Abstract
Background:
Although immunomodulatory effects of anesthetics have been increasingly recognized, their underlying molecular mechanisms are not completely understood. Toll-like receptors (TLRs) are one of major receptors to recognize invading pathogens and danger signals from damaged host tissues to initiate immune responses. Among TLR family, TLR2 and TLR4 recognize a wide range of ligands and are considered to be important players in perioperative pathophysiology. Based on our recent finding that volatile anesthetics modulate TLR4 function, we tested our hypothesis that they would also modulate TLR2 function.
Methods:
The effect of anesthetics isoflurane, sevoflurane, propofol and dexmedetomidine on TLR2 activation was examined by reporter assays. An anesthetic that affected the activation was subjected to in silico rigid docking simulation on TLR2. To test our prediction that sevoflurane and a TLR1/TLR2 ligand Pam3CSK4 would compete for the same pocket of TLR2, we performed Pam3CSK4 competitive binding assay to TLR2 using HEK cells stably transfected with TLR2 (HEK-TLR2) with or without sevoflurane. We examined the effect of different anesthetics on the functions of human neutrophils stimulated with TLR2 ligands. Kruskal-Wallis test and Mann-Whitney test were used for statistical analysis.
Results:
We observed that the attenuation of TLR1/TLR2 activation was seen upon sevoflurane exposure, but not upon isoflurane, propofol, or dexmedetomidine exposure. The attenuation of TLR2/TLR6 activation was not seen in any of the anesthetics tested. The rigid docking simulation predicted that sevoflurane and Pam3CSK4 bound to the same pocket of TLR1/TLR2 complex. The binding of Pam3CSK4 to HEK-TLR2 cells was impaired in the presence of sevoflurane, indicating that sevoflurane and Pam3CSK4 competed for the pocket, as predicted in silico. The stimulation of neutrophils with Pam3CSK4 induced L-selection shedding, but did not affect phagocytosis and reactive oxygen species production. L-selectin shedding from neutrophils was attenuated only by sevoflurane, consistent with the result of our reporter assays.
Conclusions:
We found that TLR1/TLR2 activation was attenuated by sevoflurane, but we found no evidence for an attenuation by isoflurane, propofol or dexmedetomidine at clinically relevant concentrations. Our structural analysis and competition assay supported that sevoflurane directly bound to TLR2 at the interphase of TLR1/TLR2 complex. Sevoflurane attenuated neutrophil L-selectin shedding, an important step for neutrophil migration.
Introduction
Immune cells are mounted with a number of pattern recognition receptors (PRRs) that recognize endogenous danger signals from injured tissues and foreign pathogens (‘alarmins’). Toll-like receptors (TLRs) are part of the PRR family and evolutionarily ancient mediators for innate host defense 1. So far 10 human TLRs (TLR1-TLR10) and 12 mouse TLRs (TLR1-9, TLR11-13) are identified. Neutrophils are the most abundant leukocytes in human blood, and the first host defense innate immune cells reacting to stress such as surgery and infection. Human neutrophils express most of the TLRs (TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9 and TLR10) 2. Among them, TLR2 and TLR4 are unique that they bind to a variety of ligands. TLR4 binds to Gram negative bacterial constituent lipopolysaccharide and a number of endogenous alarmins. TLR2 is best known for its binding to ligands from Gram positive bacteria. It also binds to Gram negative bacteria, virus, fungus, helminth and many endogenous alarmins 3.
Clearly TLR2 is important in infection. TLR2 knockout mice inoculated with Staphylococcus aureus (S. aureus), Mycoplasma and Mycobacterium suffered from higher bacterial loads and death compared to wild type mice 3–5. Patients with TLR2 polymorphism were susceptible to tuberculosis 5. TLR2 is also involved in the development of ischemia-reperfusion injury, a neutrophil driven pathophysiology often experienced intraoperatively 6. It is therefore important to know if TLR2 functions are affected in the perioperative setting. Anesthesia is a requisite component of surgery. General anesthesia is typically provided by volatile and intravenous anesthetics. We and others have reported that these anesthetics can be immunomodulatory 7–14. Recently we found that volatile anesthetics isoflurane and sevoflurane directly bound to TLR4 and inhibited its function 15. TLRs have characteristic leucine-rich-repeat (LRRs) 16. The ectodomain of TLR2 and TLR4 can be split into three subdomains; N-terminal, central and C-terminal subdomains. Because TLR2 and TLR4 are structurally alike (Fig. 1), we hypothesized that volatile anesthetics would also affect TLR2 function as they did on TLR4. We examined the effect of anesthetics on TLR2 activation and their mechanism. We also studied the effect of anesthetics on the function of neutrophils stimulated with TLR2 ligands.
Figure 1. Horseshoe structure of TLR2 and TLR4.
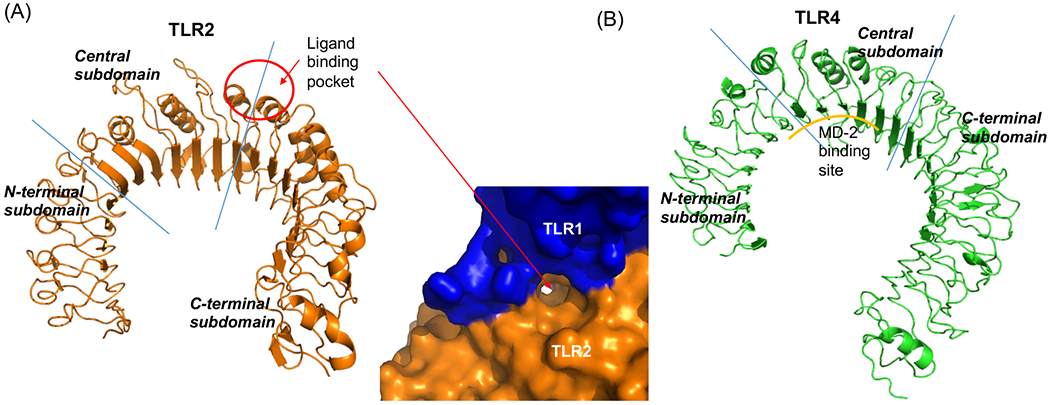
Structures of (A) TLR2 (left, PBD: 2Z7X, TLR1/2 ligand Pam3CSK4 was removed from the original structure) and (B) TLR4 (right, PBD: 3FXI) were shown. Blue lines indicated the borders between N-terminal and central subdomains or between central and C-terminal subdomains. The red circle indicated the binding site of TLR2 ligands Pam3CSK4 and Pam2CSK4. In (A), The pocket in TLR2 dimerized with TLR1 was shown.
Methods
The nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) activation by TLR ligands
THP-1 blue cells (InvivoGen; San Diego, CA, USA) are human monocytic leukemia cell line with an NFκB-inducible secreted embryonic alkaline phosphate (SEAP) reporter 17. Similarly, TLR2 reporter cell lines, HEK-TLR2 cells containing an NFκB-inducible SEAP reporter (InvivoGen) were used. Both cells were cultured per the company’s protocol. They were stimulated with Pam3CSK4 (100 ng/mL) or lipoteichoic acid (LTA, 10 μg/mL) (both from InvivoGen) for 6 hours. In the experiments where cells were exposed to volatile anesthetics, they were placed in an air-tight chamber with inflow and outflow ports. Isoflurane or sevoflurane was introduced from the inflow port connected to the vaporizer with air passing through as a carrier and they were exposed for 6 hours as we previously performed 18. The concentration of volatile anesthetics was measured using infrared spectroscopy (Ultima; Datex Instrument Corp, Helsinki, Finland) as previously performed19. Intravenous anesthetics (propofol, dexmedetomidine) were added to corresponding wells for 6 hours. Stock solutions for propofol and dexmedetomidine were dissolved in dimethylsulfoxide (DMSO), and the final concentration of DMSO was kept the same (0.1%) for propofol and dexmedetomidine experiments. Clinically used intralipid containing propofol was used in some of the experiments. NF-κB activation was assessed by using the reaction buffer Quanti-Blue to quantitate SEAP in the medium per the company protocol. Samples were subjected to a spectrophotometer analysis at 655 nm.
Determination of isoflurane and sevoflurane concentrations in culture media
The concentrations of dissolved isoflurane and sevoflurane were determined by liquid chromatography with mass spectrometry detection (UPLC/MS). Culture medium was collected immediately following exposure to isoflurane and sevoflurane in air-tight glass containers without air space as we previously performed20. Samples were diluted in mobile phase comprised of Part A pure methanol MS grade and Part B 0.1% formic acid in MilliQ water. All samples were assayed with an Acquity H-Class UPLC with a XEVO TQ triple quadrupole mass spectrometry detector (Waters CO., Milford, MA). We used an Acquity UPLC BEH 18 1.7 μm 2.1 x 100 mm column, with a VanGuard Pre-Column 2.1 x 5 mm guard. The mobile phase was run on a gradient from 95% B to 90% A for 5.5 minutes. The flow rate was 0.3 mL/min and the injection volume was 3 μL. The detection was carried out by Multiple Reaction Monitoring (MRM) under positive mode. For isoflurane we used the transitions 185.067 > 99.867 and 185.067 > 143.927, while for sevoflurane we used 201.039 > 153.867 and 201.039 > 168.896. Data acquisition and instrument control was done by Masslynx 4.1 software (Waters Co.). The accuracy and precision (% coefficient of variation) were 97.2-99.5% and 1.8-4.7%, respectively.
Rigid docking simulation of sevoflurane on TLR1/TLR2
Potential sevoflurane binding sites on human TLR1/TLR2 were predicted in silico using SiteMap (Schrodinger LLC; Cambridge, MA, USA). First, Pam3CSK4 was removed from PDB: 2Z7X21 to create apo-TLR1/TLR2 structure. The protein structure was prepared using the built-in protein preparation wizard. “Site” was defined as the protein surface suitable for binding of a ligand to the receptor. SiteMap was set as follows: 15 site points per reported site, report up to 5 sites, using a restrictive definition of hydrophobicity, standard grids and cropping site maps at 4 angstroms from the nearest site point. The OPLS_2005 force field was used for calculation. An estimated binding site with the highest score was then embedded into the grid with the size of 20 x 20 x 20 cubic angstroms for docking with sevoflurane using Autodock Vina (The Scripps Research Institute; La Jolla, CA, USA).
Pam3CSK4 competitive binding assay using HEK cells stably transfected with TLR2
HEK cells stably transfected with human TLR2 (HEK-TLR2) were kindly given by Dr. Dennis Kasper (Brigham and Women’s Hospital, Boston, MA, USA). HEK and HEK-TLR2 cells were maintained in RPMI1640 with 10% heat-inactivated FBS/ 2 mM L-glutamine. Pam3CSK4-biotin and streptavidin-allophycocyanin (APC) were co-incubated for multimer formation. The multimer was co-incubated with either HEK cells or HEK-TLR2 cells for 60 min at 37°C. Some cells were co-incubated with the multimer under isoflurane or sevoflurane for 60 min using an air-tight chamber as above. After washing, cells were subjected to flow cytometry analysis using Accuri 6 (BD Biosciences; Franklin Lakes, NJ, US). Binding% of Pam3CSK4 under sevoflurane was calculated as [mean fluorescence intensity (MFI) of HEK-TLR2 cells – MFI of HEK cells]/ [average MFI of HEK-TLR2 cells without sevoflurane – average MFI of HEK cells without sevoflurane] x 100(%).
L-selectin shedding assay in neutrophils
Blood was obtained from healthy volunteers and heparinized. The protocol was approved by the Institutional Review Board at Boston Children’s Hospital. Whole blood was stimulated with Pam3CSK4 (concentration; 100 ng/mL) and LTA (concentration; 10 μg/mL) under isoflurane, sevoflurane, propofol or dexmedetomidine, and the degree of L-selectin shedding from neutrophils was probed with anti-CD62L antibody (DERG-56; Biolegend, San Diego, CA, USA). Blood was subjected to lysis using FACS lysis buffer (BD; Franklin Lakes, NJ, USA) and flow cytometry analysis. Neutrophil population was gated from forward and side scatters as we previously performed 22.
CD11b expression analysis on neutrophils
CD11b is a proinflammatory molecule, and its expression level increases upon inflammation. Heparinized blood was stimulated with Pam3CSK4 or LTA (final concentration: 100 ng/mL) under different anesthetics, and CD11b expression was probed with anti-CD11b antibody (ICRF44, Biolegend). Blood was lyzed and subjected to flow cytometry analysis as above. Neutrophils were gated from forward and side scatters.
Whole blood phagocytosis assay
Heparinized blood was subjected to phagocytosis assay using latex beads labeled with fluorescein isothiocyanate (FITC). Latex beads were opsonized with human plasma. The opsonized latex beads were co-incubated with Pam3CSK4 (100 ng/mL) or LTA (10 μg/mL) -stimulated whole blood under anesthetics for 30 min at 37°C. After quenching, samples were subjected to lysis and washing, and analyzed by flow cytometry analysis. Neutrophils were gated from forward and side scatters.
Whole blood reactive oxygen species (ROS) production measurement
Heparinized blood was stimulated with Pam3CSK4 (100 ng/mL) or LTA (10 μg/mL). Blood was further stimulated with N-formylmethionine-leucyl-phenylalanine (fMLP, 100 ng/mL). ROS production was examined using rhodamine 123 (Invitrogen). Samples were subjected to lysis and washing, and analyzed by flow cytometry. Neutrophils were gated as forward and side scatters.
Statistical analysis
Statistical analysis methods were performed using PRISM 5 software (GraphPad; La Jolla, CA, USA). Detailed statistical analysis was described in the corresponding figure legends. Briefly, we used Kruskal-Wallis test to compare the groups in analyses involving more than two groups, and Dunn’s post hoc test was subsequently used for pairwise comparisons between specific groups. For analyses in which only two groups were compared, a Mann-Whitney test was used. Statistical significance was considered when p< 0.05. An a priori sample size calculation was not performed, and we empirically chose 6-8 replicates per group.
Results
Sevoflurane attenuated TLR1/TLR2 activation
TLR2 is unique among TLRs to form functional heterodimers with other TLR members (TLR1 and TLR6) 3. The heterodimer formation may allow it to bind to a diverse array of ligands. TLR2 binds to Gram positive wall products lipoproteins and LTA 3. Pam3CSK4 is a triacyl lipoprotein /lipopeptide analogue and a TLR1/TLR2 agonist. LTA is a TLR2/TLR6 agonist. First, THP-1 cells were stimulated with Pam3CSK4 and LTA at a range of concentrations for 6 hours (Supplemental Fig. 1). Based on this result, 100 ng/mL for Pam3CSK4 and 10 μg/mL for LTA were used in subsequent reporter assays. THP-1 blue cells were stimulated with Pam3CSK4 or LTA under isoflurane, sevoflurane, dexmedetomidine or propofol at a range of concentrations. Pam3CSK4 induced-TLR2 activation was attenuated by sevoflurane, but we did not observe attenuation by LTA stimulation (Fig. 2A–B). Attenuation of TLR2 activation by isoflurane was observed in neither Pam3CSK4- nor LTA-stimulated samples. Of note, we examined isoflurane and sevoflurane concentrations in the culture medium, which were 0.34 mM for 1% isoflurane and 0.28 mM for 1% sevoflurane, respectively. We did not find any evidence that intravenous anesthetics dexmedetomidine and propofol affected TLR2 activation within their clinically relevant concentrations (the clinically relevant concentrations of dexmedetomidine and propofol are < 0.01 μM 23–25 and up to ~50 μM 26–28, respectively). Both dexmedetomidine and propofol attenuated TLR2 activation only at the very high concentration (100 μM). We also tested clinically used propofol, containing intralipid. We did not observe change even at 100 μM (Supplemental Fig. 2). In addition, we tested the effect of sevoflurane and isoflurane in HEK-TLR2 reporter system. Sevoflurane attenuated TLR1/TLR2 activation (Fig. 3A). We did not observe the change of TLR2/TLR6 activation by sevoflurane (Fig. 3B), supporting our findings in THP-1 cell system.
Figure 2. The effect of anesthetics on TLR2 activation in THP-1 cells.
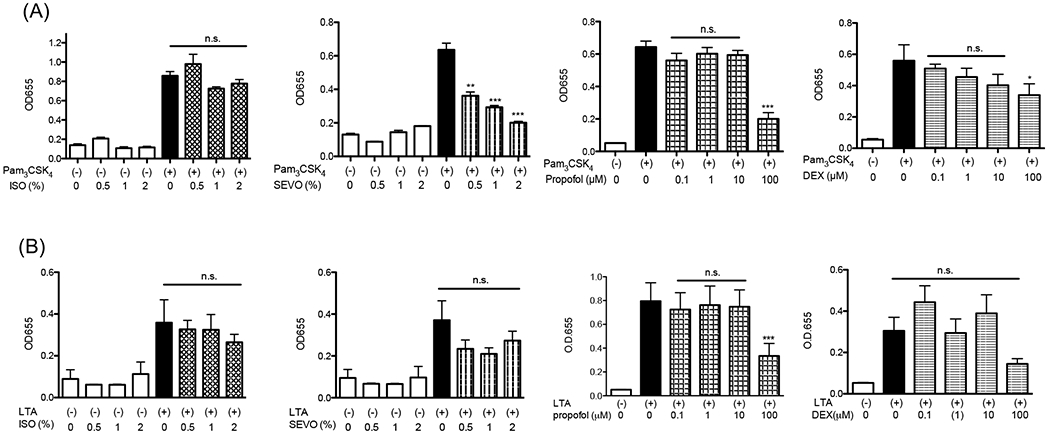
TLR2 activation by (A) TLR1/TLR2 agonist Pam3CSK4 (100 ng/mL) and (B) TLR2/TLR6 agonist LTA (10 μg/mL) was examined under anesthetics (isoflurane, sevoflurane, dexmedetomidine or propofol) in THP-1 blue cell system. Data were shown as mean +/− S.D. 8 independent replicates were used per group. Statistical analysis was performed using Kruskal-Wallis test with Dunns post hoc analysis. *, ** and *** adjusted p< 0.05, < 0.01, and < 0.001 vs samples stimulated with TLR2 ligands and without anesthetics. n.s = not significant.
Fig. 3. The effect of volatile anesthetics on TLR1/TLR2 activation in HEK-TLR2 cells.
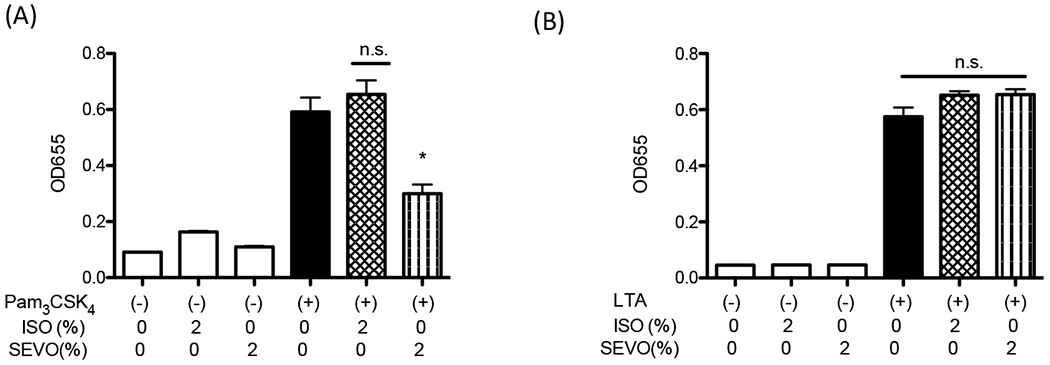
TLR1/TLR2 activation by Pam3CSK4 (100 ng/mL) and TLR2/TLR6 activation by LTA (10 μg/mL) was examined under isoflurane and sevoflurane using HEK-TLR2 reporter cells. Data were shown as mean +/− S.D. 8 independent replicates were used per group. Statistical analysis was performed using Kruskal-Wallis test with Dunns post hoc analysis. * denotes adjusted p< 0.05 vs samples stimulated with Pam3CSK4 and without anesthetics.
Rigid docking simulation predicted that sevoflurane bound to the internal pocket of TLR2
Data so far showed that sevoflurane attenuated TLR2 activation by TLR1/2 agonist. Pam3CSK4 bind to the pocket in TLR2 at the interphase between TLR1 and TLR2 (we call ‘internal pocket’) (Fig. 1A). Because this pocket is very hydrophobic, we hypothesized that sevoflurane directly bound to this pocket and impaired its function. First, we performed in silico docking simulation to examine if sevoflurane would bind to TLR2 using the previously solved TLR1/TLR2 structure (PDB: 2Z7X). Sevoflurane was predicted to bind to the internal pocket (Fig. 4A–B). Many hydrophobic residues were nearby from the docked sevoflurane (i.e. within 4 angstroms from the docked sevoflurane), which included Ile-319, Phe-322, Phe-325, Leu-328, Ser-346, Lys-347, Val-348, Phe-349 and Leu-350 (Fig. 4C). Pam3CSK4 interacts with Ile-319, Phe-325, Leu-328, Val-348 and Leu-350 via hydrophobic interactions and with Phe-439 via hydrogen bond 21. This fitted with our hypothesize that sevoflurane would compete with Pam3CSK4 at the internal pocket for TLR2 binding.
Figure 4. Sevoflurane interaction with TLR2.
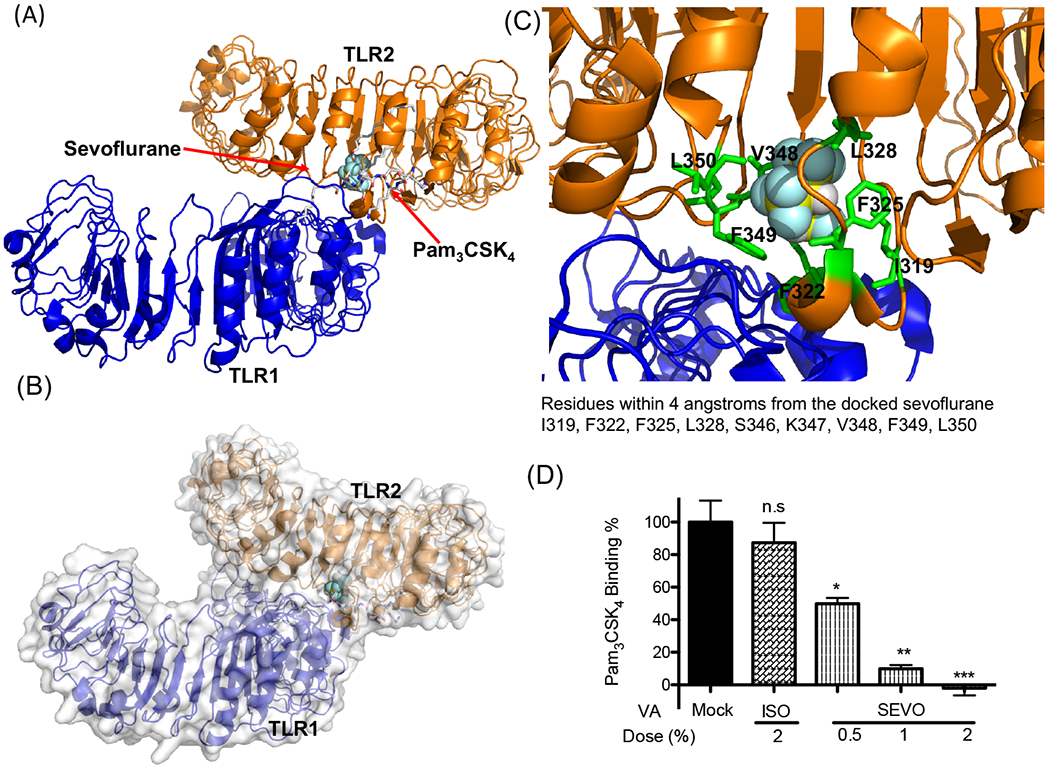
(A, B, C) Rigid docking simulation was performed to predict sevoflurane binding on TLR1/TLR2. Sevoflurane bound to the internal pocket on TLR2 that Pam3CSK4 binds to (A, B). Blow-out image of the sevoflurane binding site. The nearby residues around sevoflurane (within 4 angstroms from the sevoflurane) are shown with sticks.
(D) Pam3CSK4 binding to HEK-TLR2 cells was tested with or without volatile isoflurane or sevoflurane. Data were shown as mean +/− S.D. 6 independent replicates were used per group. Kruskal-Wallis test with Dunns post hoc analysis was performed for statistical analysis. *, **, and *** denote adjusted p< 0.05, 0.01, and 0.001 vs mock, respectively. n.s.; not significant, ISO; isoflurane, SEVO; sevoflurane, VA; volatile anesthetic.
Sevoflurane attenuated Pam3CSK4 binding to TLR2
To test our hypothesis, we performed competitive binding assay of Pam3CSK4 to HEK-TLR2 cells with or without volatile anesthetics. Knowing that Pam3CSK4 multimer effectively bound to TLR2 as non-multimer Pam3CSK4 (Supplemental Figure 3), we used multimer system for volatile anesthetic competition assay. Our data showed that sevoflurane significantly reduced the binding of Pam3CSK4 to TLR2, but isoflurane did not (Fig. 4D), compatible with our reporter assay experiments (Fig. 2A, 3A). IC50 of sevoflurane was 1.4 (95% Confidence Intervals: 0.15-12.9). In summary, our data supported that sevoflurane directly bound to TLR2 to attenuate its activation.
Sevoflurane attenuated neutrophil L-selectin shedding in human whole blood
Neutrophils are critical for first-line defense during infection but also cause tissue injury such as ischemia-reperfusion injury in the intraoperative setting, where TLR2 is involved in these processes 29. Therefore, we examined the effect of anesthetics on neutrophils stimulated with TLR2 ligands. Circulating neutrophils are typically attracted to the site of infection/ inflammation via the following mechanism; When neutrophils become activated with proinflammatory mediators, L-selectin on neutrophils binds to glycans on the endothelial cells to initiate neutrophil tethering and rolling along the luminal walls of post-capillary venules 30. Then, they make an arrest on the endothelial cells before migrating to the target site. L-selectin is shed from the surface of rolling neutrophils, which is the step necessary for an efficient transendothelial migration of neutrophils 31,32. Shedding occurs by proteolytical cleavage of L-selectin 33. Once neutrophils are recruited to their destination, they kill bacteria by phagocytosis or ROS production. First, we examined the effect of anesthetics on shedding. Only sevoflurane attenuated neutrophil L-selectin shedding induced by Pam3CSK4 (Fig. 5A). We did not observe the attenuation of LTA-induced L-selectin shedding by sevoflurane. CD11b expression is often used as a neutrophil activation marker. CD11b expression was upregulated after both Pam3CSK4 and LTA stimulation (Fig. 5B). The upregulation of CD11b expression was attenuated by isoflurane and sevoflurane under Pam3CSK4 and LTA stimulation (Fig. 5B). Isoflurane inhibits CD11b directly 11. This might explain the results of isoflurane experiment. We also examined neutrophil ROS formation and phagocytosis. A significant ROS formation and phagocytosis were elicited by neither Pam3CSK4 nor LTA (Supplemental Fig. 4).
Figure 5. The effect of anesthetics on neutrophil functions.
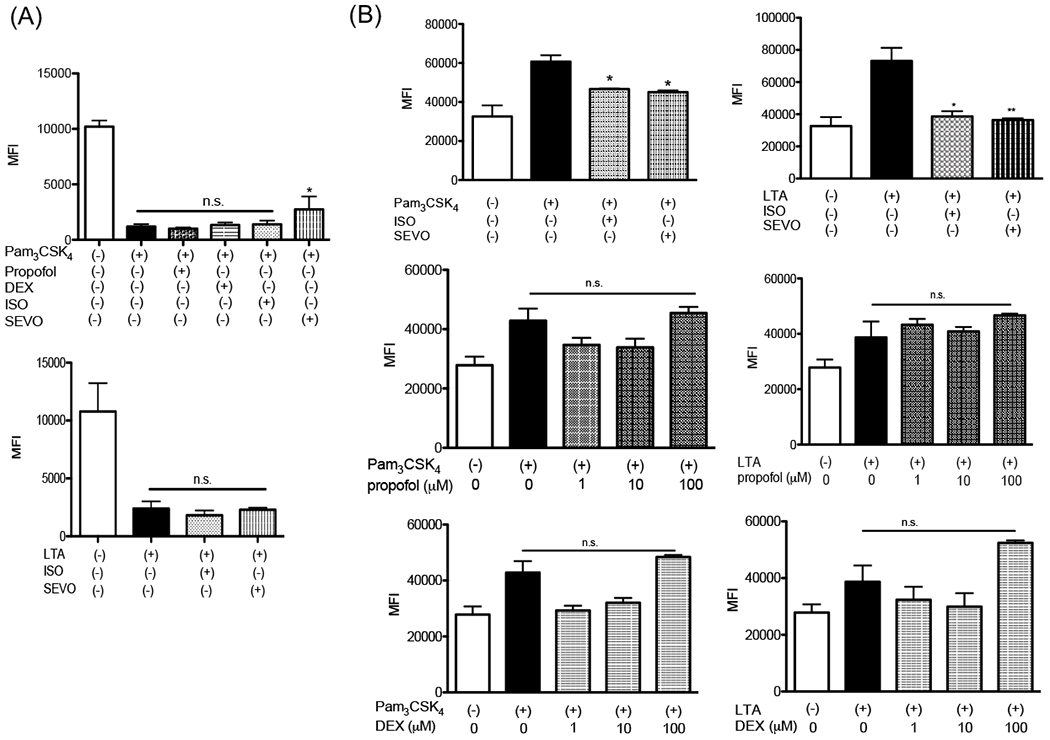
The effect of anesthetics (isoflurane, sevoflurane, dexmedetomidine or propofol) on neutrophil L-selectin shedding (A) and CD11b expression (B) was examined. Data were shown as mean +/− S.E. of mean of 6 independent experiments. Statistical analyses were performed using Kruskal-Wallis test with Dunns post hoc analysis. n.s.= not significant. * and ** adjusted p<0.05 and < 0.01 vs TLR2 ligand stimulation groups without anesthetics, respectively. ISO; isoflurane, SEVO; sevoflurane, DEX; dexmedetomidine.
Discussion
Here we found that sevoflurane attenuated TLR1/TLR2 activation by competing with Pam3CSK4 for the internal pocket on TLR2. However, we did not observe the attenuation of TLR1/TLR2 activation by another ether-derivative volatile anesthetic isoflurane. We did not find any evidence that intravenous anesthetics dexmedetomidine and propofol attenuated TLR2 activation within their clinically relevant concentrations. The shedding of Pam3CSK4-induced L-selectin was attenuated by sevoflurane. We did not find that LTA-induced L-shedding was affected by sevoflurane, in line with our reporter assays. The upregulation of CD11b expression in human neutrophils were attenuated by isoflurane and sevoflurane.
TLR2 is predominantly expressed on myeloid cells and endothelial cells. Neutrophils are the most abundant myeloid cells and protect host from infection. Neutrophils are double-edge swords because they can also cause organ injury such as ischemia-reperfusion injury during surgery, which could contribute to significant perioperative morbidity and mortality 29. Alarmins such as high-mobility group box 1 (HMGB1) and heat shock proteins (HSPs) are TLR2 ligands and produced from tissues that experience ischemia and cell death 34. Migration of overwhelmingly activated neutrophils to the previously ischemic tissues upon reperfusion can lead to a significant tissue injury. Thus tight regulation of neutrophil function is necessary to allow neutrophils to effectively eradicate invading pathogens while limiting tissue damage and organ dysfunction 29. The inhibition of L-selectin shedding reduces leukocyte migration. CD11b is not only used as an activation maker, but also works as complement receptor for microbial phagocytosis 35,36. In the setting of infection, the attenuation of neutrophil functions by sevoflurane may not be necessarily favorable in S. aureus, Mycoplasma and Mycobacterium infections as TLR2 knockout mice showed worse outcomes 3–5. However, the effect of sevoflurane on TLR2 may be beneficial to mitigate neutrophil-driven tissue damage in the setting of ischemia-reperfusion injury. Knowing that the attenuation of L-selectin shedding by TLR2 ligands was not observed by isoflurane, it will be clinically important to compare the effect of isoflurane and sevoflurane on ischemia-reperfusion injury. HMGB-1 is one of major alarmins, and binds to both TLR2 and TLR4 on neutrophils 37. Although it is reported that TLR2 and TLR4 respond to HMGB-1 differently regarding cytokine release 38, it remains to be examined if HMGB1 has a distinct role in TLR2 and TLR4 functions in the setting of ischemia-reperfusion injury. HSPs also interact with TLR2 and TLR4. We did not examine the impact of HMGB1 and HSPs on neutrophils and the effect of anesthetics. Because both isoflurane and sevoflurane attenuated TLR4 function 15, whether or not the distinct difference between isoflurane and sevoflurane in the effect on TLR2 function will translate into pathophysiological difference needs to be investigated in the future. In addition to its role in leukocytes, the activation of endothelial cells by TLR2 ligands promotes them to release proinflammatory cytokines, which enhance vascular leakage 39. We did not study the effect of anesthetics on TLR2 on the endothelial cells, but we expect that sevoflurane exposure will attenuate TLR2 function on the endothelial cells and limit proinflammatory cytokine release.
As a target of sevoflurane on TLR2 signaling, we primarily focused on the cell surface receptor. This was primarily due to that fact that we observed the attenuation of TLR1/TLR2-mediated NFκB activation by sevoflurane but did not see the attenuation of TLR2/TLR6-mediated activation with the assumption that TLR1/TLR2- and TLR2/TLR6-mediated NFκB activation uses the same signaling pathway. It is possible that sevoflurane can interact with intracellular signaling molecules, but we consider this as a future study. The upregulation of CD11b expression by Pam3CSK4 and LTA was attenuated by both isoflurane and sevoflurane. The phenotype of isoflurane may be explained by its direct effect on CD11b 11. Because our study was done in vitro, in vivo validation of our findings needs to be done in the future.
Isoflurane and sevoflurane are structurally similar, derived from ether as their prototype. However, we found that only sevoflurane interacted with TLR2. Sevoflurane (154 cubic angstroms)40 is slightly larger than isoflurane (144 cubic angstroms)40. Because the predicted binding pocket size of TLR2 was large enough (174 cubic angstroms) to accommodate isoflurane as well, it still remains to be determined about the pocket’s selectivity. Our recent investigation showed that Fas death domain (DD) and Fas-associated DD (FADD) interaction was inhibited by sevoflurane, not by isoflurane 12. It will be important to delineate the underlying mechanism of this selectivity to further understand the interaction of volatile anesthetics with proteins, which may help to decipher the yet-to-be determined mechanism of anesthesia.
In our THP-1 reporter assay, both propofol and dexmedetomidine at 100 μM attenuated Pam3CSK4-mediated NFκB activation. However, we did not find that clinically used propofol attenuated NFκB activation even at 100 μM. Clinically used propofol contains lipid emulsion (intralipid). Because THP-1 cells express lipase, fatty acids can be produced from intralipid by their lipase activity. Saturated fatty acids are known ligands for TLR2 and TLR4. Thus, propofol dissolved in intralipid may have very different effects on TLRs under different in vitro conditions and in vivo.
In summary, we have shown that sevoflurane reduced TLR1/TLR2 activation and attenuated neutrophil shedding associated with it. However, the implication of our in vitro findings needs further validation in clinical settings.
Supplementary Material
Key points.
Question:
What is the effect of anesthetics on Toll-like receptor 2, an important receptor to recognize invading pathogens and endogenous danger signals?
Findings:
Sevoflurane bound to Toll-like receptor 2, attenuated its activation, and mitigated neutrophil L-selectin shedding.
Meaning:
Toll-like receptor 2 is one of sevoflurane targets for immunomodulation.
Acknowledgement
We thank Dr. Hasan Babazada (University of Pennsylvania) for the technical support.
Financial Support: This work was in part supported by CHMC Anesthesia Foundation (K.Y., K.O.), NIH R01GM118277 (K.Y.)
Glossary of Terms
- PRR
pattern recognition receptor
- TLR
Toll-like receptor
- S. aureus
Staphylococcus aureus
- LRR
leucine-rich repeat
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells
- SEAP
embryonic alkaline phosphate
- LTA
lipoteichoic acid
- APC
allophycocyanin
- fMLP
N-formylmethionine-leucyl-phenylalanine
- ROS
reactive oxygen species
- HMBG1
high-mobility group box 1
- HSP
heat shock protein
- DD
death domain
- FADD
Fas-associated DD
Footnotes
Conflict of Interest: None
Contributor Information
Yusuke Mitsui, Postdoctoral research fellow of Anaesthesia, Harvard Medical School; Department of Anesthesiology, Critical Care and Pain Medicine, Cardiac Anesthesia Division, Boston Children’s Hospital; Department of Anesthesiology and Intensive Care Medicine, Tokyo Medical and Dental University
Lifei Hou, Instructor of Anaesthesia, Harvard Medical School; Department of Anesthesiology, Critical Care and Pain Medicine, Cardiac Anesthesia Division, Boston Children’s Hospital
Xiayi Huang, Research Assistant, Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children’s Hospital
Kirsten C. Odegard, Associate Professor of Anaesthesia, Harvard Medical School; Department of Anesthesiology, Critical Care and Pain Medicine, Cardiac Anesthesia Division, Boston Children’s Hospital
Luis M. Pereira, Instructor of Anaesthesia, Harvard Medical School; Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children’s Hospital
Koichi Yuki, Associate Professor of Anaesthesia, Harvard Medical School; Department of Anesthesiology, Critical Care and Pain Medicine, Cardiac Anesthesia Division, Boston Children’s Hospital
References
- 1.Leulier F & Lemaitre B Toll-like receptors--taking an evolutionary approach. Nat Rev Genet 9, 165–178, doi: 10.1038/nrg2303 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Hayashi F, Means TK & Luster AD Toll-like receptors stimulate human neutrophil function. Blood 102, 2660–2669, doi: 10.1182/blood-2003-04-1078 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Oliveira-Nascimento L, Massari P & Wetzler LM The Role of TLR2 in Infection and Immunity. Front Immunol 3, 79, doi: 10.3389/fimmu.2012.00079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Hoshino K & Akira S Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165, 5392–5396, doi: 10.4049/jimmunol.165.10.5392 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Yim JJ et al. The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun 7, 150–155, doi: 10.1038/sj.gene.6364274 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Romics L Jr., Szabo G, Coffey JC, Wang JH & Redmond HP The emerging role of toll-like receptor pathways in surgical diseases. Arch Surg 141, 595–601, doi: 10.1001/archsurg.141.6.595 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Stollings LM et al. Immune Modulation by Volatile Anesthetics. Anesthesiology 125, 399–411, doi: 10.1097/ALN.0000000000001195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbo C, Yuki K, Demers M, Wagner DD & Shimaoka M Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J Anesth 27, 261–268, doi: 10.1007/s00540-012-1508-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuki K, Bu W, Xi J, Shimaoka M & Eckenhoff R Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth Analg 117, 803–811, doi: 10.1213/ANE.0b013e3182a00ae0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutsogiannaki S et al. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol Sci 156, 402–411, doi: 10.1093/toxsci/kfw261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung S & Yuki K Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen. J Immunotoxicol 13, 148–156, doi: 10.3109/1547691X.2015.1019596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutsogiannaki S et al. The volatile anesthetic sevoflurane reduces neutrophil apoptosis via Fas death domain-Fas-associated death domain interaction. FASEB J, fj201901360R, doi: 10.1096/fj.201901360R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JR, Yuki K, Baek C, Han XH & Soriano SG Dexmedetomidine-Induced Neuroapoptosis Is Dependent on Its Cumulative Dose. Anesth Analg 123, 1008–1017, doi: 10.1213/ANE.0000000000001527 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Tazawa K, Koutsogiannaki S, Chamberlain M & Yuki K The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett 266, 23–31, doi: 10.1016/j.toxlet.2016.12.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuno T, Koutsogiannaki S, Hou L, Bu W, Mitsui Y, Ohto U, Eckenhoff RG, Yokomizo T, Yuki K Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system. FASEB J (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botos I, Segal DM & Davies DR The structural biology of Toll-like receptors. Structure 19, 447–459, doi: 10.1016/j.str.2011.02.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandyopadhaya A, Tsurumi A & Rahme LG NF-kappaBp50 and HDAC1 Interaction Is Implicated in the Host Tolerance to Infection Mediated by the Bacterial Quorum Sensing Signal 2-Aminoacetophenone. Front Microbiol 8, 1211, doi: 10.3389/fmicb.2017.01211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuki K et al. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J 26, 4408–4417, doi: 10.1096/fj.12-212746 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuki K et al. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J 22, 4109–4116, doi: 10.1096/fj.08-113324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bu W, Pereira LM, Eckenhoff RG & Yuki K Stereoselectivity of isoflurane in adhesion molecule leukocyte function-associated antigen-1. PLoS One 9, e96649, doi: 10.1371/journal.pone.0096649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin MS et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082, doi: 10.1016/j.cell.2007.09.008 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Koutsogiannaki S, Bernier R, Tazawa K & Yuki K Volatile Anesthetic Attenuates Phagocyte Function and Worsens Bacterial Loads in Wounds. J Surg Res 233, 323–330, doi: 10.1016/j.jss.2018.07.075 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weerink MAS et al. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet 56, 893–913, doi: 10.1007/s40262-017-0507-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita Y et al. Relationship between dexmedetomidine dose and plasma dexmedetomidine concentration in critically ill infants: a prospective observational cohort study. Korean J Anesthesiol 70, 426–433, doi: 10.4097/kjae.2017.70.4.426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishikawa H et al. The effects of dexmedetomidine on human neutrophil apoptosis. Biomed Res 29, 189–194 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Albanese J et al. Pharmacokinetics of long-term propofol infusion used for sedation in ICU patients. Anesthesiology 73, 214–217, doi: 10.1097/00000542-199008000-00004 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Short TG et al. A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br J Anaesth 72, 302–306, doi: 10.1093/bja/72.3.302 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Gepts E, Camu F, Cockshott ID & Douglas EJ Disposition of propofol administered as constant rate intravenous infusions in humans. Anesth Analg 66, 1256–1263 (1987). [PubMed] [Google Scholar]
- 29.Meier A & Nizet V Impact of Anesthetics on Human Neutrophil Function. Anesth Analg 128, 569–574, doi: 10.1213/ANE.0000000000003927 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Ivetic A A head-to-tail view of L-selectin and its impact on neutrophil behaviour. Cell Tissue Res 371, 437–453, doi: 10.1007/s00441-017-2774-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smalley DM & Ley K L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med 9, 255–266, doi: 10.1111/j.1582-4934.2005.tb00354.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzeniewicz K et al. L-selectin shedding is activated specifically within transmigrating pseudopods of monocytes to regulate cell polarity in vitro. Proc Natl Acad Sci U S A 112, E1461–1470, doi: 10.1073/pnas.1417100112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschon JJ et al. An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284, doi: 10.1126/science.282.5392.1281 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Yang, Han Z & Oppenheim JJ Alarmins and immunity. Immunol Rev 280, 41–56, doi: 10.1111/imr.12577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zha H et al. Volatile anesthetics affect macrophage phagocytosis. PLoS One 14, e0216163, doi: 10.1371/journal.pone.0216163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JR, Han X, Soriano SG & Yuki K The role of macrophage 1 antigen in polymicrobial sepsis. Shock 42, 532–539, doi: 10.1097/SHK.0000000000000250 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Park JS et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279, 7370–7377, doi: 10.1074/jbc.M306793200 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Yu M et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179, doi: 10.1097/01.shk.0000225404.51320.82 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Shin HS et al. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol 186, 1119–1130, doi: 10.4049/jimmunol.1001647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins A et al. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci 21, RC136 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


