joint destruction in OA.14,34 Among them, IL-1β is an important cytokine participant in catabolism and degeneration of cartilage during OA development.13,35 IL-1β can not only increase the expression and activation of MMPs,36 but also accelerate the breakdown of collagen II and proteoglycans.37 Furthermore, IL-1β is able to augment inflammation by inducing the production of pro-inflammatory cytokines, such as COX-2 and iNOS, at the early stage of OA.38 In this present study, we found that less mechanical loading significantly reduced the level of IL-1β compared to OA rats with normal mechanical loading, as well as COX-2 and iNOS.
The secretion and activation of IL-1β is mainly controlled by the NLRP3/caspase-1/IL-1β axis.39 The activation of NLRP3 inflammasome was thought to have an important role in the pathology of OA17,18 and other degenerative diseases.39 Moreover, some reports suggested that activation of caspase-1 contributed to the development of OA by accelerating cartilage destruction.40,41 However, there is no research, to our knowledge, regarding the relationship between the NLRP3/caspase-1/IL-1β axis and cartilage degeneration under different mechanical loading. The data presented in the current study suggest that this axis is activated in OA development, while less mechanical loading could downregulate the expression levels of NLRP3, caspase-1, and IL-1β and thus reduce inflammation status of OA joints.
Tail suspension model is widely used to create a disuse osteoporosis animal model.42,43 In this study, this model was adapted to remove the weight loading for OA joint and intermittent model was used to avoid the disuse atrophy. In order to test the effects of intermittent tail suspension on the bony structure of rats, the BMD of rats treated with intermittent weight unloading was compared with another six rats with full-time suspension without modelling surgery for OA. Data showed that the BMD of rats with intermittent unloading was comparable with that of normal controls, while being significantly greater than that of rats with full-time suspension (Supplementary Figure c). In addition, weight and obesity are important risk factors during the development of OA,34 and our data reveal that the weight of rats was comparable among the three groups at the endpoint of the experiment (Supplementary Figure d).
In conclusion, less mechanical loading of joints can alleviate cartilage destruction, subchondral bone destruction, and secondary inflammation during OA development, which may be due to the downregulation of NLRP3/caspase-1/IL-1β. Therefore, less mechanical loading rest can be a strategy for slowing down OA progression and may have clinical benefits for OA patients.
Author contributions
Z. He: Performed the experiments, Analyzed the results, Wrote the manuscript.
P. Nie: Performed the experiments, Collected and analyzed the data.
J. Lu: Performed the experiments, Collected the data.
Y. Ling: Performed the experiments, Collected and analyzed the data.
J. Guo: Performed the experiments, Collected the data.
B. Zhang: Performed the experiments, Analyzed the data.
J. Hu: Performed the experiments.
J. Liao: Performed the experiments, Collected the data.
J. Gu: Performed the experiments, Analyzed the data.
B. Dai: Performed the experiments.
Z. Feng: Designed the study, Performed the experiments, Analyzed the data, Wrote the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
Z. He reports an institutional grant (paid to Beilun District People’s Hospital) from The Program of Technology Bureau of Ningbo (No.2017A61018), related to this study. Z. Feng reports institutional grants (paid to Zhejiang University) from the Public Welfare Program of Science and Technology Department of Zhejiang Province (No. LGF19H060012), the Medicine and health science and technology plan in Zhejiang province (No. 2020RC058), and the Technology Program of Traditional Chinese Medicine Department of Zhejiang Province (No. 2017ZZ011), all related to this study.
Ethical review statement
All experiments were carried out following the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and all the procedures were preapproved by Beilun District People’s Hospital, Ningbo, China.
Supplementary material
Protocols of different experimental groups, the negative control for immunohistochemical examination, the microarchitectural indices of rats treated with intermittent weight unloading and full-time weight unloading, and the weight of rats among the experiments.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Martel-Pelletier J, Boileau C, Pelletier J-P, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. [DOI] [PubMed] [Google Scholar]
- 4.Kinds MB, Welsing PMJ, Vignon EP, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthritis Cartilage. 2011;19(7):768–778. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier J-P, Raynauld J-P, Berthiaume M-J, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove SE, Calcaterra SL, Brooker RM, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton CB, Pest MA, Pitelka V, et al. Weight-bearing asymmetry and vertical activity differences in a rat model of post-traumatic knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(7):1178–1185. [DOI] [PubMed] [Google Scholar]
- 8.Shimomura K, Kanamoto T, Kita K, et al. Cyclic compressive loading on 3D tissue of human synovial fibroblasts upregulates prostaglandin E2 via COX-2 production without IL-1β and TNF-α. Bone Joint Res. 2014;3(9):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiegant K, van Roermund PM, Intema F, Roermund PV, et al. Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(11):1660–1667. [DOI] [PubMed] [Google Scholar]
- 10.Intema F, Wiegant K, Roermund PMV, et al. Tissue structure modification in end-stage knee osteoarthritis by use of joint distraction. Ann Rheum Dis. 2011;70:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennell KL, Nelligan RK, Kimp AJ, et al. Comparison of weight bearing functional exercise and non-weight bearing quadriceps strengthening exercise on pain and function for people with knee osteoarthritis and obesity: protocol for the TARGET randomised controlled trial. BMC Musculoskelet Disord. 2019;20(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Huang P, Li G, et al. Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis. Bone Joint Res. 2019;8(9):425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blom AB, van der Kraan PM, van den Berg WB. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8(2):283–292. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- 15.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L-R, Xing R-L, Wang P-M, et al. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol Med Rep. 2018;17(4):5463–5469. [DOI] [PubMed] [Google Scholar]
- 18.McAllister MJ, Chemaly M, Eakin AJ, Gibson DS, McGilligan VE. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthritis Cartilage. 2018;26(5):612–619. [DOI] [PubMed] [Google Scholar]
- 19.Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3(2):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S, Songlin P, Zhang G, et al. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone. 2009;45(3):534–544. [DOI] [PubMed] [Google Scholar]
- 21.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–S34. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017;44(4):410–417. [DOI] [PubMed] [Google Scholar]
- 24.Xu B, Xing R, Huang Z, et al. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019;228:158–166. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R-K, Li G-W, Zeng C, et al. Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1). Bone Joint Res. 2018;7(11):587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafeber FPJG, Intema F, Van Roermund PM, Marijnissen ACA. Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol. 2006;18(5):519–525. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Sun Y, Pan X, Ho K, Li G. Joint distraction attenuates osteoarthritis by reducing secondary inflammation, cartilage degeneration and subchondral bone aberrant change. Osteoarthritis Cartilage. 2015;23(10):1728–1735. [DOI] [PubMed] [Google Scholar]
- 28.Intema F, Thomas TP, Anderson DD, et al. Subchondral bone remodeling is related to clinical improvement after joint distraction in the treatment of ankle osteoarthritis. Osteoarthritis Cartilage. 2011;19(6):668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flouzat-Lachaniette C-H, Roubineau F, Heyberger C, Bouthors C. Distraction to treat knee osteoarthritis. Joint Bone Spine. 2017;84(2):141–144. [DOI] [PubMed] [Google Scholar]
- 30.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2):204–211. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Kiraly AJ, Sun AR, et al. Effects of a phosphocitrate analogue on osteophyte, subchondral bone advance, and bone marrow lesions in Hartley guinea pigs. Bone Joint Res. 2018;7(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhen G, Wen C, Jia X, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McErlain DD, Ulici V, Darling M, et al. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res Ther. 2012;14(1):R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sansone V, Applefield RC, De Luca P, et al. Does a high-fat diet affect the development and progression of osteoarthritis in mice?: a systematic review. Bone Joint Res. 2019;8(12):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei L, Fleming BC, Sun X, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res. 2010;28(7):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanbe K, Takemura T, Takeuchi K, et al. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2004;86-B(2):296–300. [DOI] [PubMed] [Google Scholar]
- 37.Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Guan P-P, Guo C, et al. Fluid shear stress-induced osteoarthritis: roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. Faseb J. 2013;27(12):4664–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. [DOI] [PubMed] [Google Scholar]
- 40.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–1821. [DOI] [PubMed] [Google Scholar]
- 41.Joosten LAB, Netea MG, Fantuzzi G, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60(12):3651–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang R, Supowit SC, Klein GL, et al. Rat tail suspension reduces messenger RNA level for growth factors and osteopontin and decreases the osteoblastic differentiation of bone marrow stromal cells. J Bone Miner Res. 1995;10(3):415–423. [DOI] [PubMed] [Google Scholar]
- 43.Ho M-L, Tsai T-N, Chang J-K, et al. Down-regulation of N-methyl D-aspartate receptor in rat-modeled disuse osteopenia. Osteoporos Int. 2005;16(12):1780–1788. [DOI] [PubMed] [Google Scholar]