Abstract
Current reports concerning cardiac involvement in the novel corona virus disease (COVID-19) mostly document acute myocardial injury at presentation. Here, we present a healthy young male, with presumed acute myocarditis, presenting 20 days after initial diagnosis of COVID-19 – and after a clinical, and apparent laboratory, resolution of the original episode. His sole substantial clinical finding upon admission was fever, which was followed by a witnessed elevation in troponin-I.
Key Indexing Terms: COVID-19, SARS-CoV-2, Myocarditis, Myocardial injury, Viral cardiovascular complications
Introduction
The novel corona virus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originated in China during December 2019, and rapidly became a worldwide pandemic. Evidence of cardiac involvement of SARS-CoV-2 infection is growing as the disease continues to spread.1 While the incidence of SARS-CoV-2-positive patients presenting with a spectrum of cardiovascular syndromes is in the rise, various mechanisms for the pathophysiology underlying such myocardial injury were postulated.1, 2, 3 Interestingly, hypothesized mechanisms include a direct viral insult to the heart, as well as an indirect myocardial injury secondary to the overwhelming inflammatory response.1 While such mechanisms are yet to be established, empiric data currently suggest a negative correlation between an elevation of cardiac biomarkers and patients’ prognosis.1 , 4 , 5 Notably, reports thus far have focused mostly on the immediate and severe cardiac manifestations of SARS-CoV-2 infection, rather than the delayed cardiac complications of the disease.
Case presentation
Here, we present the case of a healthy and physically active 21-year-old male, with unremarkable medical history with the exception of social smoking who presented to the Corona Department with a fever twenty days after first being diagnosed with COVID-19.
The patient was initially tested for SARS-CoV-2 based on his complaints of fever, headache, cough and a concomitant mild pleuritic chest pain, all of which had elapsed within 3 days. Following a positive COVID-19 diagnosis, he was monitored in the setting of COVID-19 ambulatory services of the Israeli Health Maintenance Organization. Eight days after his first positive SARS-CoV-2 test, he tested negative twice for SARS-CoV-2 with a 72-hour interval between these tests. All SARS-CoV-2 examinations mentioned in this case were performed using a polymerase chain reaction evaluation of nasopharyngeal and oropharyngeal swabs. One week after his second negative test, he experienced a loss of smell, based on which he was re-tested for SARS-COV-2, and was found positive. Shortly afterwards he also became feverish again, and following three consecutive days with high fevers, he was referred to our hospital.
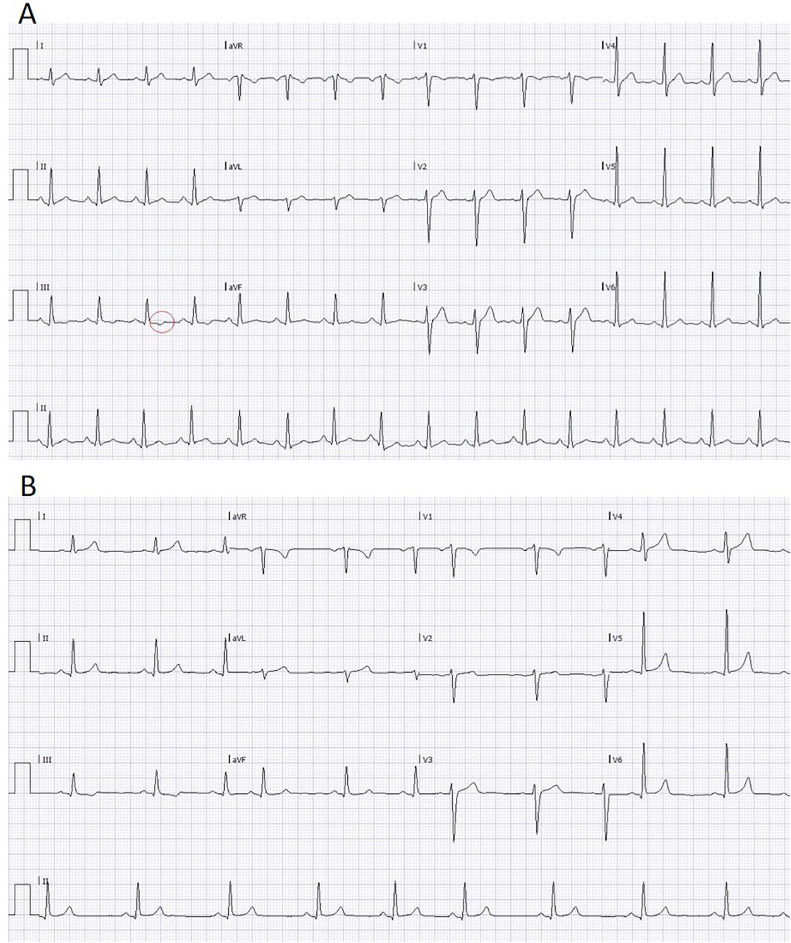
Upon admission he was stable, with vital signs as following: body temperature of 38.5 °C, heart rate of 110 beats per minute (BPM), oxygen saturation of 96% in room air, blood pressure of 130/70 mmHg. In between febrile episodes he remained relatively tachycardic with a basal heart rate of ~90 BPM. Physical examination revealed a slight hepatosplenomegaly, with no other abnormal findings. Initial laboratory at admission showed a normal, yet measurable Troponin-I (Trop-I) (17 ng/L; normal value <34), as well as elevated C-reactive protein (CRP) (3.87 mg/dl; normal value <0.5) and d-Dimer (2463 ng/ml; normal value <500). Electrolytes, creatinine, and blood count were all within the normal range. Repeated blood panels 36 h after admission were significant for high Trop-I (965 ng/L) and elevated hepatocellular transaminases, up to twice the upper limit of the norm (aspartate transaminase was 52 U/L [0–34] and alanine transaminase was 68 U/L [0–55]). Electrocardiograms (ECG), from both his admission and during his hospitalization, did not demonstrate changes consistent with pericarditis and/or myocardial infarction, and the patient did not suffer from any chest discomfort or dyspnea at any time during his stay in our department. Nonspecific findings in his ECGs, inconsistent with criteria of the above diagnoses, included a minimal ST-depressions and T-wave inversions in lead III, and one-millimeter PR depressions on leads II and III. On subsequent ECGs, PR intervals were normalized within 24 h, while T wave inversions remained unchanged (Fig. 1 ).
Figure 1.
Electrocardiogram during peak illness and following resolution.
A. An ECG, conducted on the day of peak Trop-I levels, demonstrating sinus tachycardia and minimal ST depressions with T wave inversions on lead III. In addition, ~1 mm PR depressions are evident on leads II and III. B. An ECG, conducted on the day of patient's discharge, demonstrating normalization of PR interval changes denoted in A, while the previously noted T wave inversions have persisted.
Other causative viruses of myocarditis were investigated (cytomegalovirus, Epstein-Barr virus, West Nile virus, Coxiella burnetii, Parvovirus B19, Human herpesvirus 6, Toxoplasma, Hepatitis B virus, Hepatitis C virus, and Human immunodeficiency virus), all of which were excluded by serologic tests. Immunoglobulins G (IgG) for SARS-CoV-2 were detected 2 days following his admission. Bacterial blood cultures were all negative. Computed tomography angiography exhibited no evidence of a pulmonary embolism. Likewise, no pulmonary infiltrates, pericardial or pleural effusion, signs of congestion, or enlargement of the heart's chambers were demonstrated.
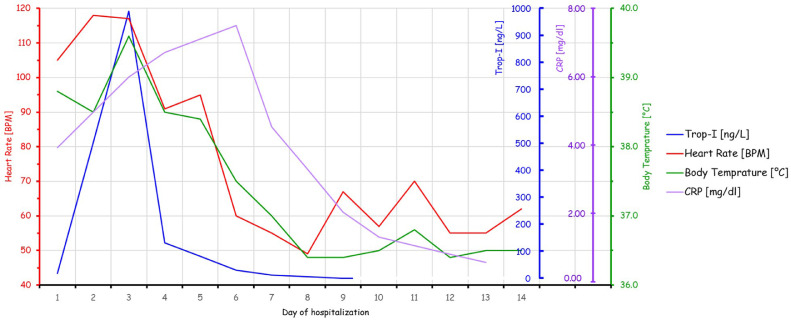
The patient was treated symptomatically, and both Trop-I and CRP have gradually decreased. On the sixth day of hospitalization – concomitantly with Trop-I level dropping to 29 ng/L, and NT-proBNP level of 29.9 pg/mL (normal value <300) – the patient reported a significant clinical improvement, and was no longer febrile for the first time in a nine days. Trop-I was further monitored down to a level <5 ng/L at the ninth day of hospitalization, at which time basal heart-rate decreased to 60 BPM underscoring that his previous heart rate recordings of 90 BPM at rest, indeed reflected a significant relative tachycardia. Interestingly, his heart rate, body temperature, and Trop-I levels, all trended in a synchronized manner during hospitalization, while his CRP level seemed to follow a similar course – with a delay of ~1 day (Fig. 2 ). Taken together, these findings point out that the initial detected Trop-I level, though seemingly within the norm, was in fact an ominous sign. Ten days after the resolution of his symptoms, and with 3 negative tests for SARS-CoV-2, a transthoracic echocardiogram demonstrated a normal left ventricular ejection fraction of 65%, and a normal global contraction with no wall-motion abnormalities.
Figure 2.
Trends in heart rate, body temperature, Trop-I, and CRP during hospitalization.
Trop-I and CRP levels were obtained on days 1,3,4,6,7,9, while CRP was obtained also on days 10 and 13. As Trop-I levels lower than 5 ng/L are undetectable, a value of 0 was assigned when the laboratory result was unmeasurable on day 9. Heart rate and temperature were measured daily. Day 1 represents the patient's admission to our hospital, while on day 14 he was discharged.
Discussion
Clinical and laboratory findings in this case, support a diagnosis of subclinical acute myocarditis. The timely dynamics of fever, heart rate, Trop-I, and CRP seems to best fit a myocardial injury secondary to an inflammatory response, hypothesized to underlie extrapulmonary manifestations of SARS-COV-2 infection.1, 2, 3
Other reports concerning cardiac manifestations of COVID-19, published thus far, mostly describe moderate to severely ill patients, many of whom had symptoms suggestive of cardiac and respiratory compromise at the time of initial presentation and COVID-19 diagnosis.6, 7, 8, 9, 10 In this case, however, cardiac damage was isolated; developed approximately 3 weeks following the initial diagnosis; and was preceded by a latent, asymptomatic period with laboratory results indicative of a cure. Furthermore, with the exception of fever and tachycardia, no other symptoms suggestive of a cardiac etiology, were present.
Given the existence of an asymptomatic period and documented negative SARS-CoV-2 PCR tests, followed by the reappearance of fever and a positive SARS-CoV-2 PCR test, the question of a possible reinfection is inevitable. There are few reports concerning patients tested positive for the second time – after a documented resolution of a SARS-CoV-2 infection by means of a negative PCR test, accompanied also by a clinical resolution of symptoms.11, 12, 13 Conflicting evidence exist concerning the role of the initial immune response in these cases: while the immune response to SARS-CoV-2 and generation of immunoglobulins have been investigated,14 , 15 their implications during re-exposure remain uncertain. Some studies suggest that such antibodies may play a protective role in face of re-exposure, as presented in a case report of a 69-year-old female patient,11 and in a controlled experiment in Rhesus Macaques monkeys.16 On the other hand, reports presenting a significant number of patients have described a second infection, with a prominent clinical presentation and a positive PCR test for SARS-CoV-2 – after resolution of the original episode, by means of both aspects.12 , 13 Importantly, for most documented cases of such re-infection, the presence (or absence) of an acquired immune response to the primary infection was not evaluated. It is still unclear whether the immune protection following convalescence is, in some cases, insufficient, or whether an infection and resolution do not necessarily result in the acquisition of immunity. It is also uncertain whether negative PCR tests in-between episodes, reflect viral loads below detection threshold as part of a gradual, yet incomplete, recovery (i.e. a false negative), or indeed a complete resolution.17
Based on the short asymptomatic period, and the presumed myocarditis presenting ~3 weeks following the initial infection, we tend to assume reactivation of a non-resolved viral infection, rather than re-infection, as the etiology in this case. A definite distinction between a false negative PCR test during the asymptomatic period as part of a continuous illness vs re-infection after a true convalescence is yet to be determined.
Notably, if re-infection was indeed the underlying cause in the case presented herein, it may represent an unusual case of myocarditis as the main presentation following re-infection which, to our knowledge, has yet to be documented.
In conclusion, the case described herein suggests that fever and tachycardia may be the only clues for a COVID-19-related myocarditis, which can present late in the course of the disease, isolated from other organ damage, and despite apparent resolution of the primary viral infection.
Footnotes
The authors declare that there is no conflict of interest.
Funded by Internal Medicine “A” department foundation.
References
- 1.Akhmerov A., Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendren NS, Drazner MH, Bozkurt B, Cooper, Jr. LT. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020:1903–1914. 10.1161/circulationaha.120.047349 [DOI] [PMC free article] [PubMed]
- 3.Siripanthong B., Nazarian S., Muser D. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Hear Rhythm. May 2020 doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoso A, Pranata R, Wibowo A, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. 10.1016/j.ajem.2020.04.052 [DOI] [PMC free article] [PubMed]
- 5.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng J-H, Liu Y-.X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 1:3. 10.1007/s15010-020-01424-5 [DOI] [PMC free article] [PubMed]
- 7.Doyen D, Moceri P, Ducreux D, Dellamonica J. Clinical picture myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes A C B D SV1 RV5. 2020. 10.1016/S0140-6736(20)30912-0 [DOI] [PMC free article] [PubMed]
- 8.Sala S. Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020:1–2. 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed]
- 9.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luetkens J.A., Isaak A., Zimmer S. CARDIOVASCULAR IMAGES diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging circulation: cardiovascular imaging. Circ Cardiovasc Imaging. 2020;13:10897. doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 11.Bentivegna E., Sentimentale A., Luciani M., Speranza M.L., Guerritore L., Martelletti P. New IgM seroconversion and positive RT-PCR test after exposure to the virus in recovered COVID-19 patient. J Med Virol. June 2020 doi: 10.1002/jmv.26160. jmv.26160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludovic L, Thomas C, Luc G, et al. Recurrence or relapse of COVID-19 in older patients: a description of three cases. Available at:https://onlinelibrary-wiley-com.rambam-ez.medlcp.tau.ac.il/doi/epdf/10.1111/jgs.16728. Accessed August 19, 2020. [DOI] [PMC free article] [PubMed]
- 13.Batisse D., Assistance M.D., Benech N. Journal pre-proof clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020 doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2019;1:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grifoni A., Weiskopf D., Ramirez S.I., Smith D.M., Crotty S., Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. .e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrashekar A, Liu J, Martinot AJ, et al.SARS-CoV-2 Infection Protects Against Rechallenge in Rhesus Macaques. Available at: http://science.sciencemag.org/. Accessed August 19, 2020. [DOI] [PMC free article] [PubMed]
- 17.Kang H., Wang Y., Tong Z., Liu X. Retest positive for SARS-CoV-2 RNA of “recovered” patients with COVID-19: persistence, sampling issues, or re-infection? J Med Virol. 2020;1 doi: 10.1002/jmv.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]