Abstract
In this work, we present a novel model-based material decomposition (MBMD) approach for x-ray CT that includes system blur in the measurement model. Such processing has the potential to extend spatial resolution in material density estimates - particularly in systems where different spectral channels exhibit different spatial resolutions. We illustrate this new approach for a dual-layer detector x-ray CT and compare MBMD algorithms with and without blur in the reconstruction forward model. Both qualitative and quantitative comparisons of performance with and without blur modeling are reported. We find that blur modeling yields images with better recovery of high-resolution structures in an investigation of reconstructed line pairs as well as lower cross-talk bias between material bases that is ordinarily found due to mismatches in spatial resolution between spectral channels. The extended spatial resolution of the material decompositions has potential application in a range of high-resolution clinical tasks and spectral CT systems where spectral channels exhibit different spatial resolutions.
1. INTRODUCTION
Spectral CT is finding increasing application in research and clinical applications due to its ability to use energy-dependent measurements to discriminate and quantify different materials. Applications include the ability to produce virtual non-contrast images, improved contrast discrimination (e.g., in angiography), single-shot multiphase studies, and quantitative imaging studies (e.g. bone mineral density estimation). There are various ways to capture the varied spectral measurements required for material decomposition including kV-switching, multiple x-ray sources (and detectors), photon-counting detectors, and multi-layer detectors. Many of these techniques have (or have the potential for) a difference in spatial resolution between spectral channels. For example, dual-layer detectors1 can be constructed with a relatively thin scintillator in the first (entrance) layer and a thicker scintillator in the second layer. While this helps to separate the low-energy x-ray measurements in the first layer from the high-energy x-rays measurements in the second layer, the first layer induces less scintillator blur and consequently higher spatial resolution measurements than the second layer. Similar discrepancies can be found in dual-source/detector systems with varied size focal spots or different detector characteristics in each imaging path, focal spot size differences for kV-switching methods, etc. Such mismatched spatial resolutions have the potential to limit the accuracy of material decomposition.
Historically, material decomposition has been performed via 1) image-domain decomposition (IDD) that reconstructs images separately from each energy channel following by linear decomposition using effective attenuation coefficients of material basis characterized in each energy channel; or 2) projection-domain decomposition (PDD) that first decomposes energy-dependent measurements into material density sinograms which are subsequently reconstructed into material density maps. A third, emerging option is Model-Based Material Decomposition (MBMD)2 that directly estimates material densities from spectral CT acquisition without an intermediate step. Moreover, MBMD uses a general objective function that can incorporate a measurement model with various physical and statistical aspects of the acquisition process as well as direct regularization of the material density maps.
In previous work3, we have demonstrated that model-based reconstruction methods can be adapted to incorporate source and detector blur to recover spatial resolution that is lost in ordinary processing. In this work, we combine system blur modeling with MBMD permitting different spatial resolutions in different spectral channels. Specifically, we introduce a system blur kernel in the acquisition model that allows for different resolution between energy channels. We demonstrate the approach in a dual-layer flat-panel detector system and illustrate that explicit blur modeling allows increased spatial resolution in material density maps over more conventional processing. The proposed methods have the potential to enable high spatial resolution material decomposition for applications including fine vessel angiography, high-resolution bone mineral density maps, etc.
2. METHODS
2.1. Model-based material decomposition with a system blur model
We adopt the following generalized polyenergetic forward model for x-ray CT:
| (1) |
where ρjk denotes the material density maps to be estimated for material basis k and voxel j; and αij represents the forward projection and the path length contribution of voxel j to pixel i. Material mass attenuation coefficients qwk relate energy-dependence in the mth energy bin which are summed over all materials. Energy-dependent transmissivity is computed using Beer’s law and then weighted and summed over projection-dependent spectral sensitivities Siw. The novel element to the above forward mode lies in the next sum. The raw (predetection) image is blurred according to a spatial kernel whose coefficients are given by bin. (An additional projection-dependent gain term is given by gi.) Note that this system model permits different spectral sensitivities for all (or any combination of) measurements as well as complex shift-variant blurs. The forward model may be represented compactly in matrix form as
| (2) |
where B is the blur operator, G is a diagonal gain matrix, S represents the spectral sensitivity matrix, Q is a matrix of energy-dependent mass attenuation coefficients for all materials, A is a system matrix that performs linear projection of the stack of all material density maps in the vector ρ. For MBMD without a system blur model (as in previous work), B is the identity matrix.
Presuming measurements follow a multivariate Gaussian distribution with covariance Ky, an objective function
| (3) |
may be written where the mean of the measurements is approximated with the model in Equation (2). R(ρ) is a regularization term. In this work we consider both quadratic and Huber regularization - applied separately in each material density map parameterized by regularization strength (β) and Huber shape parameter (δ)4. We note that while the above objective function is novel, the overall mathematical form is the same as that used in Tilley et al.2 and we may use the same iterative algorithm for estimating ρ as used in that work.
2.2. Dual-layer flat-panel detector simulation study
We simulated a spectral CT system with a 120 kVp X-ray source (2 mm aluminium filtration) and a prototype Varex dual-layer detector (2880 × 2880 pixel matrix, 150 μm pixelsize)1. The source-to- axis distance (SAD) was 400 mm and the source-to-detector distance (SDD) was 600 mm. Measurements were generated using a soft tissue-iodine digital phantom (axial slice shown in Figure 1a) with 1500 × 1500 × 5, 0.02 mm cubic voxels. Measurements were simulated using Equation (2). The dual-layer detector is modeled with different scintillator thicknesses for each layer. The low-energy (LE) detector on the top layer has a 200 μm CsI scintillator, while the high-energy (HE) detector on the bottom layer has a 550 μm CsI scintillator. We modeled detector blur in each detector using the Modulation Transfer Functions (MTFs) and gains characterized in previous work1. The detector MTFs are shown in Figure 1b, while the gains of LE and HE channels are 533 e-/X-ray and 227 e-/X-ray, respectively. The incident spectrum and material basis functions were simulated using Spektr5 and Poisson noise was added to the projection data.
Figure 1:
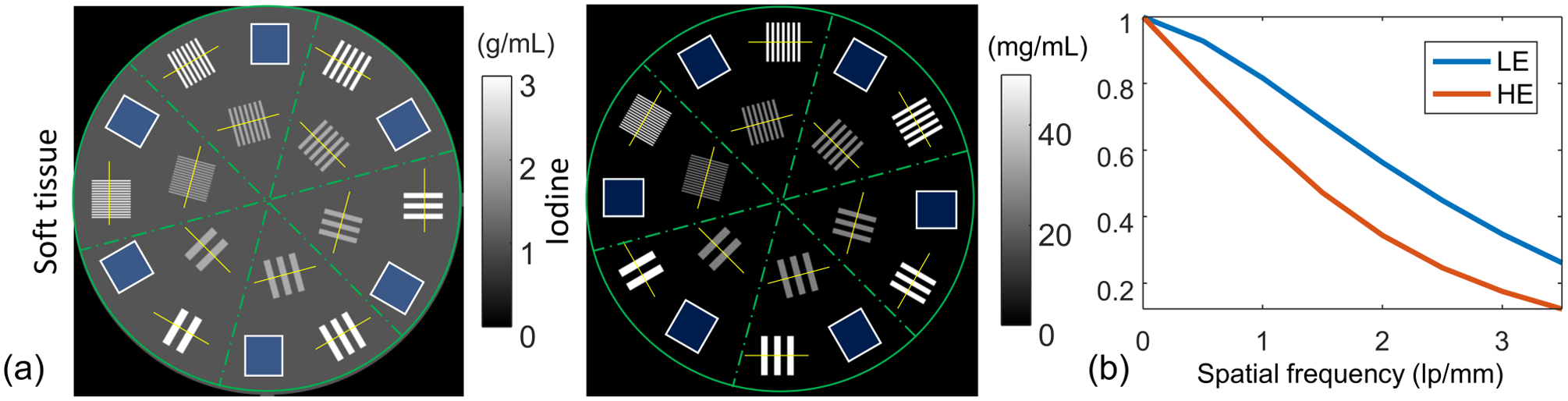
(a) A high-resolution dual-material phantom consisting of soft tissue and iodine bases. The soft tissue basis consists of a 30 mm-diameter cylinder with 1 g/mL concentration. Six groups of line pairs varying from 0.2 mm/lp to 1.2 mm/lp are inserted in each section. (b) Detector MTFs in LE and HE channels of the dual-layer flat-panel detector.
The spectral measurements were reconstructed into volumes with 300 × 300 × 1, 0.1 mm cubic voxels using MBMD with either the idealized (B = I) forward model (iMBMD) or the forward model with system blur (bMBMD). Quadratic and Huber penalties were applied using regularization parameters in the soft tissue basis (βs) between 1 × 104 and 1 × 106, and in the iodine basis (βI) between 1 × 107 and 1 × 109. Each reconstruction used 400 iterations of the separable surrogates algorithms2 with 10 subsets.
We present a qualitative comparison of decomposition results as well as quantitative comparisons of line pair modulation and cross-basis bias. Line pair modulation was measured as the normalized difference between the highest and the lowest value of the profile projected onto the perpendicular virtual axis marked with the yellow line in Figure (1a). The bias was measured as the absolute difference of the sums in uniform phantom regions (identified with blue squares in Figure) from the true uniform value.
3. RESULTS
3.1. Qualitative assessment
The ground truth, iMBMD, and bMBMD results are shown in Figure 2. We adopted quadratic regularization with regularization parameters (βs = 104, βI = 107) in iMBMD and (βs = 106, βI = 107) in bMBMD to match the noise level in the soft tissue basis in a central region-of-interest. The top row shows the soft tissue basis and the bottom shows the iodine basis. The yellow squares highlight the 2.5lp/mm dual-material line pairs in the soft tissue basis, and the cross-talk bias from the 1.25lp/mm single-material soft tissue line pairs in the iodine basis. (Note the display window for the zoom-in regions in the iodine basis is [0,10] mg/mL for better visualization of the bias.) One can see that the iMBMD results suffer from lower spatial resolution, contrast loss, and more cross-basis bias due to the mismatch between the acquisition and reconstruction models.
Figure 2:

Ground truth and MBMD results with a quadratic penalty.
3.2. Modulation of line pairs
Line pair modulation results are summarized in Figure 3, where red and magenta lines show bMBMD performance, and blue and cyan lines show iMBMD, respectively. Quadratic penalty results are represented with solid lines, while Huber penalty results are shown with dashed lines. Each profile shows the relative modulation at different in-basis regularization strengths, while the regularization in the other material basis is fixed (values shown below each set of plots). The bMBMD results show higher spatial resolution for both material bases as compared with the iMBMD results. In the iodine basis, the spatial resolution decreases with increasing regularization strength, while the Huber penalty outperforms the quadratic regularizer with the same regularization parameters. The soft tissue performance is less sensitive to the particular regularizer.
Figure 3:
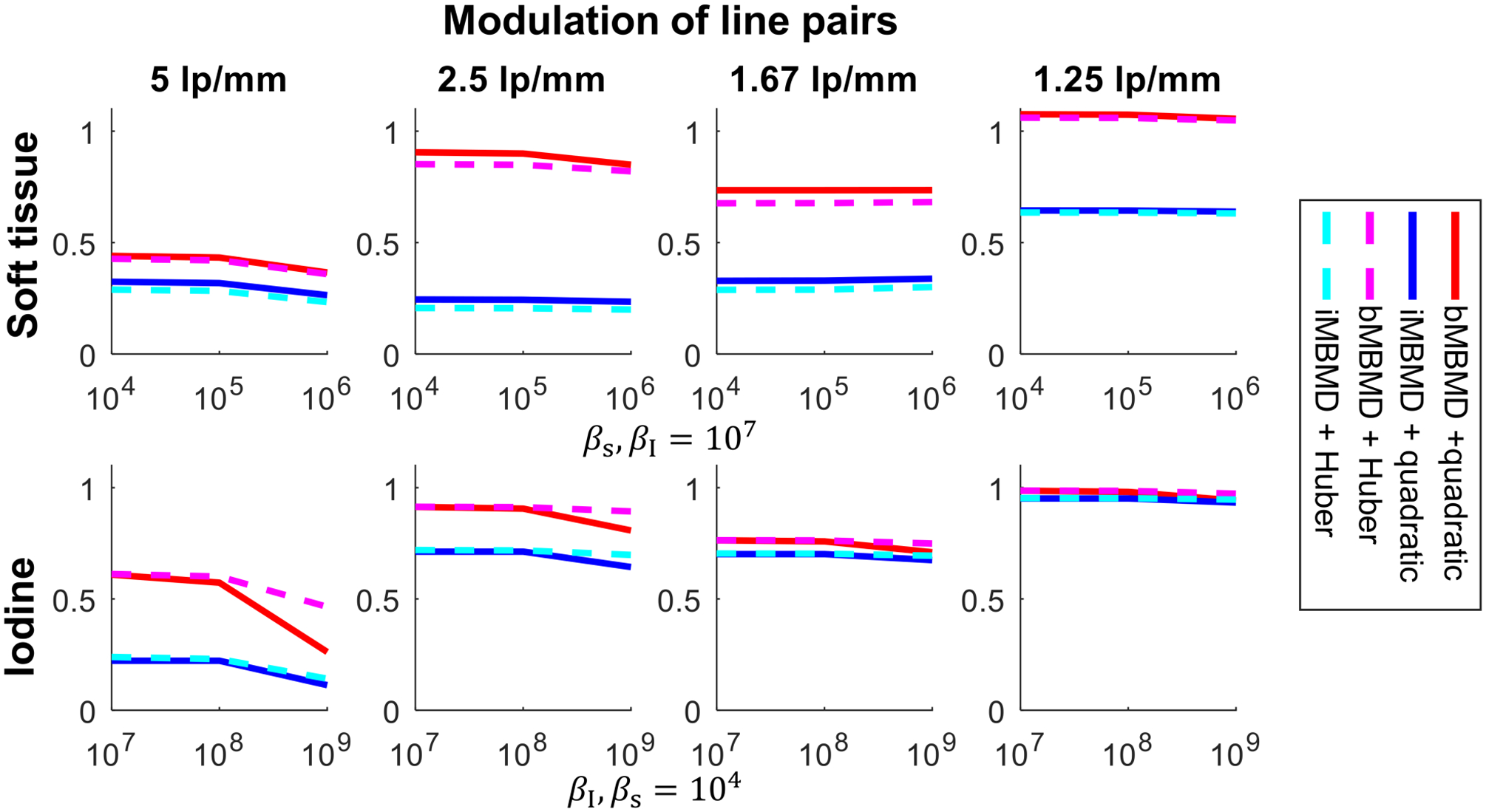
Line pair modulations. Top: the modulations of line pairs in the soft tissue with varying regularization strength in the soft tissue and fixed strength in the iodine. Bottom: the modulations in the iodine with varying regularization strength in the iodine and fixed strength in the soft tissue.
3.3. Cross-talk between material bases
Estimated biases due to cross-talk are summarized in Figure 4. In each plot, a 3 × 3 map shows reconstruction bias with varying regularization strengths in both material bases (varying βI on x-axis and βs on the y-axis). In general, the bias in one material basis increases with increasing regularization strength in the other material basis. More significantly, compared with the iMBMD results, the bMBMD results have lower bias with the same regularization settings.
Figure 4:
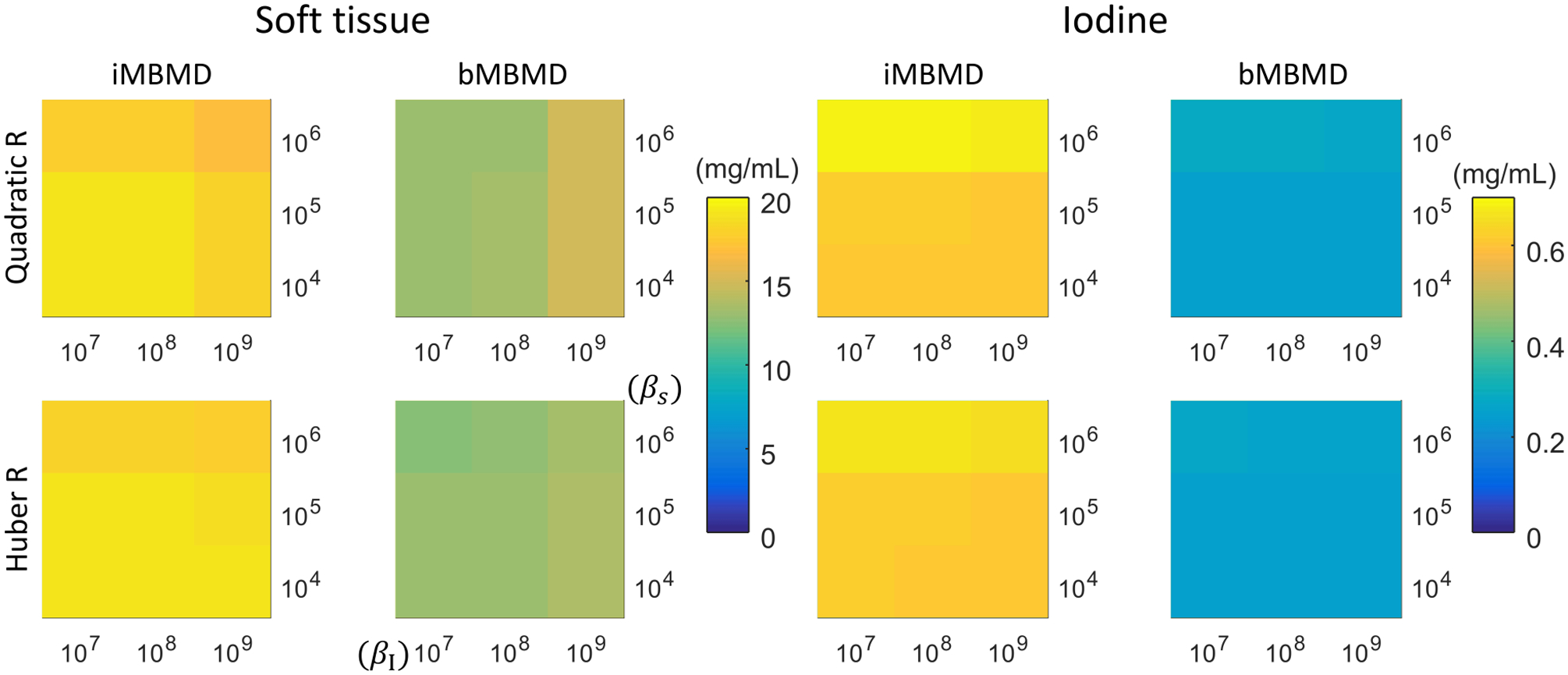
Bias evaluations in soft tissue (left) or iodine (right). Each subplot shows bias measurement with varying regularization strengths in soft tissue and iodine basis. Top: quadratic regularization; bottom: Huber regularization.
4. CONCLUSIONS
In this work, we presented a generalized model-based material decomposition algorithm incorporating a high-fidelity acquisition model that characterizes the varied system blur in different energy channels. We evaluated the approach in a dual-layer flat-panel detector CT system using a high-resolution dual-material phantom. The study shows incorporation of a blue model permits higher resolution material decompositions with lower cross-talk bias than approaches that do not model this blur. This suggests that the model-based algorithm is able to deconvolve the system detector blurs as part of the decomposition, eliminate biases associated with the resolution mismatch between channels, and balance high-resolution information in one channel with lower resolution information in the other channel. High-resolution material decomposition has potential application in a number of clinical targets and the proposed methods have the potential to extend the high-resolution capabilities of mixed resolution data like that provided by multi-layer flat-panel detectors. Applying this methodology to experimental data from a dual-layer detector is ongoing.
ACKNOWLEDGMENTS
This work was supported, in part, by NIH grant R01EB025470 and in collaboration with Varex Imaging Corporation.
References
- [1].Lu M, Wang A, Shapiro E, Shiroma A, Zhang J, Steiger J, and Star-Lack J, “Dual energy imaging with a dual-layer flat panel detector,” 1094815(March), 40 (2019). [Google Scholar]
- [2].Tilley II S, Zbijewski W, and Stayman JW, “Model-based material decomposition with a penalized nonlinear least- squares CT reconstruction algorithm,” Phys Med Biol 64(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tilley S, Jacobson MW, Cao Q, Brehler M, Sisniega A, Zbijewski W, and Stayman JW, “Penalized-likelihood reconstruction with high-fidelity measurement models for high-resolution cone-beam ct imaging,” IEEE Transactions on Medical Imaging 37(4), 988–999 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huber PJ, [Robust statistics], Springer; (2011). [Google Scholar]
- [5].Punnoose J, “Technical Note: SPEKTR 3.0 - A Computational Tool for X-Ray Spectrum Modeling and Analysis,” Medical Physics 4711(April), 4711–4717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


