Abstract
SARS-CoV-2 is responsible for the current COVID-19 pandemic. On the basis of our analysis of hepatitis C virus and coronavirus replication, and the molecular structures and activities of viral inhibitors, we previously demonstrated that three nucleotide analogues (the triphosphates of Sofosbuvir, Alovudine, and AZT) inhibit the SARS-CoV RNA-dependent RNA polymerase (RdRp). We also demonstrated that a library of additional nucleotide analogues terminate RNA synthesis catalyzed by the SARS-CoV-2 RdRp, a well-established drug target for COVID-19. Here, we used polymerase extension experiments to demonstrate that the active triphosphate form of Sofosbuvir (an FDA-approved hepatitis C drug) is incorporated by SARS-CoV-2 RdRp and blocks further incorporation. Using the molecular insight gained from the previous studies, we selected the active triphosphate forms of six other antiviral agents, Alovudine, Tenofovir alafenamide, AZT, Abacavir, Lamivudine, and Emtricitabine, for evaluation as inhibitors of the SARS-CoV-2 RdRp and demonstrated the ability of these viral polymerase inhibitors to be incorporated by SARS-CoV-2 RdRp, where they terminate further polymerase extension with varying efficiency. These results provide a molecular basis for inhibition of the SARS-CoV-2 RdRp by these nucleotide analogues. If sufficient efficacy of some of these FDA-approved drugs in inhibiting viral replication in cell culture is established, they may be explored as potential COVID-19 therapeutics.
Keywords: COVID-19, SARS-CoV-2, RNA-dependent RNA polymerase, nucleotide analogues
Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has already infected more than 14 million people worldwide resulting in over 600 000 reported deaths, with severe social and economic ramifications. SARS-CoV-2 is a new member of the subgenus Sarbecovirus in the Orthocoronavirinae subfamily, which also includes MERS-CoV and SARS-CoV.1 The coronaviruses are single-strand RNA viruses, sharing properties with other single-stranded RNA viruses such as hepatitis C virus (HCV), West Nile virus, Marburg virus, HIV virus, Ebola virus, dengue virus, and rhinoviruses. SARS-CoV-2 is a positive-sense single-strand RNA virus like HCV and other flaviviruses;2,3 these viruses share a similar replication mechanism requiring an RNA-dependent RNA polymerase (RdRp).
There are currently no effective FDA-approved drugs to specifically treat coronavirus infections such as SARS, MERS, and now COVID-19. Components of nearly every stage of the coronavirus replication cycle have been targeted for drug development.2 In particular, the coronavirus RdRp is a well-established drug target. This polymerase shares similar catalytic mechanisms and displays active site conservation among different positive-sense RNA viruses, including coronaviruses and HCV.4 Like RdRps in other viruses, the coronavirus enzyme is highly error-prone,5 which might increase its ability to accept modified nucleotide analogues as substrates. Nucleotide analogues that inhibit polymerases are an important group of antiviral agents.6−9
On the basis of our analysis of hepatitis C virus and coronavirus replication, and the molecular structures and activities of viral inhibitors, we previously proposed Sofosbuvir triphosphate as a candidate inhibitor of the SARS-CoV-2 RdRp.10,11 Elfiky used a molecular docking study to predict that Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir may have inhibitory activity against SARS-CoV-2 RdRp.12 Remdesivir, a phosphoramidate prodrug containing a 1′-cyano modification on the sugar, is converted into an adenosine triphosphate analogue inside virus-infected cells, which inhibits the RdRps of MERS-CoV, SARS-CoV, and SARS-CoV-2.13,14 Recently, the FDA issued an emergency use authorization for Remdesivir for potential COVID-19 treatment.15 On the basis of a comparison of the positive-strand RNA genomes of HCV and SARS-CoV-2, Buonaguro et al. postulated that Sofosbuvir might be an optimal nucleotide analogue to repurpose for COVID-19 treatment.16 After considering the potential advantages of Sofosbuvir, including its low toxicity, its ability to be rapidly activated to the triphosphate form by cellular enzymes, and the high stability of this active molecule intracellularly, Sayad et al. have initiated a clinical trial with Sofosbuvir for treatment of COVID-19.17 However, a recent kinetic analysis of Sofosbuvir triphosphate with SARS-CoV-2 polymerase indicated that it has lower incorporation activity than UTP.14
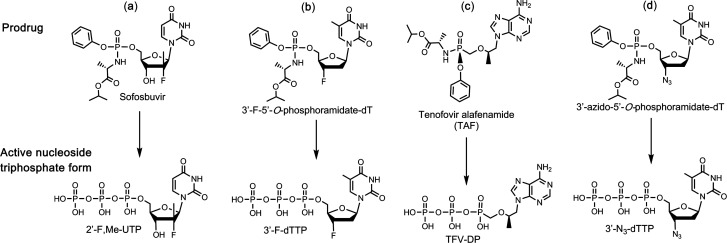
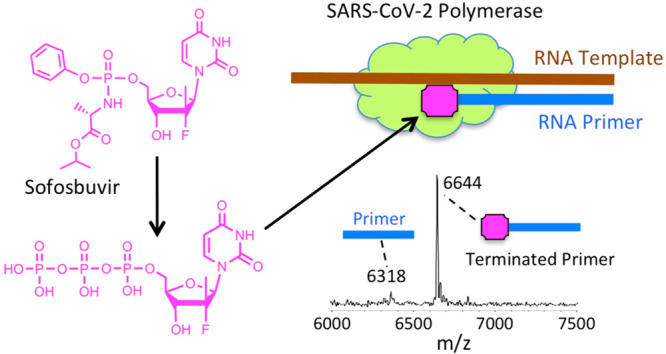
We previously demonstrated that the triphosphates of Sofosbuvir, Alovudine (3′-F-dT), and AZT (3′-N3-dT) (Figure 1a,b,d) inhibit the SARS-CoV RdRp.11 On the basis of the molecular rationale above, we conducted polymerase primer extension experiments with Sofosbuvir triphosphate (2′-F,Me-UTP, Figure 1a) and demonstrated that it was incorporated by SARS-CoV RdRp and blocked further incorporation. Using the same molecular insight, we selected two HIV reverse transcriptase (RT) inhibitors, Alovudine and AZT, for evaluation as inhibitors of SARS-CoV RdRp. Alovudine and AZT share a similar backbone structure (base and ribose) with Sofosbuvir but have fewer modification sites (Figure 1b,d). Furthermore, because these modifications on Alovudine and AZT are on the 3′ position of the sugar ring in place of the 3′–OH group, if they are accepted as substrates by the RdRp, they will prevent further incorporation of nucleotides leading to obligate termination of RNA synthesis. We demonstrated the ability of the active triphosphate forms of Alovudine and AZT, 3′-F-dTTP (Figure 1b) and 3′-N3-dTTP (Figure 1d), respectively, to be incorporated by SARS-CoV RdRp where they also terminated further polymerase extension.11 We also demonstrated that a library of additional nucleotide analogues terminate RNA synthesis catalyzed by the SARS-CoV-2 RdRp.18
Figure 1.
Structures of four prodrug viral inhibitors. Top: Prodrug (phosphoramidate) form; Bottom: Active triphosphate form.
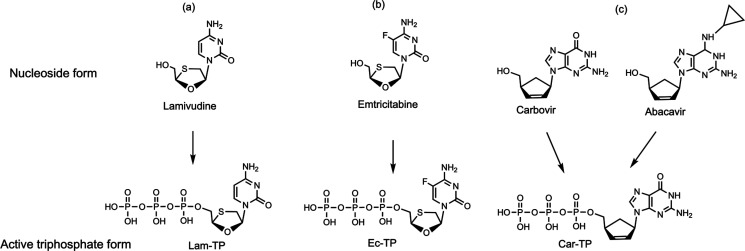
We first constructed SARS-CoV-2 RdRp using a similar procedure to that of SARS-CoV,19,20 and then we demonstrated that the above three nucleotide analogues (Figure 1a,b,d) are inhibitors of SARS-CoV-2 RdRp. Using structure–activity-based molecular insight, we selected the active triphosphate form of Tenofovir alafenamide (TAF, Vemlidy, an acyclic adenosine nucleotide) (Figure 1c), which is an FDA approved drug for the treatment of HIV and hepatitis B virus (HBV) infection, for evaluation as a SARS-CoV-2 RdRp inhibitor. Similarly, we also selected the triphosphates of three HIV RT inhibitors, Lamivudine triphosphate (Lam-TP, Figure 2a), Emtricitabine triphosphate (Ec-TP, Figure 2b) and Carbovir triphosphate (Car-TP, Figure 2c) to test their ability to inhibit the SARS-CoV-2 RdRp. The results indicated that the active triphosphate forms of Tenofovir, Lamivudine, Emtricitabine, and Abacavir (the prodrug of Car-TP) inhibited this polymerase with varying efficiency. The properties of these four viral inhibitors are described below.
Figure 2.
Structures of three viral inhibitors. Top: Nucleoside form; Bottom: Active triphosphate form.
TAF, a prodrug form of the nucleotide analogue viral polymerase inhibitor Tenofovir (TFV), shows potent activity for HIV and HBV but only limited inhibition of host nuclear and mitochondrial polymerases.21,22 It is activated by a series of hydrolases to the deprotected monophosphate form, TFV, and then by two consecutive kinase reactions to the triphosphate form Tenofovir diphosphate (TFV-DP).23 TFV-DP is an acyclic nucleotide and does not have a 3′–OH group. Remarkably, this molecule is incorporated by both HIV and HBV polymerases, terminating nucleic acid elongation and viral replication.21,23 In addition, resistance mutations were rarely seen in patients treated with regimens including TAF.24 In view of the fact that the active triphosphate form of TAF, TFV-DP, is much smaller than natural nucleoside triphosphates, we expect that it can easily fit within the active site of the SARS-CoV-2 RdRp. As a noncyclic nucleotide, TFV-DP lacks a normal sugar ring configuration, and thus, we reasoned that it is unlikely to be recognized by 3′-exonucleases involved in SARS-CoV-2 proofreading processes, decreasing the likelihood of developing resistance to the drug.25
The oral drug Lamivudine (3TC) is a cytidine analogue containing an oxathiolane ring with an unnatural (−)-β-L-stereochemical configuration, making it a poor substrate for host DNA polymerases.26 This prodrug, which can be taken orally and has low toxicity, is converted by cellular enzymes, first to a monophosphate, then to the active triphosphate form, Lam-TP. Emtricitabine (Emtriva, FTC) has a similar structure to Lamivudine but with a fluorine at the 5-position of the cytosine.27 Conversion of the prodrug form to the active triphosphate is analogous to the activation mechanism for Lamivudine. Like TAF, 3TC and FTC are effective against HBV.28 The absence of an OH group at the 3′ position of both Lam-TP and Ec-TP ensures that once these nucleotide analogues are incorporated into the primer in the polymerase reaction, no further incorporation of nucleotides by the polymerase can occur. Car-TP is a carbocyclic guanosine didehydro-dideoxynucleotide. The parent prodrug, Abacavir (Ziagen), is an FDA-approved nucleoside RT inhibitor used for HIV/AIDS treatment.29,30 We previously studied Car-TP as an inhibitor of the SARS-CoV and SARS-CoV-2 RdRp using a higher concentration than in the current study.18
Experimental Section
Materials
Nucleoside triphosphates and nucleoside triphosphate analogues were purchased from TriLink BioTechnologies (CTP, ATP and UTP), Sierra Bioresearch (2′-F,Me-UTP), Amersham Life Sciences (3′-F-dTTP, 3′-N3-dTTP), Toronto Research Chemicals (Lamivudine-TP, Emtricitabine-TP), or Santa Cruz Biotechnology (Carbovir-TP). Oligonucleotides were purchased from Integrated DNA Technologies, Inc. or Dharmacon, Inc.
Recombinant Protein Expression of RdRp (nsp12) and Cofactors (nsp7 and nsp8) for SARS-CoV-2
SARS-CoV-2 nsp12
The SARS-CoV-2 nsp12 gene was codon optimized and cloned into pFastBac with C-terminal additions of a thrombin site and double strep tags (Genscript). The pFastBac plasmid and DH10Bac E. coli (Life Technologies) were used to create recombinant bacmids. The bacmid was transfected into Sf9 cells (Expression Systems) with Cellfectin II (Life Technologies) to generate recombinant baculovirus. The baculovirus was amplified through two passages in Sf9 cells and then used to infect 1 L of Sf21 cells (Expression Systems) and incubated for 48 h at 27 °C. Cells were harvested by centrifugation and resuspended in wash buffer (25 mM HEPES pH 7.4, 300 mM NaCl, 1 mM MgCl2, 5 mM DTT) with 143 μL of BioLock per liter of culture. Cells were lysed via microfluidization (Microfluidics). Lysates were cleared by centrifugation and filtration. The protein was purified using Strep Tactin superflow agarose (IBA). Strep Tactin eluted protein was further purified by size exclusion chromatography using a Superdex 200 Increase 10/300 column (GE Life Sciences) in 25 mM HEPES, 300 mM NaCl, 100 μM MgCl2, 2 mM TCEP, at pH 7.4. Pure protein was concentrated by ultrafiltration prior to flash freezing in liquid nitrogen.
SARS-CoV-2 nsp7 and nsp8
The SARS-CoV-2 nsp7 and nsp8 genes were codon optimized and cloned into pET46 (Novagen) with an N-terminal 6x histidine tag, an enterokinase site, and a TEV protease site. Rosetta2 pLys E. coli cells (Novagen) were used for bacterial expression. After induction with isopropyl β-D-1-thiogalactopyranoside (IPTG), cultures were grown at 16 °C for 16 h. Cells were harvested by centrifugation, and pellets were resuspended in wash buffer (10 mM Tris pH 8.0, 300 mM NaCl, 30 mM imidazole, 2 mM DTT). Cells were lysed via microfluidization and lysates were cleared by centrifugation and filtration. Proteins were purified using Ni-NTA agarose beads and eluted with wash buffer containing 300 mM imidazole. Nsp7 and nsp8 proteins were cleaved with 1% (w/w) TEV protease overnight and passed back over Ni-NTA agarose. Cleaved proteins were further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column (GE Life Sciences). Purified proteins were concentrated by ultrafiltration prior to flash freezing with liquid nitrogen.
Extension Reactions with SARS-CoV-2 RNA-Dependent RNA Polymerase
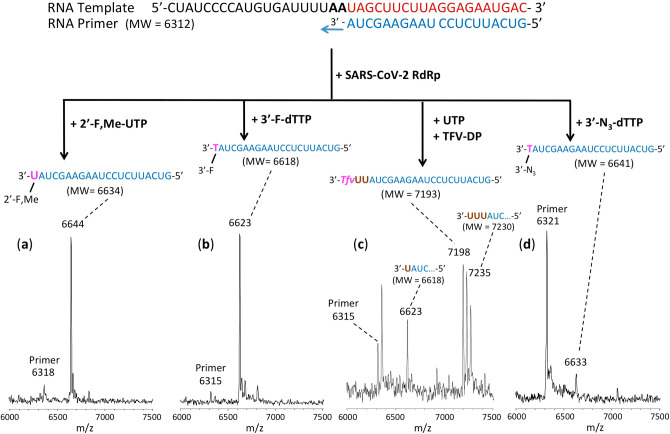
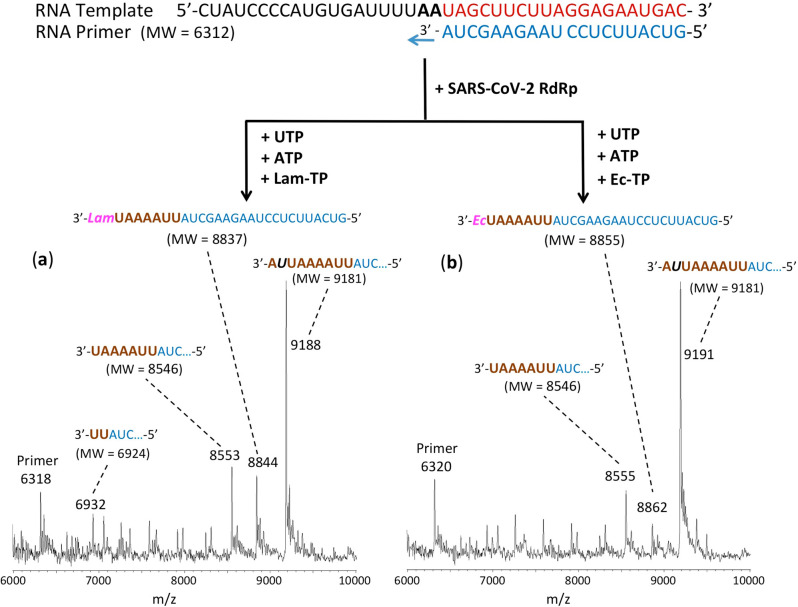
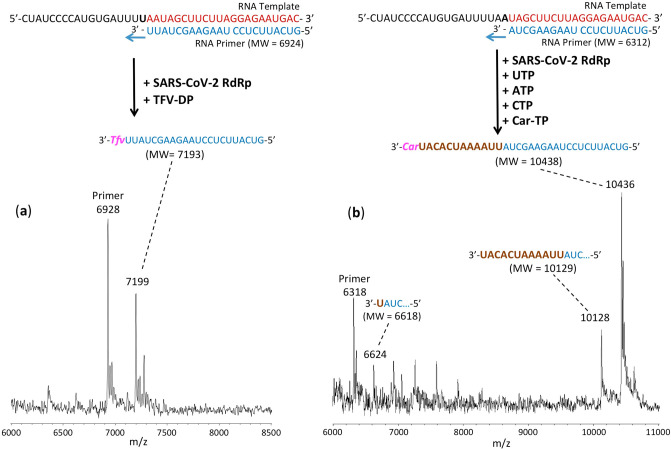
The RNA primers and template (sequences shown in Figures 3–5) were annealed by heating to 70 °C for 10 min and cooling to room temperature in 1× reaction buffer. For reactions in Figure 3, the RNA polymerase mixtures consisting of 6 μM nsp12 and 18 μM each of cofactors nsp7 and nsp8 were incubated for 15 min at room temperature in a 1:3:3 ratio in 1× reaction buffer. For reactions in Figures 4 and 5, higher concentrations of nsp 12, nsp7, and nsp8 were used (10, 30, and 60 μM, respectively). Then 5 μL of the annealed template primer solution containing 2 μM template and 1.7 μM primer in 1× reaction buffer was added to 10 μL of the RNA polymerase mixture and incubated for an additional 10 min at room temperature. Finally, 5 μL of a solution containing either 2 mM 2′-F,Me-UTP (Figure 3a), 2 mM 3′-F-dTTP (Figure 3b), 2 mM TFV-DP + 200 μM UTP (Figure 3c), 2 mM 3′-N3-dTTP (Figure 3d), 2 mM TFV-DP (Figure 4a), 400 μM UTP + 400 μM ATP + 400 μM CTP + 1 mM Car-TP (Figure 4b), 400 μM UTP + 400 μM ATP + 2 mM Lam-TP (Figure 5a) or 400 μM UTP + 400 μM ATP + 2 mM Ec-TP (Figure 5b) in 1× reaction buffer was added, and incubation was carried out for 2 h at 30 °C. The final concentrations of reagents in the 20 μL extension reactions were 3 μM nsp12, 9 μM nsp7, 9 μM nsp8 (Figure 3) or 5 μM nsp12, 15 μM nsp7, 30 μM nsp8 (Figures 4 and 5), 425 nM RNA primer, 500 nM RNA template, and either 500 μM 2′-F,Me-UTP (Figure 3a), 500 μM 3′-F-dTTP (Figure 3b), 500 μM TFV-DP + 50 μM UTP (Figure 3c), 500 μM 3′-N3-dTTP (Figure 3d), 500 μM TFV-DP (Figure 4a), 100 μM UTP + 100 μM ATP + 100 μM CTP + 250 μM Car-TP (Figure 4b), 100 μM UTP + 100 μM ATP + 500 μM Lam-TP (Figure 5a) or 100 μM UTP + 100 μM ATP + 500 μM Ec-TP (Figure 5b). The 1× reaction buffer contains the following reagents: 10 mM Tris-HCl pH 8, 10 mM KCl, 2 mM MgCl2, and 1 mM β-mercaptoethanol. Following desalting using an Oligo Clean & Concentrator (Zymo Research), the samples were subjected to MALDI-TOF-MS (Bruker ultrafleXtreme) analysis.
Figure 3.
Incorporation of 2′-F,Me-UTP, 3′-F-dTTP, TFV-DP, and 3′-N3-dTTP by SARS-CoV-2 RdRp to terminate the polymerase reaction. The sequences of the primer and template used for these extension reactions, which are at the 3′ end of the SARS-CoV-2 genome, are shown at the top of the figure. Polymerase extension reactions were performed by incubating (a) 2′-F,Me-UTP, (b) 3′-F-dTTP, (c) UTP + TFV-DP, and (d) 3′-N3-dTTP with preassembled SARS-CoV-2 polymerase (nsp12, nsp7, and nsp8), the indicated RNA template and primer, and the appropriate reaction buffer, followed by detection of reaction products by MALDI-TOF MS. The accuracy for m/z determination is ±10 Da.
Figure 5.
Incorporation of Lam-TP and Ec-TP by SARS-CoV-2 RdRp catalyzed reaction. The sequences of the primer and template used for these extension reactions, which are at the 3′ end of the SARS-CoV-2 genome, are shown at the top of the figure. Polymerase extension reactions were performed by incubating (a) UTP + ATP + Lam-TP and (b) UTP + ATP + Ec-TP with preassembled SARS-CoV-2 polymerase (nsp12, nsp7, and nsp8), the indicated RNA template and primer, and the appropriate reaction buffer, followed by detection of reaction products by MALDI-TOF MS. The accuracy for m/z determination is ±10 Da.
Figure 4.
Incorporation of TFV-DP and Car-TP by SARS-CoV-2 RdRp to terminate the polymerase reaction. The sequences of the primers and template used for these extension reactions, which are at the 3′ end of the SARS-CoV-2 genome, are shown at the top of the figure. Polymerase extension reactions were performed by incubating (a) TFV-DP and (b) UTP + ATP + CTP + Car-TP with preassembled SARS-CoV-2 polymerase (nsp12, nsp7, and nsp8), the indicated RNA template and primers, and the appropriate reaction buffer, followed by detection of reaction products by MALDI-TOF MS. The accuracy for m/z determination is ±10 Da.
Results and Discussion
Given the 98% amino acid similarity of the SARS-CoV and SARS-CoV-2 RdRps and our previous inhibition results on SARS-CoV and SARS-CoV-2 RdRps,11,18 we reasoned that the nucleotide analogues listed in Figures 1 and 2 should also inhibit the SARS-CoV-2 polymerase. We thus assessed the ability of 2′-F,Me-UTP, 3′-F-dTTP, TFV-DP, and 3′-N3-dTTP (the active triphosphate forms of Sofosbuvir, Alovudine, TAF, and AZT, respectively), along with Lam-TP, Ec-TP, and Car-TP (the active triphosphate forms of Lamivudine, Emtricitabine, and Carbovir/Abacavir), to be incorporated by SARS-CoV-2 RdRp into an RNA primer to terminate the polymerase reaction.
The RdRp of SARS-CoV-2, referred to as nsp12, and its two protein cofactors, nsp7 and nsp8, whose homologues were shown to be required for the processive polymerase activity of nsp12 in SARS-CoV,19,20 were cloned and purified as described in the Experimental Section. These three viral gene products in SARS-CoV-2 have high homology (e.g., 96% identity and 98% similarity for nsp12, with similar homology levels at the amino acid level for nsp7 and nsp8) to the equivalent gene products from SARS-CoV, the causative agent of SARS.11
We performed polymerase extension assays with 2′-F,Me-UTP, 3′-F-dTTP, 3′-N3-dTTP, or TFV-DP + UTP, following the addition of a preannealed RNA template and primer to a preassembled mixture of the SARS-CoV-2 RdRp (nsp12) and two cofactor proteins (nsp7 and nsp8). The primer extension products from the reaction were subjected to MALDI-TOF-MS analysis. The RNA template and primer, corresponding to the 3′ end of the SARS-CoV-2 genome, were used for the polymerase reaction assay; their sequences are indicated at the top of Figure 3. 2′-F,Me-UTP has a 3′–OH group, but because of 2′ modification with a fluorine and methyl group, it acts as a nonobligate terminator for HCV RdRp.8 3′-F-dTTP and 3′-N3-dTTP do not have a 3′–OH, and we previously demonstrated that they are obligate terminators of the SARS-CoV RdRp.11
For the data presented in Figure 3, because there are two As in a row in the next available positions of the template for RNA polymerase extension downstream of the priming site, if 2′-F,Me-UTP, 3′-F-dTTP or 3′-N3-dTTP are incorporated by the viral RdRp and terminate the polymerase reaction, a single nucleotide analogue will be added to the 3′-end of the primer strand. Because the two As in the template are followed by four Us, in the case of the TFV-DP/UTP mixture, two UTPs should be incorporated prior to the incorporation and termination by TFV-DP, which is an ATP analogue and an obligate terminator due to the absence of an OH group. As shown in Figure 3, this is exactly what we observed. In the MALDI-TOF MS trace in Figure 3a, a peak indicative of the molecular weight of a single nucleotide (2′-F,Me-UMP) primer extension product was obtained (6644 Da observed, 6634 Da expected). Similarly, in the trace in Figure 3b, a single extension peak indicative of a single base extension by 3′-F-dTMP is revealed (6623 Da observed, 6618 Da expected), with no further incorporation. In both of the above cases, the primer was nearly completely depleted, indicating that 2′-F,Me-UTP and 3′-F-dTTP are efficient substrates of the RdRp. In the trace in Figure 3d, a single extension peak indicative of a single-base extension by 3′-N3-dTMP is seen (6633 Da observed, 6641 Da expected), with no evidence of further incorporation, though the incorporation efficiency was lower than for 2′-F,Me-UTP and 3′-F-dTTP; further optimization may be required. Finally, in the trace in Figure 3c, a peak indicative of the molecular weight of a primer extension product formed by incorporating 2 Us and 1 TFV (an A analogue) is found (7198 Da observed, 7193 Da expected), in addition to other peaks representing partial incorporation (one U, 6623 Da observed, 6618 Da expected) or misincorporation (3 Us, 7235 Da observed, 7230 Da expected). Importantly, once the TFV was incorporated, there was no further extension, indicating it was an obligate terminator for the RdRp. The result of an additional experiment with TFV-DP is shown in Figure 4a, in which a longer RNA primer was used with the same template RNA, allowing direct incorporation of TFV. Again, only a single TFV was incorporated (7199 Da observed, 7193 Da expected), despite the presence of 3 additional Us in the template.
The results for Car-TP, which is a G analogue, are shown in Figure 4b. The most prominent extension peak observed indicates extension by UTP, ATP, and CTP followed by complete termination with a Car-TP (10 436 Da observed, 10 438 Da expected). Despite the inclusion of UTP, ATP and CTP in the mixture with Car-TP, no extension past this point was observed, indicating that Car-TP was an obligate terminator of the SARS-CoV-2 RdRp. In addition, some partial extension peaks were seen, e.g., incorporation of one U (6624 Da observed, 6618 Da expected), and extension up to the position just before the first C in the template strand (10 128 Da observed, 10 129 Da expected). These results are consistent with previous results obtained using a higher concentration of Car-TP.18
MALDI-TOF MS results for extension by the CTP analogues Lam-TP and Ec-TP are shown in panels a and b, respectively, of Figure 5. There was relatively poor incorporation by these nucleotide analogues. With Lam-TP, a small peak was observed at 8844 Da (8837 Da expected) indicating the incorporation of Lam-TP following multiple incorporated Us and As. In addition, partial extension peaks were observed at 6932 Da indicating extension by two Us (6924 Da expected) and at 8553 Da indicating extension by 2 Us, 4 As, and 1 U (8546 Da expected). However, the most prominent peak was observed at 9188 Da, indicating misincorporation by a U at the position where the C analogue Lam-TP would be expected to be incorporated followed by incorporation of the subsequent A (9181 Da expected). Similar results were obtained for Ec-TP. Minimal extension by Ec-TP is indicated by the peak at 8862 Da (8855 Da expected), but a partial extension peak indicating incorporation by 2 Us, 4 As and 1 U at 8555 Da (8546 Da expected), and a prominent peak indicating misincorporation by a U at the position where the C analogue Ec-TP should be incorporated and a subsequent A at 9191 Da (9181 Da expected) were also present. These misincorporation results for both Lam-TP and Ec-TP indicate that SARS-CoV-2 RdRp has low fidelity, which is consistent with the known low fidelity of RdRps.5
Note Added in Revision
Data for four of the nucleotide analogues included in this paper were presented in a preprint posted on bioRxiv on March 20, 2020.31 This field is moving rapidly, and while the current paper was under review and revision, numerous additional publications and preprints have appeared. Sofosbuvir has been shown to inhibit SARS-CoV-2 replication in Huh-2 (human hepatoma-derived) and Calu-3 (Type II pneumocyte-derived) cells with EC50 values of 6.2 and 9.5 μM, respectively, but not in Vero-E6 cells.32 Sofosbuvir was also shown to protect human brain organoids from infection by SARS-CoV-2.33 A recent preprint provides K1/2 values (the concentration leading to 50% SARS-CoV-2 polymerase extension) for a library of nucleotide analogues including Sofosbuvir and others examined in this paper.34 Recently, results from a cohort study comparing COVID-19 outcomes in over 77,000 HIV patients taking combination drugs including Tenofovir and Emtricitabine, among other protocols, indicated that these individuals had a somewhat lower COVID-19 diagnosis rate and suggested that Tenofovir disoproxil fumarate led to the best overall COVID-19 results.35
Conclusions
In summary, these results demonstrate that the nucleotide analogues 2′-F,Me-UTP, 3′-F-dTTP, TFV-DP, and Car-TP terminate the RNA synthesis catalyzed by SARS-CoV-2 RdRp. In contrast, 3′-N3-dTTP, Lam-TP, and Ec-TP were poor RdRp substrates. Sofosbuvir, Tenofovir alafenamide, and Abacavir, the prodrugs of 2′-F,Me-UTP, TFV-DP, and Car-TP, respectively, are FDA-approved oral drugs for treatment of other viral infections, and their safety profiles are well-established. The phosphoramidate prodrugs for Alovudine and Abacavir can be readily synthesized using the ProTide prodrug approach.36 The results presented here, coupled with those we obtained previously,18 provide a molecular basis for inhibition of the SARS-CoV-2 RdRp by a library of nucleotide analogues. If these FDA-approved drugs display efficacy in inhibiting SARS-CoV-2 replication in cell culture, as recently demonstrated for Sofosbuvir in virus infected lung cells32 and brain organoids,33 they can be considered as potential candidates in clinical trials for the treatment and prevention of COVID-19.
Author Contributions
J.J. and R.N.K. conceived and directed the project; the approaches and assays were designed and conducted by J.J., X.L., S.K., S.J., J.J.R., M.C. and C.T., and SARS-CoV-2 polymerase and associated proteins nsp7 and 8 were cloned and purified by T.K.A. and R.N.K. Data were analyzed by all authors. All authors wrote and reviewed the manuscript.
This research is supported by Columbia University, a grant from the Jack Ma Foundation, a generous gift from the Columbia Engineering Member of the Board of Visitors Dr. Bing Zhao, and Fast Grants to J.J. and a National Institute of Allergy and Infectious Disease grant AI123498 to R.N.K.
The authors declare no competing financial interest.
References
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; Niu P.; Zhan F.; Ma X.; Wang D.; Xu W.; Wu G.; Gao G. F.; Tan W. for the China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A.; Chan J. F. W.; Azhar E. I.; Hui D. S. C; Yuen K.-Y. Coronaviruses – drug discovery and therapeutic options. Nat. Rev. Drug Discovery 2016, 15, 327–347. 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin L. B.; Bartolini B.; Capobianchi M. R.; Pistello M. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin. Microbiol. Infect. 2016, 22, 826–832. 10.1016/j.cmi.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A. J. W. Common and unique features of viral RNA-dependent polymerases. Cell. Mol. Life Sci. 2014, 71, 4403–4420. 10.1007/s00018-014-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selisko B.; Papageorgiou N.; Ferron F.; Canard B. Structural and functional basis of the fidelity of nucleotide selection by Flavivirus RNA-dependent RNA polymerases. Viruses 2018, 10, 59. 10.3390/v10020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna C. E.; Levy J. N.; Khawli L. A.; Harutunian V.; Ye T.-G.; Starnes M. C.; Bapat A.; Cheng Y.-C. Inhibitors of viral nucleic acid polymerases. Pyrophosphate analogues. ACS Symp. Ser. 1989, 401, 1–16. 10.1021/bk-1989-0401.ch001. [DOI] [Google Scholar]
- Öberg B. Rational design of polymerase inhibitors as antiviral drugs. Antiviral Res. 2006, 71, 90–95. 10.1016/j.antiviral.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Eltahla A. A.; Luciani F.; White P. A.; Lloyd A. R.; Bull R. A. Inhibitors of the hepatitis C virus polymerase; mode of action and resistance. Viruses 2015, 7, 5206–5224. 10.3390/v7102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E.; Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J.; Kumar S.; Li X.; Jockusch S.; Russo J. J. Nucleotide analogues as inhibitors of viral polymerases. bioRxiv. 2020, 10.1101/2020.01.30.927574. [DOI] [Google Scholar]
- Ju J.; Li X.; Kumar S.; Jockusch S.; Chien M.; Tao C.; Morozova I.; Kalachikov S.; Kirchdoerfer R. N.; Russo J. J. Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020, 10.1101/2020.03.12.989186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A. A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Feng J. Y.; Porter D. P.; Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Woolner E.; Perry J. K.; Feng J. Y.; Porter D. P.; Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman R. T.; Roth J. S.; Brimacombe K. R.; Simeonov A.; Shen M.; Patnaik S.; Hall M. D. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L.; Tagliamonte M.; Tornesello M. L.; Buonaguro F. M. SARS-CoV-2 RNA polymerase as target for antiviral therapy. J. Transl. Med. 2020, 18, 185. 10.1186/s12967-020-02355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayad B.; Sobhani M.; Khodarahmi R. Sofosbuvir as repurposed antiviral drug against COVID-19: why were we convinced to evaluate the drug in a registered/approved clinical trial?. Arch. Med. Res. 2020, 10.1016/j.arcmed.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch S.; Tao C.; Li X.; Anderson T. K.; Chien M.; Kumar S.; Russo J. J.; Kirchdoerfer R. N.; Ju J. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 2020, 180, 104857. 10.1016/j.antiviral.2020.104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subissi L.; Posthuma C. C.; Collet A.; Zevenhoven-Dobbe J. C.; Gorbalenya A. E.; Decroly E.; Snijder E. J.; Canard B.; Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, E3900–E3909. 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R. N.; Ward A. B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019, 10, 2342. 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou L. Advances in nucleotide antiviral development from scientific discovery to clinical applications: Tenofovir disproxil fumarate for hepatitis B. J. Clin. Translat. Hepatol. 2013, 1, 33–38. 10.14218/JCTH.2013.004XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 2016, 119, 1–7. 10.1016/j.bcp.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Birkus G.; Bam R. A.; Willkom M.; Frey C. R.; Tsai L.; Stray K. M.; Yant S. R.; Cihlar T. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob. Agents Chemother. 2016, 60, 316–322. 10.1128/AAC.01834-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margot N.; Cox S.; Das M.; McCallister S.; Miller M. D.; Callebaut C. Rare emergence of drug resistance in HIV-1 treatment-naïve patients receiving elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide for 144 weeks. J. Clin. Virol. 2018, 103, 37–42. 10.1016/j.jcv.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Smith E. C.; Blanc H.; Vignuzzi M.; Denison M. R. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013, 9, e1003565. 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quercia R.; Perno C.-F.; Koteff J.; Moore K.; McCoig C.; St. Clair M.; Kuritzkes D. Twenty-five years of lamivudine: current and future use for the treatment of HIV-1 infection. JAIDS, J. Acquired Immune Defic. Syndr. 2018, 78, 125–135. 10.1097/QAI.0000000000001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M.; Tokarsky E. J.; Lagpacan L.; Zhang L.; Suo Z.; Lansdon E. B. Elucidating molecular interactions of L-nucleotides with HIV-1 reverse transcriptase and mechanism of M184V-caused drug resistance. Commun. Biol. 2019, 2, 469. 10.1038/s42003-019-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. G.; Ng T. M.; Kung N.; Krastev Z.; Volfova M.; Husa P.; Lee S. S.; Chan S.; Shiffman M. L.; Washington M. K.; Rigney A.; Anderson J.; Mondou E.; Snow A.; Sorbel J.; Guan R.; Rousseau F. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch. Intern. Med. 2006, 166, 49–56. 10.1001/archinte.166.1.49. [DOI] [PubMed] [Google Scholar]
- Faletto M. B.; Miller W. H.; Garvey E. P.; St. Clair M. H.; Daluge S. M.; Good S. S. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob. Agents Chemother. 1997, 41, 1099–1107. 10.1128/AAC.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A. S.; Basavapathruni A.; Anderson K. S. Mechanistic studies to understand the progressive development of resistance in human immunodeficiency virus type 1 reverse transcriptase to abacavir. J. Biol. Chem. 2002, 277, 40479–40490. 10.1074/jbc.M205303200. [DOI] [PubMed] [Google Scholar]
- Chien M.; Anderson T. K.; Jockusch S.; Tao C.; Kumar S.; Li X.; Russo J. J.; Kirchdoerfer R. N.; Ju J.. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase. bioRxiv. 2020. 10.1101/2020/03/18.997585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento C. Q.; Fintelman-Rodrigues N.; Temerozo J. R.; da Silva Gomes Dias S.; Ferreira A. C.; Mattos M.; Pão C. R. R.; de Freitas C. S.; Cardoso Soares V.; Bozza F. A.; Bou-Habib D. C.; Bozza P. T.; Souza T. M. L. The in vitro antiviral activity of the anti-hepatitis C virus (HCV) drugs daclatasvir and sofosbuvir against SARS-CoV-2. bioRxiv. 2020, 10.1101/2020.06.15.153411. [DOI] [Google Scholar]
- Mesci P.; Macia A.; Saleh A.; Martin-Sancho L.; Yin X.; Snethlage C.; Avansini S.; Chanda S. K.; Muotri A. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRxiv. 2020, 10.1101/2020.05.30.125856. [DOI] [Google Scholar]
- Lu G.; Zhang X.; Zheng W.; Sun J.; Hua L.; Xu L.; Chu X.; Ding S.; Xiong W. Development of a simple in vitro assay to identify and evaluate nucleotide analogs against SARS-CoV-2 RNA-dependent RNA polymerase. bioRxiv. 2020, 10.1101/2020.07.16.205799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Amo J.; Polo R.; Moreno S.; Díaz A.; Martinez E.; Arribas J. R.; Jarrin I.; Hernán M. A. The Spanish HIV/COVID-19 Collaboration. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy. A cohort study. Ann. Intern. Med. 2020, 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A. S.; James E.; Mehellou Y. The ProTide prodrug technology: where next?. ACS Med. Chem. Lett. 2019, 10, 2–5. 10.1021/acsmedchemlett.8b00586. [DOI] [PMC free article] [PubMed] [Google Scholar]