Abstract
A sequence of consensus-based Rome criteria for irritable bowel syndrome (IBS) has been published since 1989. The fundamental definition based on abdominal pain in association with bowel dysfunction has been consistent. However, two major changes occurred in the Rome II and IV criteria. The former change involved “splitting off” of symptoms that were not consistently associated with pain, such as functional, constipation, diarrhea, and bloating. In Rome IV, the main changes were the exclusion of discomfort (in contrast to pain) and the more stringent frequency criteria for the pain to be eligible for diagnosis of IBS (specifically, on average, at least 1 day per week in the last 3 months). Validation studies of the consensus, symptom-based criteria have identified multiple deficiencies that question the rationale for “splitting” the different syndromes, and favor a simpler identification of the classical symptoms of abdominal pain, bowel dysfunction, and bloating, and exclusion of alarm symptoms. Advances in the identification of actionable biomarkers related to the symptoms suggestive of functional gastrointestinal disorders have the potential to usher a change in practice from positive diagnosis of symptom complexes followed by empirical treatment to identification of the mechanisms causing the symptoms and targeted therapy.
Keywords: bloating, constipation, diarrhea, mood, pain, psychology, sex, somatization
1 |. INTRODUCTION
The concept of positive diagnosis of irritable bowel syndrome (IBS) based on symptoms kicked off with studies from the University of Bristol; Manning et al identified 4 symptoms that were clearly more common in patients with IBS: abdominal distension as evidenced by tight clothing or visible appearance; pain relief with bowel action; more frequent stools with the onset of pain; and looser stools with the onset of pain.1
Justification for the development of symptom-based criteria for irritable bowel syndrome is based on the perspectives of several stakeholders: From the patient perspective, effective diagnostic criteria would be expected to improve outcomes by enabling clinicians to diagnose and choose treatments that are likely to be effective. For researchers, accepted criteria would be expected to stratify patient cohorts for research studies. For industry involved in the development of novel approaches to treatment, the availability of effective, evidence-based criteria enables the development of such treatments and convinces regulators and payers that functional gastrointestinal disorders and, specifically, irritable bowel syndrome are real disorders that are amenable to efficacious treatments. A sequence of consensus-based Rome criteria for IBS has been published since 19892–5 (Table 1). The fundamental definition based on abdominal pain in association with bowel dysfunction has been consistent. However, 2 major changes occurred in the Rome II and IV criteria. The former change involved “splitting off” of symptoms that were not consistently associated with pain, such as functional, constipation, diarrhea, and bloating. In Rome IV, the main changes have been the exclusion of discomfort (in contrast to pain) and the more stringent frequency criteria for the pain to be eligible for diagnosis of IBS (specifically, on average, at least 1 day per week in the last 3 months).
TABLE 1.
Summary of Rome I-IV criteria
| Rome I. IBS criteria (from ref. 2) |
| Continuous or recurrent symptoms of: |
| 1. Abdominal pain, relieved with defecation, or associated with a change in frequency or consistency of stool; AND/OR |
| 2. Disturbed defecation (2 or more of): a. Altered stool frequency, b. Altered stool form (hard or loose/watery), c. Altered stool passage (straining or urgency, feeling of incomplete evacuation), d. Passage of mucus; USUALLY WITH |
| 3. Bloating or feeling of abdominal distension. |
| Rome II criteria (from ref. 3) |
| Functional bowel disorders recognized: irritable bowel syndrome, functional abdominal bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder |
| IBS criteria: At least 12 wk, which need not be consecutive, in the preceding 12 mo of abdominal discomfort or pain that has 2 or 3 features: |
| 1. Relieved with defecation; and/or |
| 2. Onset associated with a change in frequency of stool; and/or |
| 3. Onset associated with a change in form (appearance) of stool. |
| Rome II “Splitting” of non-IBS criteria: At least 12 wk, which need not be consecutive, in the preceding 12 mo of: |
| Functional abdominal bloating: |
| 1. Feeling of abdominal fullness, bloating or visible distension; And |
| 2. Insufficient criteria for a diagnosis of functional dyspepsia, IBS, or other functional disorder. |
| Functional constipation: |
| 1. Straining; 2. Lumpy or hard stools 3. Sensation of incomplete evacuation 4. Sensation of anorectal obstruction/blockade in >1/4 defecations; 5. Manual maneuvers to facilitate >1/4 defecations (eg, digital, evacuation, support of the pelvic floor); and/or 6. <3 defecations/wk. |
| Loose stools are not present, and there are sufficient criteria for IBS. |
| Functional diarrhea: |
| 1. Liquid (mushy) or watery stools; |
| 2. Present > 3/4 of the time; and |
| 3. No abdominal pain. |
| Rome III (from ref. 4) |
| Functional bowel disorders recognized: irritable bowel syndrome, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder. |
| IBS: Recurrent abdominal pain or discomfort (an uncomfortable sensation not described as pain) at least 3 days per month in the last 3 mo associated with 2 or more of the following: |
| 1. Improvement with defecation |
| 2. Onset associated with a change in frequency of stool |
| 3. Onset associated with a change in form (appearance) of stool |
| Criteria fulfilled for the last 3 mo with symptom onset ≥6 mo prior to diagnosis. |
| Subtyping IBS by predominant stool pattern |
| IBS-C–hard or lumpy stools ≥25% and loose (mushy) or watery stools <25% of BMs |
| IBS-C–hard or lumpy stools ≥25% and loose (mushy) or watery stools <25% of BMs |
| IBS-D–loose (mushy) or watery stools ≥ 25% and hard or lumpy stool < 25% of BMs |
| IBS-M–hard or lumpy stools ≥ 25% and loose (mushy) or watery stools ≥ 25% of BMs |
| Unsubtyped IBS–insufficient abnormality of stool consistency to meet criteria for IBS-C, D, or M |
| Functional Constipation: must include ≥2 of: |
| Straining during ≥25% of defecations |
| Lumpy or hard stools in ≥25% of defecations |
| Sensation of incomplete evacuation for ≥25% of defecations |
| Sensation of anorectal obstruction for ≥25% of defecations |
| Manual maneuvers to facilitate ≥25% of defecations (eg, digital, evacuation, support of the pelvic floor) |
| <3 defecations per week |
| Loose stools are rarely present without the use of laxatives |
| Insufficient criteria for IBS |
| Functional Diarrhea |
| Loose (mushy) or watery stools without pain in ≥75% of stools |
| Criteria fulfilled for the last 3 mo with symptom onset ≥6 mo before diagnosis. |
| Rome IV Bowel Disorders (from ref. 5) |
| Functional bowel disorders recognized: irritable bowel syndrome, functional abdominal bloating/distension, functional constipation, functional diarrhea, unspecified functional bowel disorder, opioid-induced constipation |
| IBS: Recurrent abdominal pain, on average, at least 1 day per week in the last 3 mo, associated with 2 or more of the following criteria: |
| 1. Related to defecation |
| 2. Associated with a change in frequency of stool |
| 3. Associated with a change in form (appearance) of stool |
| Criteria fulfilled for the last 3 mo with symptom onset ≥6 mo before diagnosis. |
| Rome IV Bowel Disorders: Diagnostic Criteria for IBS Subtypes (from ref. 5) |
| Predominant bowel habits are based on stool form on days with at least 1 abnormal bowel movement. |
| IBS with predominant constipation (IBS-C) |
| >25% of BMs with Bristol Stool Form Scale (BSFS) types 1 or 2 and <25% of BMs with BSFS types 6 or 7 |
| IBS with predominant diarrhea (IBS-D) |
| >25% of BMs with BSFS types 6 or 7 and <25% of BMs with BSFS types 1 or 2 |
| IBS with mixed bowel habits (IBS-M) |
| >25% of BMs with BSFS types 1 or 2 and >25% of BMs with BSFS types 6 or 7 |
| IBS unclassified (IBS-U) |
| Meets criteria for IBS, but BMs not accurately categorized into 1 of the 3 groups above. |
The consensus criteria for IBS were pivotal 3 decades ago for establishing positive clinical diagnosis of IBS based on symptoms. Epidemiological and clinical studies now call for a less stringent subclassification of symptoms and more thorough clinical evaluation of dysfunctions causing the primary symptoms, with the opportunity to individualize treatment. This review appraises important observations over the past 3 decades that evaluated the performance and utility of the stringent Rome criteria in clinical practice and in epidemiological studies.
2 |. OVERLAP AND TRANSITION OF SYMPTOMS AND SYMPTOM COMPLEXES: “TO SPLIT” OR “NOT TO SPLIT”
A major initiative that started in the Rome II criteria was the splitting off of conditions that were deemed to be non-IBS (functional abdominal bloating, functional constipation, and functional diarrhea) from IBS. Rome III subcategorized IBS based on predominant stool pattern, that is, IBS-constipation (IBS-C), IBS-diarrhea (IBS-D), IBS-mixed (IBS-M), and unsubtyped IBS.
Since those modifications to the Rome criteria, several studies have documented the overlap of symptoms suggestive of functional gastrointestinal disorders with IBS6 (Figure 1). In addition, it has become clear that, in the same patient, there could be a transition of the symptoms over time, such that a patient may bridge the separation between “diagnostic” entities. This is most clearly documented in transitions from IBS-D to IBS-M, or from both of these entities to functional diarrhea.6,7 Another example is the transition from IBS-C to functional constipation. In these transitions, a minor alteration in a patient’s perception of the bowel function or the reduced experience of pain may be sufficient for metamorphosis of the diagnoses between the mentioned categories. An additional confounder is appreciated by the differences in definitions of pain in the context of IBS in Rome III and Rome IV,8 which reduced the prevalence of IBS by half among adults in the United States, Canada, and the UK, but increased the prevalence of functional constipation and functional diarrhea.
FIGURE 1.
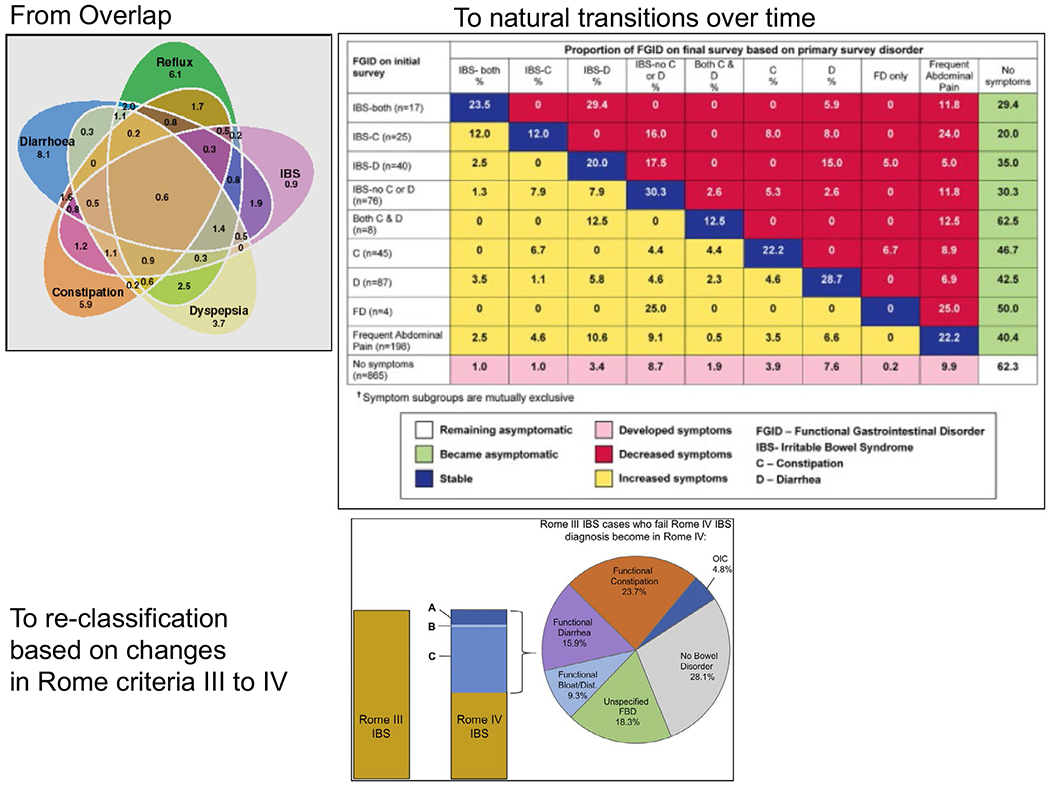
Overlap and transitions in IBS (reproduced from references 6–8)
An informative example based on a clinical study has questioned the rationale for splitting IBS-D and functional diarrhea, given similarities in clinical and psychosocial characteristics of patients in these 2 entities.9 Thus, an analysis of 48 patients with functional diarrhea and 49 patients with IBS-D at Beth Israel Hospital, Boston, showed no significant differences in the clinical symptoms of the 2 groups other than pain and discomfort (which are implicit, based on the definitions of the groups). In addition to the lack of differences in the clinical symptoms, these 2 groups of patients manifested similar scores of anxiety, depression, sleep disturbance, and somatization, leading the investigators to conclude that the 2 entities are in a continuum.9
In summary, the accumulated evidence in the literature points to the redundancy that has followed the splitting of the main bowel function-related disorders, and argues in favor of a clinicophysiological appraisal, particularly for patients presenting with chronic functional constipation or diarrhea in the absence of alarm symptoms. By the same token, it is relevant to note that there is no current fda-approved drug for chronic functional abdominal pain, and in the future, it is essential to use criteria such as those in Rome III to identify patients with functional abdominal pain, to develop robust patient response outcome endpoints and, thereby, appraise the efficacy of candidate visceral analgesics such as those reviewed elsewhere.10,11
3 |. IMPRECISION OF SYMPTOM CRITERIA USED TO SUBCATEGORIZE PATIENTS WITH CONSTIPATION: THE NEED TO IMPROVE THE CHARACTERIZATION OF PATIENTS WITH CONSTIPATION
There are several symptoms that are included in both “diagnostic criteria” of functional constipation that are highly suggestive of associated rectal evacuation disorders. Clinical experience and clinical trials have demonstrated that the latter patients respond best to retraining of the evacuation process, whether this is delivered in home or in the office, or it includes electromyography or transanal electrostimulation or sensation retraining.12–14 More importantly, it is clear that patients with constipation due to dyssynergic defecation respond better to biofeedback retraining than to standard treatment for constipation,15,16 specifically diet, exercise, and laxative or diazepam. The symptoms suggestive of dyssynergic defecation that are included in the criteria for functional constipation or in the inclusion criteria for pharmacological studies17 based on “modified Rome criteria” are straining, sensation of incomplete evacuation, sensation of anorectal obstruction, or manual maneuvers to facilitate defecation (eg, digital evacuation, support of the pelvic floor) in >25% of defecations. Failure to recognize such symptoms as part of pelvic floor dyssynergia, or descending perineum syndrome, or an anatomical cause of impaired defecation (eg, mucosal prolapse or rectocele), and attributing such symptoms to functional constipation (or to transitioning diagnosis of IBS-C) could represent a missed opportunity for effective management.
In summary, it is essential to tease out, in greater detail, symptoms that are highly suggestive of rectal evacuation disorders in the appraisal of patients who present with chronic functional constipation, so that such patients may be appropriately investigated and managed.
4 |. THE IMPACT OF VARIABLE PAIN SEVERITY IN DIFFERENT ROME CRITERIA ON DIAGNOSIS
The emphasis on pain rather than discomfort and the greater stringency regarding the frequency of pain in the Rome IV definition of IBS have resulted in halving the prevalence of IBS7 and have led to “reclassification” of patients from IBS to other functional bowel disorders. It is even more relevant to note that the requirement for recurrent abdominal pain, on average, at least 1 day per week in the Rome IV criteria has “enriched” the IBS cohort with patients with more severe symptoms, and with higher-level somatization, mood, and psychological disorders,18–20 and has led to the same patients being reclassified into different subcategories of lower functional gastrointestinal disorders. This is supported by several independent studies, 3 of which are described in more detail below.
Firstly, among 542 Swedish patients, 15% of IBS patients who were positive on Rome III criteria were negative on Rome IV criteria. Rome IV–positive patients were significantly more likely to be female, have poorer quality of life, and have greater pain severity, bloating, somatization, fatigue, and rectal sensitivity than Rome IV–negative patients.18
Secondly, in a cross-sectional survey of 1368 individuals in the United Kingdom who self-identify as having IBS, applying the Rome IV criteria instead of Rome III reduced the proportion of patients who would receive a diagnosis of IBS from almost 79% (fulfilling Rome III criteria) to 51% (positive based on Rome IV criteria). Individuals with Rome IV–defined IBS had more severe symptoms and higher levels of mood disorder and poorer psychological health, compared with people who only met the Rome III criteria for IBS. Moreover, agreement between the Rome III and Rome IV criteria was only moderate [Kappa (0.50)]. Among those 269 who no longer had IBS according to the Rome IV criteria, 11.5% met Rome IV criteria for functional constipation, 41.3% for functional diarrhea, 23.8% for functional abdominal bloating or distension, and 23.4% for an unspecified functional bowel disorder.19 This is consistent with the analysis of Palsson et al who reported that those Rome III–positive patients from the United States, Canada, and the UK who did not fulfill Rome IV criteria for IBS were classified as 23.7% functional constipation, 15.9% functional diarrhea, 9.3% functional abdominal bloating or distension, 18.3% an unspecified functional bowel disorder, 28.1% no bowel disorder, and 4.8% opioid-induced constipation.8
Thirdly, a study was conducted in the Netherlands in 404 patients who were positive for IBS by Rome III criteria and underwent a 14-day daily diary assessment of symptoms, and the Hospital Anxiety and Depression (HAD) and quality of life questionnaires. In this study, the patients who fulfilled Rome IV criteria were more often female, younger, recruited from secondary/tertiary care, had higher abdominal pain scores and gastrointestinal symptom severity, higher psychological symptom scores, and lower quality of life compared with Rome IV–negative subjects.20
In summary, while it could be argued that the more stringent Rome IV criteria have achieved the objective of reducing the overall prevalence of IBS, an unintended consequence is that the patients fulfilling the classical criteria for IBS may be denied treatments that are approved by regulatory agencies, based on clinical trials performed using Rome II and Rome III criteria for IBS.
5 |. DIAGNOSTIC PERFORMANCE OF SYMPTOM-BASED CRITERIA OF IBS IN CLINICAL PRACTICE
The diagnostic performance21 of symptom-based criteria for IBS has been assessed in 318 consecutive adults with lower gastrointestinal symptoms, all of whom underwent complete colonoscopy to cecum or terminal ileum. The sensitivity and specificity of Rome III criteria for IBS were 69.6% and 82.0%, respectively, with positive and negative likelihood ratios of 3.87 and 0.37, respectively. The diagnostic performance was considerably enhanced by additional history (nocturnal stools, somatization, and affective disorders) and measurements of hemoglobin and CRP. Indeed, individually or in combination, these items enhanced the symptom-based Rome III criteria for IBS with positive likelihood ratios to ≥5 (defining a potentially useful attribute) and increased specificity to ≥95%: HAD score alone; combination of normal hemoglobin and CRP with a high level of somatization; and combination of no nocturnal passage of stool with a high level of somatization. This is particularly relevant, as screening for anxiety, depression, and somatization is relatively easily achieved in clinical practice.
It is intriguing to note that, in 1984, Kruis et al22 proposed a scoring system for the diagnosis of IBS incorporating blood count (both hemoglobin level and total leukocyte count) and erythrocyte sedimentation rate, in addition to clinical features. The Kruis scoring system included features from the case history (abdominal pain, irregular bowel function, >2-year history, descriptors of the pain, alternating bowel function, fever > 38.5 degrees centigrade during the last week, weight loss > 5 kg in the past 6 months), history of blood in the stool, abnormal physical examination, and some basic investigations including erythrocyte sedimentation rate (>20 mm in the first hour), hemoglobin (<12 g/dL in females and <14 g/dL in males), and leucocytosis > 10 000/μL.
In summary, it is essential to incorporate the recommendations to assess additional history (nocturnal stools, somatization, and affective disorders) and measurements of hemoglobin and CRP in patients in whom IBS is suspected, in order to enhance the diagnostic capability of the symptom-based criteria.
6 |. IMPLICATIONS OF CHANGES IN IBS CRITERIA FOR PHARMACOLOGICAL TREATMENTS
Splitting the lower functional gastrointestinal disorders (FGIDs) with the same bowel dysfunction, for example, IBS-C and functional constipation, was embraced by regulatory agencies such as the USA Food and Drug Administration and the European Medicines Agency and has essentially doubled the size and increased the costs of drug development programs for medications that were ultimately approved for both IBS-C and functional (chronic idiopathic) constipation. These drugs include17,23–38 lubiprostone, linaclotide, plecanatide, and, initially, tegaserod (which is now only approved for IBS-C).
It is also relevant to note that the continued modification of the criteria over the decades has posed questions as to the applicability of results from prior clinical trials conducted in patients who fulfilled an earlier version of the Rome criteria. This would be apparent if one were to diagnose clinical patients using the most recent Rome IV criteria for IBS, and utilize the evidence from trials of patients who fulfilled Rome III criteria. As indicated above, the severity of symptoms and psychological and somatization disorders associated with Rome IV–positive patients may render them less likely to respond to treatments based on clinical trials conducted in patients with criteria consistent with Rome II or Rome III.
In summary, the regulatory agencies should revisit the question as to whether duplication of drug development programs is really warranted in the context of lower functional gastrointestinal disorders associated with constipation or diarrhea.
7 |. LOOKING BACK AND THE STRAIGHT WAY FORWARD FROM ROME CRITERIA
A comment39 published in 1998 after Rome II criteria were communicated seems to still be appropriate: “The benefits from their application in research have been quite apparent to investigators in the field, to regulatory agencies, and to the pharmaceutical industry as they have, to some extent, facilitated communication and clarified the inclusion criteria for clinical trials.” However, even then, a number of deficiencies were identified: Firstly, the specificities of symptoms were intrinsically low, necessitating exclusion of organic (eg, mucosal) diseases; secondly, that “functional” disorders may result from structural disorders such as excessive perineal descent or failure of puborectalis relaxation; thirdly, that Rome criteria impose “arbitrary constraints” such as the percentage of times a symptom has to be experienced to be considered relevant; and fourthly, recall of frequency of symptoms over a 6-month period is notoriously inaccurate. Nevertheless, the comment added: “Despite these problems, the adoption of these criteria by many investigators and trials has provided a welcome, self-imposed discipline on the field of study. This discipline facilitates comparisons of results in trials or physiological observations in more mechanistic studies.”
8 |. GENERALIZABILITY OF SYMPTOM-BASED CRITERIA
An important initiative of the Rome Foundation is the study of epidemiology of functional gastrointestinal disorders. These now include worldwide studies,40 which found that more than 40% of persons worldwide have functional gastrointestinal disorders which affect quality of life and healthcare use. Such studies and others are also documenting evidence that the symptom complexes do not necessarily ring true in Asian populations.41,42
9 |. CONCLUSIONS
Three decades after the first iteration of Rome criteria, it is time to acknowledge that there have been advances that need to be incorporated into clinical practice and research.
Firstly, straightening the road from the Rome criteria should start with the appreciation that the symptoms and symptom clusters are non-specific and are never going to be diagnostic on their own.
Secondly, it is time to incorporate, in patients unresponsive to first-line therapy, recent advances in understanding of the mechanisms that result in the dysfunction and associated symptoms of constipation, diarrhea, bloating, and pain or discomfort, based on clinical research43 and validated actionable biomarkers44 (Figure 2). The latter can be applied in clinical practice to identify “organic” disorders of function such as rectal evacuation disorders (based on anorectal manometry, balloon expulsion, area of rectal gas, and stool on abdominal X-ray), slow transit constipation (based on stool burden on abdominal radiography or marker transit), fast transit diarrhea (based on scintigraphy), bile acid diarrhea (based on 75SeHCAT retention, 48-h fecal bile acid quantitation, or serum-based tests), and carbohydrate maldigestion (based on substrate-breath tests). Identification of the specific disorder of function leads to individualized treatment in the clinic, and it may help identify participants in clinical trials with greater likelihood to respond to the specific pharmacological actions of the trial medication. In addition, identification of the underlying mechanism for functional diarrhea or IBS-D using measurements of bile acid malabsorption has shown potential to markedly reduced healthcare utilization.45,46
FIGURE 2.
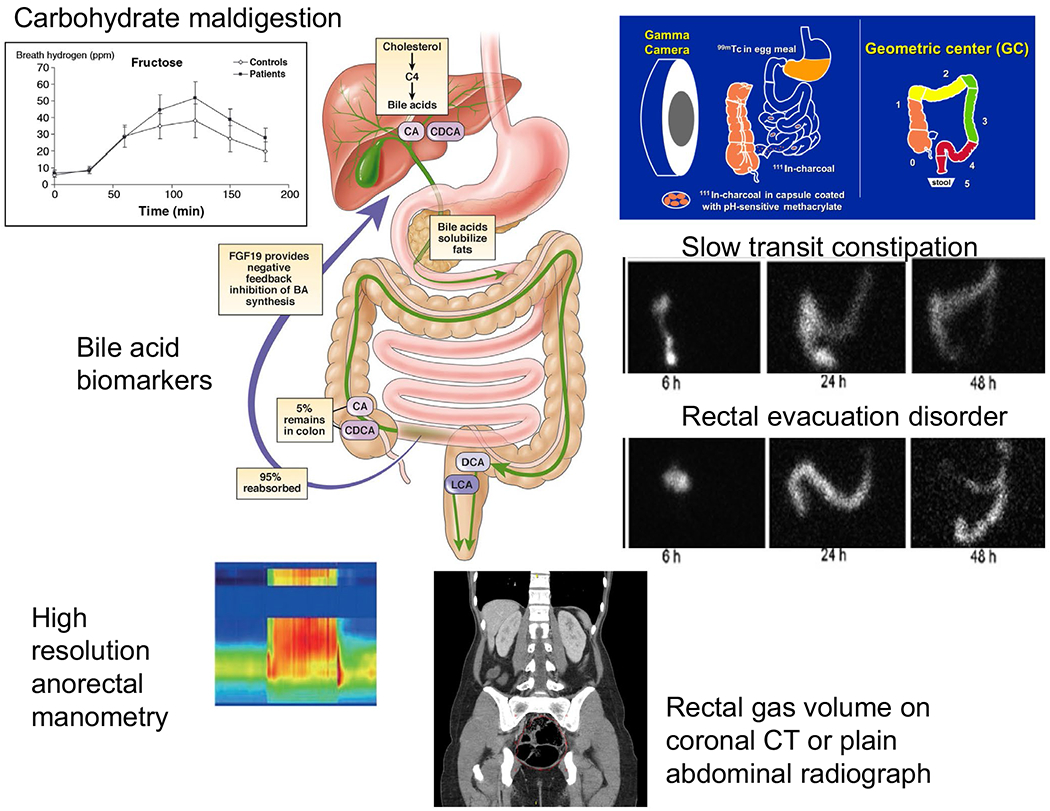
Actionable biomarkers of relevance in patients with symptoms suggestive of IBS (adapted from ref. 44, Camilleri M, Chedid V. Gut 2020 Apr 8;gutjnl-2019-320325. 10.1136/gutjnl-2019-320325. Online ahead of print)
Finally, the appraisal offered in this commentary suggests that there are opportunities for the fields of neurogastroenterology and functional gastrointestinal disorders to carefully analyze the evidence now available in the literature. This will necessitate revisiting symptom-based criteria, incorporating the advances in pathophysiology, diagnosis, and management that have transpired in the last 3 decades following the first iteration of the Rome criteria for IBS.
Key points.
Four versions of Rome criteria for IBS are appraised for their performance and utility in clinical practice and in epidemiological studies.
Validation studies identified multiple deficiencies and favor simple identification of abdominal pain, bowel dysfunction, and bloating. Actionable biomarkers related to the symptoms have potential to usher in a change in practice.
Practice changes from positive diagnosis of symptom complexes followed by empirical treatment to identification of the mechanisms causing the symptoms and targeted therapy.
Acknowledgments
Funding information
Dr Camilleri is supported by NIH grant R01-DK115950.
DISCLOSURES
Dr Camilleri is funded for single-center research studies by Allergan and Takeda. He serves as an advisor to Allergan, Takeda, Ironwood, with compensation paid to his employer, Mayo Clinic. The author served as chair of the Rome IV “Pharmacological, Pharmacokinetic, and Pharmacogenomic Aspects of Functional Gastrointestinal Disorders” Committee and was a member of the “Fundamentals of Neurogastroenterology: Physiology/Motility-Sensation” Committee.
REFERENCES
- 1.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WG, Dotevall G, Drossman DA, Heaton KW, Kruis W. Irritable bowel syndrome (guidelines for the diagnosis). Gastroenterol Int. 1989;2:92–95. [Google Scholar]
- 3.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. e5. [DOI] [PubMed] [Google Scholar]
- 6.Locke GR 3rd, Zinsmeister AR, Fett SL, Melton LJ 3rd, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Halder SL, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. [DOI] [PubMed] [Google Scholar]
- 8.Palsson OS, Whitehead W, Tornblom H, Sperber AD, Simren M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158(5):1262–1273. e3. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Lee H-N, Rangan V, et al. Similarities in clinical and psychosocial characteristics of functional diarrhea and irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2020;18(2):399–405. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M What’s in the pipeline for lower functional gastrointestinal disorders in the next 5 years? Am J Physiol Gastrointest Liver Physiol. 2019;317:G640–G650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M Toward an effective peripheral visceral analgesic: responding to the national opioid crisis. Am J Physiol Gastrointest Liver Physiol. 2018;314:G637–G646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SSC, Valestin JA, Xiang X, Hamdy S, Bradley CS, Zimmerman MB. Home-based versus office-based biofeedback therapy for constipation with dyssynergic defecation: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simón MA, Bueno AM, Otero P, Vázquez FL, Blanco V. A randomized controlled trial on the effects of electromyographic biofeedback on quality of life and bowel symptoms in elderly women with dyssynergic defecation. Int J Environ Res Public Health. 2019;16(18):3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadeddu F, Salis F, De Luca E, Ciangola I, Milito G. Efficacy of biofeedback plus transanal stimulation in the management of pelvic floor dyssynergia: a randomized trial. Tech Coloproctol. 2015;19:333–338. [DOI] [PubMed] [Google Scholar]
- 15.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–338. [DOI] [PubMed] [Google Scholar]
- 16.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis. Colon Rectum. 2007;50:428–441. [DOI] [PubMed] [Google Scholar]
- 17.Rao S, Lembo AJ, Shift SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz I, Törnblom H, Palsson OS, Whitehead WE, Simrén M. How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol. 2018;113:1017–1025. [DOI] [PubMed] [Google Scholar]
- 19.Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin Gastroenterol Hepatol. 2020;18(2):392–398. e2. [DOI] [PubMed] [Google Scholar]
- 20.Vork L, Weerts ZZRM, Mujagic Z, et al. Rome III vs Rome IV criteria for irritable bowel syndrome: a comparison of clinical characteristics in a large cohort study. Neurogastroenterol Motil. 2018;30(2):el3189. [DOI] [PubMed] [Google Scholar]
- 21.Sood R, Camilleri M, Grade DJ, et al. Enhancing diagnostic performance of symptom-based criteria for irritable bowel syndrome by additional history and limited diagnostic evaluation. Am J Gastroenterol. 2016;111:1446–1454. [DOI] [PubMed] [Google Scholar]
- 22.Kruis W, Thieme CH, Weinzierl M, Schussler P, Hall J, Paulus W. A diagnostic score for the irritable bowel syndrome: its value in the exclusion of organic disease. Gastroenterology. 1984;87:1–7. [PubMed] [Google Scholar]
- 23.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome-results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–341. [DOI] [PubMed] [Google Scholar]
- 24.Fukudo S, Hongo M, Kaneko H, Ueno R. Efficacy and safety of oral lubiprostone in constipated patients with or without irritable bowel syndrome: a randomized, placebo-controlled and dose-finding study. Neurogastroenterol Motil. 2011;23:544, e205. [DOI] [PubMed] [Google Scholar]
- 25.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170–177. [DOI] [PubMed] [Google Scholar]
- 26.Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. 2010;55:1090–1097. [DOI] [PubMed] [Google Scholar]
- 27.Fukudo S, Hongo M, Kaneko H. Takano M, Ueno R. Lubiprostone increases spontaneous bowel movement frequency and quality of life in patients with chronic idiopathic constipation. Clin Gastroenterol Hepatol. 2015;13:294–301. [DOI] [PubMed] [Google Scholar]
- 28.Lembo AJ, Kurtz CB, Macdougall JE, et al. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology. 2010;138:886–895. [DOI] [PubMed] [Google Scholar]
- 29.Lembo AJ, Schneier HA, Shift SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–536. [DOI] [PubMed] [Google Scholar]
- 30.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–1712. [DOI] [PubMed] [Google Scholar]
- 31.Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017;112:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol. 2018;113:735–745. [DOI] [PubMed] [Google Scholar]
- 33.Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796–805. [DOI] [PubMed] [Google Scholar]
- 34.Kamm MA, Müller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. [DOI] [PubMed] [Google Scholar]
- 35.Müller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–1666. [DOI] [PubMed] [Google Scholar]
- 36.Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877–1888. [DOI] [PubMed] [Google Scholar]
- 37.Tack J, Müller-Lissner S, Bytzer P, et al. A randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipation. Gut. 2005;54:1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chey WD, Paré P, Viegas A, Ligozio G, Shetzline MA. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217–1225. [DOI] [PubMed] [Google Scholar]
- 39.Camilleri M What’s in a name? Roll on Rome II. Gastroenterology. 1998;114:237. [DOI] [PubMed] [Google Scholar]
- 40.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2020;12: 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 41.Siah KTH, Gong X, Yang XJ, et al. Rome Foundation-Asian working team report: Asian functional gastrointestinal disorder symptom clusters. Gut. 2018;67:1071–1077. [DOI] [PubMed] [Google Scholar]
- 42.Holtmann GJ, Talley NJ. Inconsistent symptom clusters for functional gastrointestinal disorders in Asia: is Rome burning? Gut. 2018;67:1911–1915. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri M Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. [DOI] [PubMed] [Google Scholar]
- 44.Camilleri M, Chedid V. Actionable biomarkers: the key to resolving disorders of gastrointestinal function. Gut. 2020. 10.1136/gutjne-2019-320325. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Turner JM, Pattni SS, Appleby RN, Walters JR. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol. 2017;8:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Camilleri M. Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol. 2020;115:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]


