Abstract
Cross-sectional airway area is the main determinant of resistance to airflow in the respiratory system. In paediatric patients (<18 yr), previous evidence for sex-differences in cross-sectional airway area was limited to patients with history of pulmonary disease or cadaveric studies with small numbers of subjects. These studies either only report tracheal data and do not include a range of ages or correct for height. Therefore, we sought to assess sex-differences in airway luminal area utilizing paediatric patients of varying ages and no history of respiratory disease. Using three-dimensional reconstructions from high-resolution computed tomography scans, we retrospectively assessed the cross-sectional airway area in healthy paediatric females (n=97) and males (n=128) over a range of ages (1–17 yr). The areas of the trachea, left main bronchus, left upper lobe, left lower lobe, right main bronchus, intermediate bronchus, and right upper lobe were measured at three discrete points by a blinded investigator. No differences between the sexes were noted in the cross-sectional areas of the youngest (ages 1–12 yr.) patients (P>0.05). However, in patients greater than age ≥14 yr the cross-sectional areas were larger in the males compared to females in most airway sites. For instance, the cross-sectional size of the trachea was 25% (218± 44 vs. 163±24 mm2, P<0.01) larger in males vs. females among ages 13–17 yr. When accounting for height, these sex-differences in airway areas were attenuated, but persisted. Our results indicate that sex-differences in paediatric airway cross-sectional area manifest after age ≥14 yr and are independent of height.
Keywords: dysanapsis, exercise, airway resistance
Introduction
Respiration is a highly regulated and coordinated physiological process that matches alveolar ventilation with the metabolic demands of the body while ensuring minimal respiratory muscle work. Broadly, respiratory muscle work can be divided into viscoelastic and flow resistive work (Otis, 1954). Airflow is governed by fluid dynamic laws (e.g. Hagen-Poiseuille equation) whereby airway diameter markedly affects airway resistance (Kaminsky, 2012). Previous studies have indicated that adult females have smaller airways than adult males (Becklake & Kauffmann, 1999). However, these previous works have been limited by the assessment of older patients with significant smoking history (Sheel et al., 2009), indirect measurements of the airway (Mead, 1980; Dominelli et al., 2015a) or only tracheal area reports (Griscom & Wohl, 1986; Martin et al., 1987). Recently, we utilized computed tomography (CT) imaging in healthy adults across a range of ages (19–86 yr) to show that central airways in females were significantly smaller (~26%–35%) compared to males (Dominelli et al., 2018). Importantly, the sex-difference was preserved, albeit attenuated, in a subset of patients matched for height (Dominelli et al., 2018).
In paediatric age groups, the existing literature on sex-differences in large cross-sectional airway area is limited to indirect measurements, cadaveric studies involving a small numbers of subjects, only reporting tracheal data, a narrow range of paediatric ages, or the potential that clinical manifestations impacted the results (Butz, 1968; Thurlbeck & Haines, 1975; Wailoo & Emery, 1982; Kuo et al., 2018). The basis for these sex-differences in respiratory function is thought to be primarily from structural differences and hormonal influence (Harms et al., 2011). Puberty is a critical period during which anatomical and physiological changes occur due to hormonal surges and can significantly influence health throughout the lifetime.
The importance of airway size is numerous and has a significant impact in the integrative responses to exercise. For example, healthy adult females have been shown to have more pulmonary limitations during dynamic exercise than males and these differences are believed to be due, at least in part, to smaller airway size (Dominelli et al., 2019). Determining when this relationship is present in children is important given their high prevalence of ventilatory constraints during exercise. One example of a ventilatory constrain is expiratory flow limitation, which is the inability to generate greater expiratory flow despite increasing effort. In prepubertal children the prevalence of expiratory flow limitation during exercise is high (>90%) and independent of the aerobic capacity or sex (Swain et al., 2010). Both trained and untrained prepubescent children experience expiratory flow limitation due to both excessive minute ventilation relative to the metabolic demands during exercise and smaller airways relative to lung size (Nourry et al., 2005, 2006). If paediatric females have smaller airways than similarly aged males, it could lead to even greater ventilatory constraints during exercise.
Overall, the aim of this study was to assess potential sex-differences in the cross-sectional area of large airways in paediatric patients with no history of any potential respiratory comorbidities. Based on the adult literature and the impact of hormonal variations, we hypothesized that sex-differences in the cross-sectional airway area would exist in only post-pubertal patients.
Methods
Ethical Approval.
This retrospective study was approved by the Institutional Review Board at the Mayo Clinic (IRB #17–008537) and conformed to the standards of the Declaration of Helsinki, except registration in a database. All images were originally collected as part of routine clinical care. Informed consent was waived as no identifiers were used, the data already existed, the research did not affect patient care and the patients’ parent/legal gaurdian did not opt out of their data being used for research. This consent waiver was approved by the Mayo Clinic Institutional Review Board.
Patients.
Paediatric patients (<18 yr) who underwent a chest computed tomography (CT) scan in the emergency department as part of the trauma protocol between 03/13/2009 – 03/13/2019 were retrospectively included in the study. Only trauma not impacting the: trachea, lung, and heart (according to the radiologist report) were included. A total of n=1141 patients met the initial study criterion and a manual chart review was conducted by a physician for the exclusion criteria. Patients were excluded if they had a history or presence of any of the following: documented history of congenital heart/lung disease, respiratory conditions (e.g. cystic fibrosis, recent/ongoing infection, cancer, pulmonary nodules, asthma, interstitial lung diseases), rheumatologic disorders (e.g. systemic lupus erythematosus, limited scleroderma, systemic sclerosis, sarcoidosis, vasculitis), technical difficulties (e.g. suboptimal inspiration per radiology report, respiratory motion artefacts), tracheomalacia, diaphragmatic hernia, pulmonary hypertension of the newborn, history of tracheoesophageal fistula, prior thoracotomy or lung resection, intubation, chronic steroid therapy, rib cage abnormalities or deformities (e.g. pectus excavatum, pectus carinatum, displaced fractures), documented evidence of trauma to the chest, history of bronchopulmonary dysplasia, paediatric obstructive sleep apnoea, body mass index > 40 kg·m−2, and any smoking history. The exclusion resulted in the removal of n=881. Among the remaining n=260 eligible patients, n=32 non-trauma CT-scans (n=24 due to medical conditions, n=2 foreign objects, n=6 oncology work-up for metastases) were excluded (Figure 1). A total of n=228 images (n=97 females) were analysed for cross-sectional airway area. During the data analysis a further 3 patients (n=3 males) were excluded due to duplicate data (n=2) or poor image quality (n=1). A total of n=225 CT-scans were analysed (n=128 males and n=97 females). Forty patients were removed from height-adjusted comparisons due to missing height and/or weight data.
Figure 1.
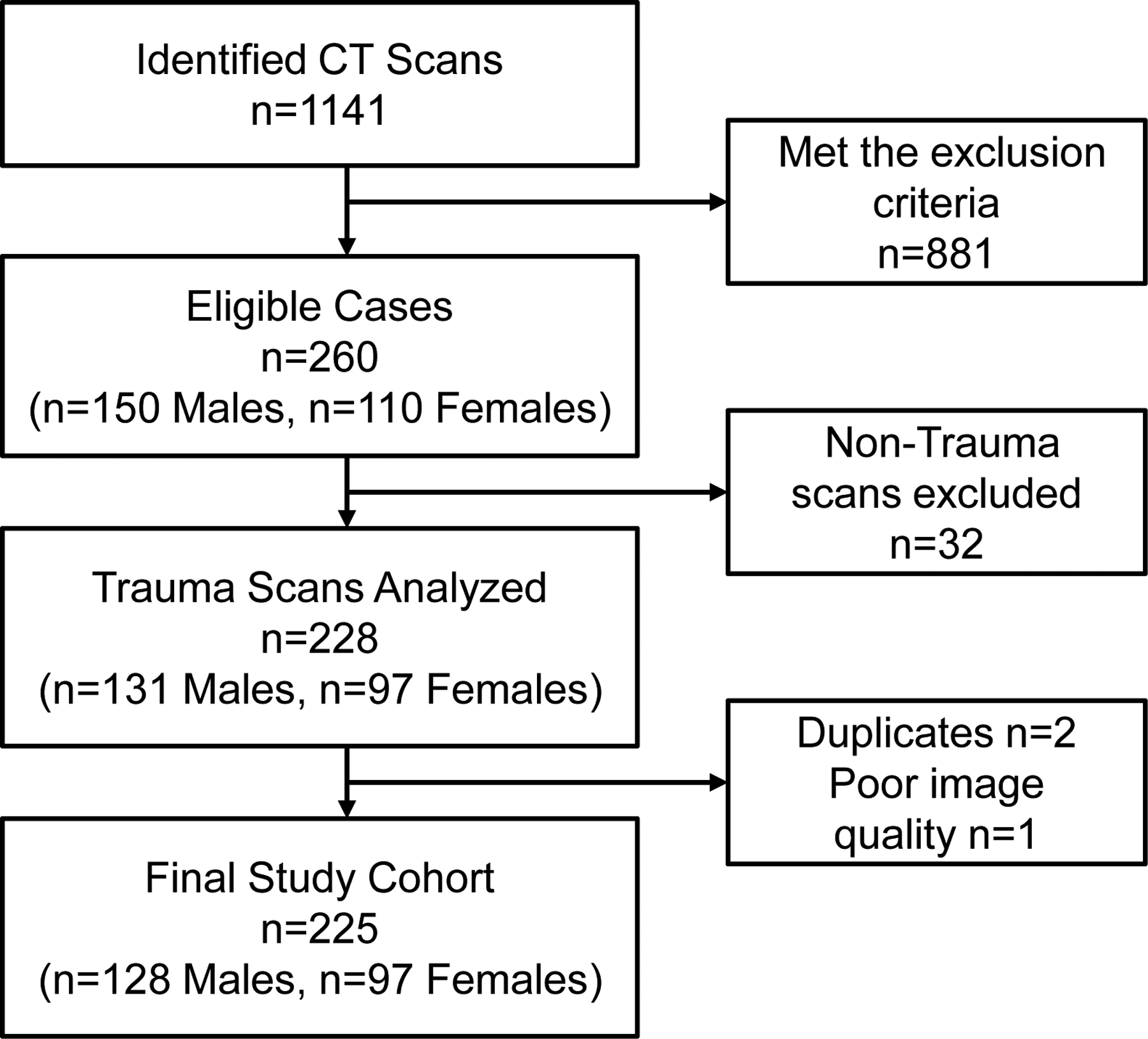
Flow of patients for the study.
Image acquisition.
The institution utilizes standardized weight-based CT algorithms for paediatric thoracic imaging in accordance with Image Gently principles (Don et al., 2013). A posterior-anterior topogram is obtained at 80 kV and 20 mA. Spiral acquisitions with a pitch of 1.4 are utilized. Kilovoltage is set at 120 with a standard milliampere-second value of 70. Images are acquired at end inspiration. Post imaging reconstructions are obtained in the axial and coronal plane using a B46 kernel. Slice thicknesses of 1.5 mm and 3 mm are reconstructed. Maximal intensity projections in the axial and coronal planes are completed with a slice thickness of 16 mm and reconstruction increment of 8 mm. Before the CT-scan, patients were asked to take a large inspiration and to hold their breath. Lung volumes were determined by image analysis (Dominelli et al., 2018).
Data acquisition and analysis
Data analysis was performed with TeraRecon (AQI, Foster City, CA), a commercially available software that allows three-dimensional reconstruction of the airway following isolation of the surrounding tissues. The airway area measurements were conducted at three points (corresponding to the beginning, middle and end of the airway) for each of the following structures: trachea, left main bronchus, left upper lobe, left lower lobe, right main bronchus, intermediate bronchus, and right upper lobe. A single investigator analysed the data and they were blinded to the sex of the patients. The beginning and the end of the airway were established based on the anatomic bifurcations. The cross-sectional area for each airway was calculated based on the averages of the three measures obtained for each anatomic airway structure. The total lung volume as well as the lengths of the trachea, left main bronchus, right main bronchus, and intermediate bronchus were also measured in every patient.
Statistical analysis.
Baseline demographics and clinical characteristics were presented as mean and standard deviation. Student’s unpaired t-test were used to compare descriptive variables. To test for differences in airway sizes as a function of sex, age and height, a linear model was fit to the data. The adjustment for height was accomplished by creating an airway index, which was the luminal area divided by the patient’s height in meters, as the dependent variable. The functional form of age was allowed to follow a third degree polynomial to allow for, if present in the data, non-linear associations of airway size by age. An (polynomial) age by sex interaction term was included in the model. Using the fitted model, model-based means (“expected values”) were generated and age-specific contrasts were constructed to test for differences by sex and age combinations. General residual analysis was conducted to examine model fit and to assess the degree to which the polynomial age fit was appropriate. Scatter plots and fitted curves with confidence bands on the expected value are presented to summarize the sex trends by age group. Significance was set at P<0.05 and values are presented as mean±SD. No multiple correction was applied to the age-sex specific contrasts.
Results
Patient descriptors.
Baseline demographics for the entire cohort are presented in Table 1. For the entire cohort, males were significantly taller than females, with no differences in body mass index, weight, lung volume and predicted total lung capacity. Demographics for the whole cohort and split into groups based on age are presented in Table 1. Across the age groups, the height and weight were similar between sexes until latter ages (13+ yr) when males were significantly taller and weighed more than females.
Table 1.
Patient demographics distributed by age categories
| All patients | < 6 yrs | 6–9 yrs | 10–12 yrs | 13–15 yrs | 16–17 yrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | F | F | F | F | F | ||||||
| N | 105 | 80 | 13 | 11 | 12 | 22 | 39 | |||||
| Age (yr) | 12.9±4.3 | 12.6±4.7 | 3.5±1.4 | 7.7±1.0 | 11.2±0.8 | 14.2±0.9 | 16.5±0.5 | |||||
| Height (cm) | 158±26 (80–198) | 150±23* (91–178) | 104±9 (91–117) | 131±11 (115–145) | 148±8 (137–163) | 161±10 (145–178) | 164±7* (145–175) | |||||
| Weight (kg) | 58±25 (11–149) | 53±21 (12–110) | 16±2 (12–20) | 35±13 (23–61) | 46±10 (35–61) | 63±17 (45–110) | 66±11* (50–100) | |||||
| BMI (kg m−2) | 22±5 (14–49) | 22±6 (12–47) | 15±0* (12–17) | 21±10 (15–47) | 21±5 (15–28) | 21±6 (17–37) | 24±4 (18–33) | |||||
| Lung volume (L) | 3.1±1.4 (0.7–6.3) | 2.3±1.0 (0.4–4.1) | 0.5±0.1* (0.4–0.7) | 1.5±0.8 (0.8–2.6) | 2.0±0.8 (1.0–3.4) | 2.4±0.7* (1.3–4.1) | 2.9±0.6* (1.6–4.1) | |||||
| Lung vol on CT (% pred TLC) | 60±18 (22–117) | 57±20 (18–120) | 29±8 (18–38) | 55±21 (25–85) | 58±30 (27–120) | 53±14* (32–74) | 63±16 (35–100) | |||||
Values are mean± SD with range in parenthesis. BMI, body mass index; Lung vol on CT, predicted total lung capacity during the computed tomography scan. TLC, total lung capacity.
Significantly different from males, P<0.05.
Absolute airway size.
Absolute airway size is presented in Figures 2 and 3. There were no sex-differences in any cross-sectional area in the patients that were less than 13 yr. At 13 yr, trachea and right main bronchus were significantly larger in females (Figure 2). However, for the older patients (≥14 yr) each airway (except bronchus intermedius) cross-sectional area was significantly larger in males. Bronchus intermedius was significantly different at age 15 years. The sex-differences were most prominent in the largest airways. Specifically, the trachea had the largest differences in the cross-sectional airway area between older males and females (ages 13–17), whereas the smallest difference was noted in the bronchus intermedius. On average, the cross-sectional airway size of the trachea was 25% (218± 44 vs. 163±24 mm2, P<0.01) larger in males compared to females among older patients (ages 13–17). The difference in the cross-sectional area of the left main bronchus, left upper lobe, and left lower lobe between sexes for age groups 13–17 yr were 24% (110±29 vs. 83±15 mm2, P<0.01), 20% (71±19 vs. 57±15 mm2, P<0.01), and 22% (63±15 vs. 49±10 mm2, P<0.01), for males and females and airway respectively. On average the cross-sectional airway size of the right main bronchus, bronchus intermedius, and right upper lobe were 20% (154±34 vs. 123±20 mm2, P<0.01), 18% (89±20 vs. 73±12 mm2, P<0.01), and 20% (59±15 vs. 47±11 mm2, P<0.01) larger in males compared to females for age groups 14–17 yr, respectively.
Figure 2.
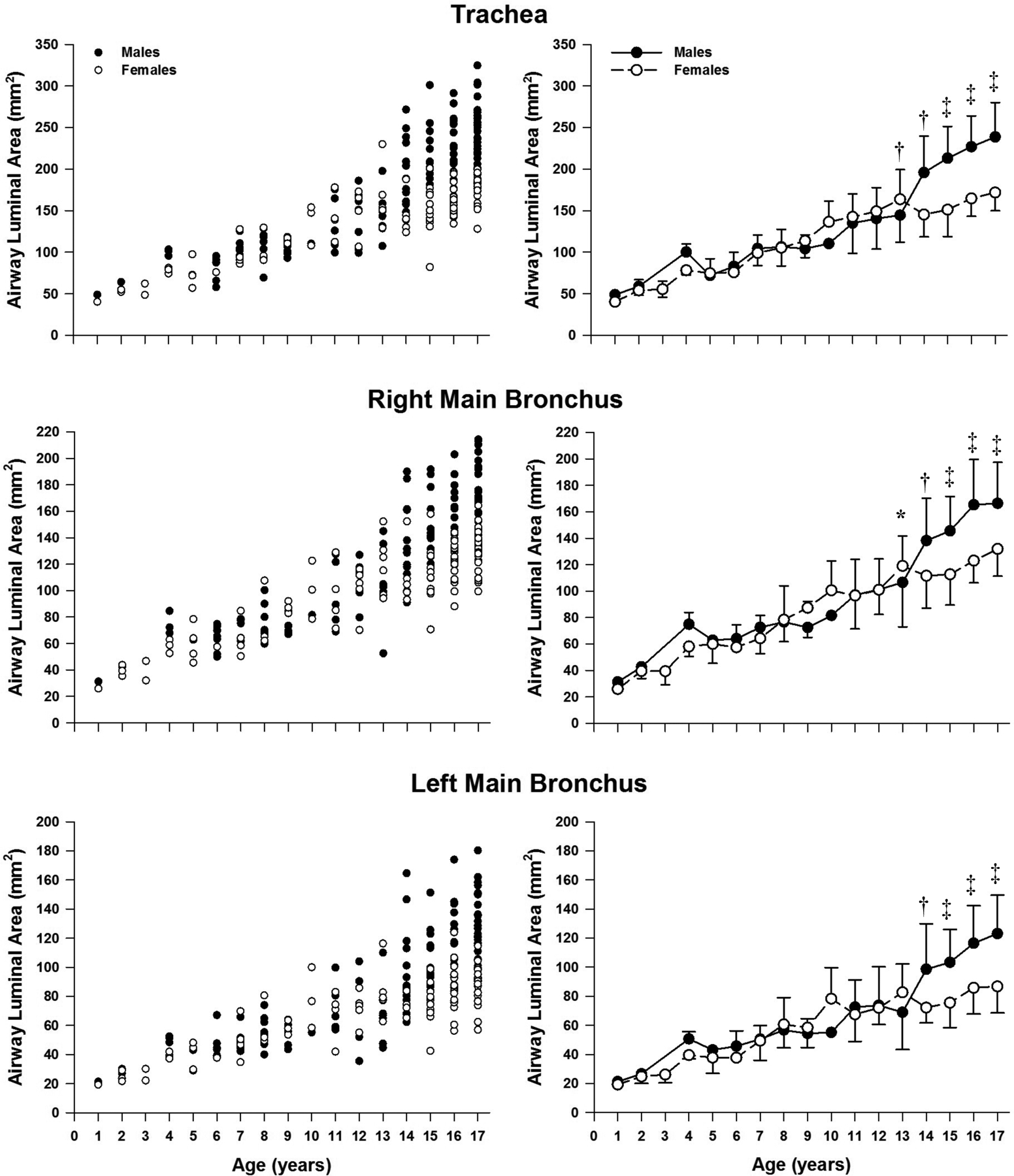
Absolute airway size for the trachea along with right and left main bronchus. Panels on the left side are individual values whereas the right side are mean ±SD. Significantly different between sexes: *, P<0.05; †, P<0.01; ‡, P<0.0001.
Figure 3.

Absolute airway size for the right and left upper lobes along with bronchus intermedius and left lower lobe. Panels on the left side are individual values whereas the right side are mean ±SD. Significantly different between sexes: *, P<0.05; †, P<0.01; ‡, P<0.0001.
Airways size - height adjusted analysis.
The height-adjusted airway size data is presented in Figures 4 and 5. When controlling for height, we found no sex-differences among patients <14 yr in the airway cross-sectional area of the trachea and first airway generation (right and left main bronchus) (Figure 4). Similarly, in the second airway generation (right upper lobe, bronchus intermedius, and left lower lobe) the cross-sectional airway size was not different between sexes in paediatric patients < 15 yr, with the exception of the left upper lobe that followed a similar growth pattern than the trachea and first generation (<14 yr) (Figure 5). In the older patients (>14 or 15 yr), males had significantly larger airways area for all measured airways. Consistent with the absolute airway size results, the trachea persisted with the largest differences in cross-sectional airway area between older males and females (>14 yr), whereas the smallest sex-difference remained in the bronchus intermedius.
Figure 4.
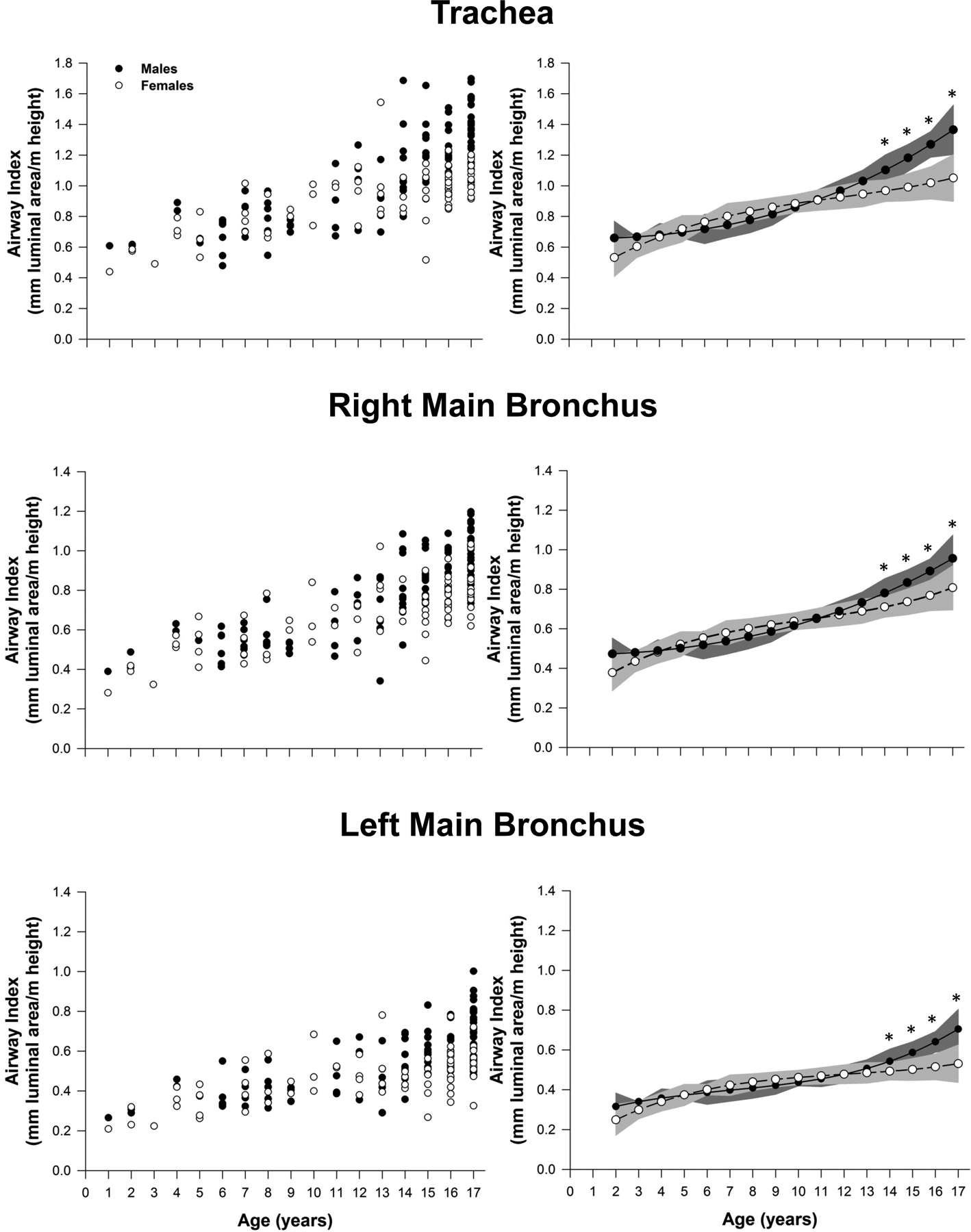
Airway size adjusted for height for the trachea along with right and left main bronchus. Leftmost panels represent the raw values for each patient. Panels on the right are model based means based on a polynomic model fit with two degrees. The shaded area on the right panels represents the confidence bands for the estimated means. Significantly different between sexes: *, P<0.05.
Figure 5.
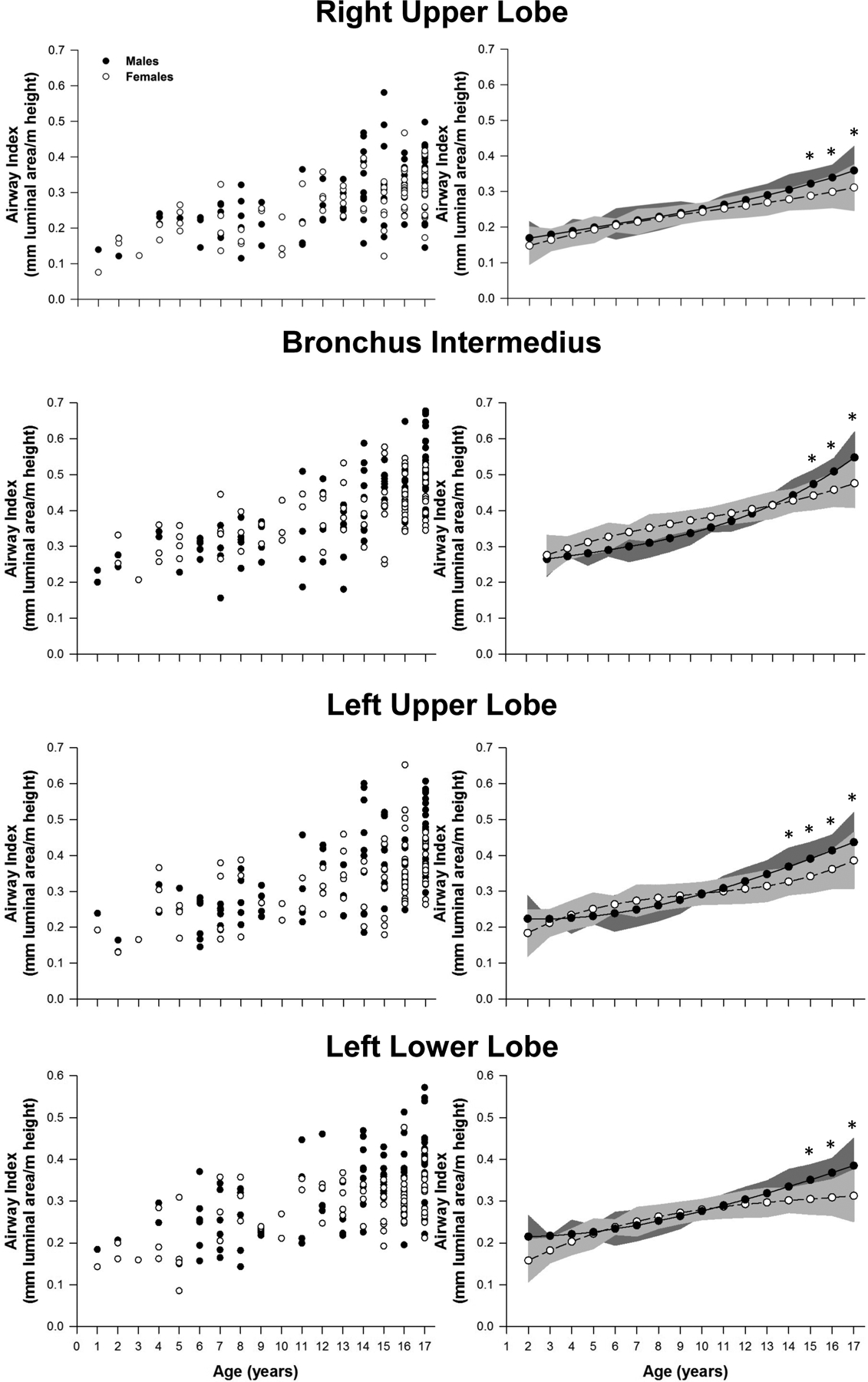
Airway size adjusted for height for the right and left upper lobes along with bronchus intermedius and left lower lobe. Leftmost panels represent the raw values for each patient. Panels on the right are model based means based on a polynomic model fit with two degrees. The shaded area on the right panels represents the confidence bands for the estimated means: *, P<0.05.
Discussion
Major findings.
The major findings from our study are two-fold. First, sex-differences in absolute airway size in a healthy paediatric cohort arise after accelerated growth in ages 14 and greater, which is associated with the typical time of puberty (Krieger et al., 2015). We interpret this to indicate that the sex-differences in airway size are not innate but rather associated with hormonal changes from puberty. Second, the sex-differences in airway area were attenuated, but persisted when controlling for height. Therefore, hormonal variation from biological sex has an independent impact on absolute airway area. Overall, we demonstrate that sex-differences in airway area are likely the result of hormonal changes around puberty and are independent of height. The effect of sex on airway growth is likely an important consideration for explaining differences in pulmonary system limitations to exercise as well as the prevalence and severity of lung disease in paediatric patients.
Sex-differences in airway anatomy.
We found that in a cohort of healthy paediatric patients of varying ages (1–17 yr), the large conducting airways were larger in males compared to females after age 14, which correlates with the average time of puberty (Krieger et al., 2015; Brix et al., 2019). Thus, the sex-differences in airway size are likely driven by the hormonal changes associated by puberty and are not innate genetic sex-differences. It is important to note that there was a considerable overlap in the average cross-sectional airway size between sexes despite males, on average, having significantly larger airways than females after puberty. Many females exhibited larger cross-sectional airway areas than males for a similar height and age (Figures 2–5). The variability in this data is consistent with that observed in indirect measurements of the airway size (Mead, 1980; Dominelli et al., 2015a). Part of the variability is most likely due to the differences in onset and peak hormonal effects of puberty, both within and between the sexes. Moreover, the variability in sex-airway anatomy following post-puberty is also consistent with our previous work that showed a similar degree of overlap in the cross-sectional airway sizes of the large conducting airway anatomy in a cohort of healthy adults of varying ages (Dominelli et al., 2018). The variability in airway size further confirms the concept of ‘dysanapsis’ (unequal airway and lung size) which is thought to explain the variability in pulmonary function between individuals of similar demographics (Green et al., 1974). It has also been noted that young males (13–18 years) have a similar dysanapsis ratio as adult females, both of which are different than adult males (Mead, 1980). That young males have relatively smaller airways or more dysanapsis further supports our finding that sex-difference in airway size develop later during pubertal growth.
When we analysed the cross-sectional airway size and adjusted for height, we found that the sex-differences in the cross-sectional airway area were attenuated, but persisted (Figure 4, 5). Given height-adjusted males had larger airways than females reveal a sex-specific effect, rather than the relationship being exclusively determined by body size. The diminished cross-sectional area would lead to increased airflow resistance and higher tendency to turbulent flow in adolescent females compared to males of similar size and age. The larger airways in anthropometrically-adjusted males are important if we take into consideration the close correlation between body size and pulmonary physiology (Becklake & Kauffmann, 1999; Dominelli et al., 2018). Prepubescent males and females of similar size would have approximately the same metabolic demand and maximal ventilation during exercise, coupled with similar airway morphology, they would be expected to have similar mechanical ventilatory constraints. However, the smaller airways in post-pubescent (but not adult) females would lead to a higher relative and absolute work of breathing that reduces exercise performance. Further research into this hypothesis is needed with patients matched for height. In adults, a reduced cross-sectional airway area in healthy females (19–55 yr) results in a greater resistance and thus increased total work of breathing (Dominelli et al., 2015a) and greater oxygen consumption of the respiratory muscles (Dominelli et al., 2015b). The increased respiratory muscle work has been shown to influence the blood flow to the respiratory (Dominelli et al., 2017) and locomotor muscles (Harms et al., 1997). Since in prepubescent children, no difference were noted in the cross-sectional airway size we would hypothesize that young prepubescent children would have no difference in ventilatory constraints. This is consistent with a prior study showing approximately 90% of prepubertal children undergoing maximal exercise experienced expiratory flow limitation, where the severity of ventilatory constraint was independent of sex (Swain et al., 2010).
While our study is the first to our knowledge to assess sex differences in airway size of a healthy paediatric population, other groups have measured airway size in paediatric pulmonary patients. Recently, a multicentre group demonstrated that up to the age of 14 yr, the diameter of the trachea and right & left main bronchi are not different between males and females (Kuo et al., 2018). Similar to our study, after 14 yrs, the males had significantly larger airway diameters. While the results from Kuo et al., are very similar to ours, their population was potentially confounded by comorbidities as the scan were performed for clinical indications that could have impacted the airways (Kuo et al., 2018). They were also unable to account for differences in height, which will influence lung and airway size. Nonetheless, their overall conclusion of sex-differences in airways after the age of 14 agrees with ours. Another prior study validated a video-bronchoscope technique to assess in-vivo airway cross-sectional areas of large conducting airway anatomy in 125 children (≤10 yr) with a history of chronic cough and concern for airway malacia disorders (Masters et al., 2006). The authors suggested that the large airways maintain constant proportional relationships to the cricoid, progressively increase in cross-sectional area size, and were sex independent across childhood (2–10 yr) (Masters et al., 2006). Our results are consistent with this study. While there are no sex-differences in the airway size among prepubescent patients (<13 yr), there is a consistent increase in the luminal area of large conducting airways across childhood (1–13 yr). In post-pubescent individuals, the airway size in older girls (ages 16–17 yr) is similar to the luminal airway size for adult females reported in our prior studies in adult subjects (Sheel et al., 2009; Dominelli et al., 2018). However, a larger difference in airway size is noted between older boys (16–17) and adult males. This is consistent with the idea that females generally go through puberty before males, such that lung development continues in males but not females as they reach adulthood. The continued lung development in males also suggests that height becomes less of an important covariate as the subjects become older. Specifically, it would appear that height increases relatively less than that of lung or airway size in the older subjects and this is more pronounced in males. As such, future work that has direct measures of lung volumes should also correct for lung size, as has been done previously in adults (Sheel et al., 2009).
Clinical and applied consequences.
During the prepubescent period, cystic fibrosis equally affects males and females (Harness-Brumley et al., 2014; Raghavan & Jain, 2016). However, current evidence suggests that there is an increased severity and rate of respiratory exacerbations in post-pubescent females compared to males. A smaller cross-sectional airway diameter observed in post-pubertal females compared to males may add to the differences observed in the rate and severity of cystic fibrosis complications. Asthma is another example of an inflammatory disease of the airways with potential sex-based differences that seem to differ across development (Vink et al., 2010; Townsend et al., 2012). It is possible that our observed sex-difference in airways size could contribute to this finding in asthmatics.
Recently it was shown that there were sex-differences in elite swimming performance in youth (Senefeld et al., 2019). In it, the authors demonstrate that prepubescent females have faster or similar swimming times compared to prepubescent males. However, after puberty the sex-difference in performance reverses and males become faster than females. Although airway size is certainly not the primary limitation to exercise, it is noteworthy that the sexes had similar divergence in both airway size and excise performance around age 14.
Methodological consideration.
Our study has methodological considerations that warrant discussion. First, the distribution of our study population was uneven across the individual age groups studied, particularly for the younger children (<6 yr). Nevertheless, our primary research hypothesis was if sex-differences existed in airway size and we had sufficient patients to perform these comparisons. In addition, the main sex-differences in airway size were noted in older children (14–15 yr), thus our main results would be minimally impacted by this limitation. Second, pulmonary function (via plethysmography or spirometry) was not available for our cohort. Thus, we are not able to determine if our sex-difference in airway size is a function of differences in lung size. Third, the end-inspiratory lung volume was not standardized to total lung capacity due to the emergency nature of the CT-scans. Older (≥6 yr) children were instructed to inspire and hold their breath. In younger children (<6 yr), the radiology technician always attempted to scan the inspiratory phase of the respiratory cycle. It should be noted however that there were no differences in the relative lung volume between sexes and it is unlikely that this random effect disproportionally impacted one sex more than the other. It has also been previously demonstrated that the influence of the respiratory cycle (inspiration and expiration) in the assessment of the cross-sectional airway area is more prominent in the more distal airways compared to the more proximal airways (Kambara et al., 2014). As a result, the sex-differences comparisons in the proximal airways size in our study cohort would likely remain unaffected by this limitation. Fourth, a single blinded investigator analysed our data and this was done to strengthen consistency. However, this approach introduces potential bias that would be minimized using several investigators. Finally, we were unable to determine maturation stages (i.e. puberty) in our cohort. As such, we had to rely on normative data on average age of puberty and relate that to when sex-differences in airway cross-sectional area occurred.
Conclusion.
We have investigated potential sex-differences in airway cross-sectional area of healthy paediatric patients (<18 yr) with no history of respiratory comorbidities. We found no sex-differences in the cross-sectional airway area of the youngest (<13 yr) patients, but a significant effect of sex on the older (≥14 yr) group. Importantly, these sex-differences persisted after the height-adjusted analysis, with males still having larger airways. Our findings help to understand the interrelation between the airway anatomy and the sex-airway differences in respiratory physiology.
NEW FINDINGS.
-
What is the central question of this study?
Are sex-difference in the central airways present in healthy paediatric patients.
-
What is the main finding and its importance?
In patients ≤12 years we found no sex-differences in central airway luminal area. After 14 years, the males had significantly larger central airway luminal areas than the females. The sex-differences were minimized, but preserved when correcting for height. Luminal area is the main determinant of airway resistance and our finding could help explain sex-differences in pulmonary system limitations to exercise in paediatric patients.
Funding information:
National Institutes of Health Grants R35-HL139854 (MJJ). Natural Sciences and Engineering Research Council of Canada RGPIN-2019–04615 (PBD)
Abbreviations
- CT
computed tomography
Footnotes
Conflict of Interest: All authors have no conflicts to report
References
- Becklake MR & Kauffmann F (1999). Gender differences in airway behaviour over the human life span. Thorax 54, 1119–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Parner E, Støvring H, Olsen J, Henriksen TB & Ramlau-Hansen CH (2019). Timing of puberty in boys and girls: A population-based study. Ped Peri Epi 33, 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz RO (1968). Length and cross-section growth patterns in the human trachea. Pediatrics 42, 336 LP – 341. [PubMed] [Google Scholar]
- Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R & Sheel AW (2017). Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102, 1535–1547. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Cross TJ, Ripoll JG, Joyner MJ, Welch BT, Baker SE & Wiggins CC (2018). Sex differences in large conducting airway anatomy. J Appl Physiol 125, 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Molgat-Seon Y, Bingham D, Swartz PM, Road JD, Foster GE & Sheel AW (2015a). Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol 119, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Molgat-Seon Y & Sheel AW (2019). Sex Differences in the Pulmonary System Influence the Integrative Response to Exercise. Exerc Sport Sci Rev 47, 142–150. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM & Sheel AW (2015b). Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593, 1965–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don S, MacDougall R, Strauss K, Moore QT, Goske MJ, Cohen M, Herrmann T, John SD, Noble L, Morrison G, Lehman L & Whiting BR (2013). Image Gently Campaign Back to Basics Initiative: Ten Steps to Help Manage Radiation Dose in Pediatric Digital Radiography. Am J Roentgenol 200, W431–W436. [DOI] [PubMed] [Google Scholar]
- Green M, Mead J & Turner JM (1974). Variability of maximum expiratory flow-volume curves. J Appl Physiol 37, 67–74. [DOI] [PubMed] [Google Scholar]
- Griscom NT & Wohl ME (1986). Dimensions of the growing trachea related to age and gender. Am J Roentgenol 146, 233–237. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Harms CA, Cooper D & Tanaka H (2011). Exercise Physiology of Normal Development, Sex Differences, and Aging. Comp Physiol 1649–1678. [DOI] [PubMed] [Google Scholar]
- Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D & Jain R (2014). Gender Differences in Outcomes of Patients with Cystic Fibrosis. J Womens Heal 23, 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara K, Shimizu K, Makita H, Hasegawa M, Nagai K, Konno S & Nishimura M (2014). Effect of Lung Volume on Airway Luminal Area Assessed by Computed Tomography in Chronic Obstructive Pulmonary Disease. PLoS One 9, e90040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky DA (2012). What Does Airway Resistance Tell Us About Lung Function? Resp Care 57, 85 LP – 99. [DOI] [PubMed] [Google Scholar]
- Krieger N, Kiang MV, Kosheleva A, Waterman PD, Chen JT & Beckfield J (2015). Age at menarche: 50-year socioeconomic trends among US-born black and white women. Am J Pub Heal 105, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo W, Ciet P, Andrinopoulou E-R, Chen Y, Pullens B, Garcia-Peña P, Fleck RJ, Paoletti M, McCartin M, Vermeulen F, Morana G, Lee EY & Tiddens HAWM (2018). Reference Values for Central Airway Dimensions on CT Images of Children and Adolescents. Am J Roentgenol 210, 423–430. [DOI] [PubMed] [Google Scholar]
- Martin TR, Castile RG, Fredberg JJ, Wohl ME & Mead J (1987). Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63, 2042–2047. [DOI] [PubMed] [Google Scholar]
- Masters IB, Ware RS, Zimmerman PV, Lovell B, Wootton R, Francis PV & Chang AB (2006). Airway sizes and proportions in children quantified by a video-bronchoscopic technique. BMC Pulm Med 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J (1980). Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121, 339–342. [DOI] [PubMed] [Google Scholar]
- Nourry C, Deruelle F, Fabre C, Baquet G, Bart F, Grosbois J-M, Berthoin S & Mucci P (2005). Exercise flow-volume loops in prepubescent aerobically trained children. J Appl Physiol 99, 1912–1921. [DOI] [PubMed] [Google Scholar]
- Nourry C, Deruelle F, Fabre C, Baquet G, Bart F, Grosbois J-M, Berthoin S & Mucci P (2006). Evidence of Ventilatory Constraints in Healthy Exercising Prepubescent Children. Pediatr Pulmonol 41, 133–140. [DOI] [PubMed] [Google Scholar]
- Otis AB (1954). The work of breathing. Physiol Rev 34, 449–458. [DOI] [PubMed] [Google Scholar]
- Raghavan D & Jain R (2016). Increasing awareness of sex differences in airway diseases. Respirology 21, 449–459. [DOI] [PubMed] [Google Scholar]
- Senefeld JW, Clayburn AJ, Baker SE, Carter RE, Johnson PW & Joyner MJ (2019). Sex differences in youth elite swimming. PLoS One 14, e0225724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S & Coxson HO (2009). Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol 107, 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain KE, Rosenkranz SK, Beckman B & Harms CA (2010). Expiratory flow limitation during exercise in prepubescent boys and girls: prevalence and implications. J Appl Physiol 108, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM & Haines JR (1975). Bronchial Dimensions and Stature. Am Rev Resp Dis 112, 142–145. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Miller VM & Prakash YS (2012). Sex differences and sex steroids in lung health and disease. Endocr Rev 33, 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink NM, Postma DS, Schouten JP, Rosmalen JGM & Boezen HM (2010). Gender differences in asthma development and remission during transition through puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol 126, 498–504.e6. [DOI] [PubMed] [Google Scholar]
- Wailoo MP & Emery JL (1982). Normal growth and development of the trachea. Thorax 37, 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]


