Abstract
Therapeutic interventions to harness the immune system against tumor cells have provided mixed results in the past for several solid tumors and hematologic malignancies. However, immunotherapy has advanced considerably over the last decade and is becoming an integral combination for treating patients with advanced solid tumors. In particular, prostate cancer (PCa) immunotherapy has shown modest efficacy for patients in the past. With several key discoveries on immune mechanisms and advanced molecular diagnostic platforms recently, immunotherapy is re-emerging as a viable option for PCa, especially castration-resistant prostate cancer (CRPC), to stimulate anti-tumor immunity. Combination of patient-tailored immunotherapy and immune checkpoint blockers with conventional cytotoxic agents and androgen receptor (AR)-targeted therapies should move the field forward. With a recent adaptation that the application of immune checkpoint inhibitors has been successful in the treatment of more than a dozen solid tumors, including melanoma, lymphoma, liver, cervical, gastrointestinal, and breast cancers, it is a timely endeavor to harness immunotherapy for PCa. Here, we provide an account on the progression of immunotherapy with new discoveries and precision approaches for tumors, in particular CRPC, from mechanistic standpoint to emerging limitations and future directions.
INTRODUCTION
The last decade has seen a tremendous increase in the number of immunotherapy trials for various solid tumors. The advances made in cancer immunotherapy extend beyond understanding the dialog between cancer and the immune system to being used as predictors of cancer prognosis (1,2). While surgery, followed by chemotherapy and/or radiation therapy remains the mainstay of management in many solid tumors, immunotherapy is rapidly being incorporated with other therapies to improve patient survival. Although immunotherapy appears to be promising for many solid tumors, progress made in prostate cancer (PCa) is relatively moderate. Evidence from studies on genetic, epidemiologic, and pathophysiologic aspects of PCa imply that inflammation plays an important role at different stages of PCa growth and metastasis. From the onset of prostatic inflammation, leading to tumorigenesis and further evolution of the disease characterized by molecular heterogeneity of driver mutations, various signalling pathways play crucial roles the development of resistance and immunosuppression (3–6). Thus, understanding the pathophysiology of PCa, with particular emphasis on disease responsiveness to different immunomodulatory agents will shed more light on developing new combination therapy approaches.
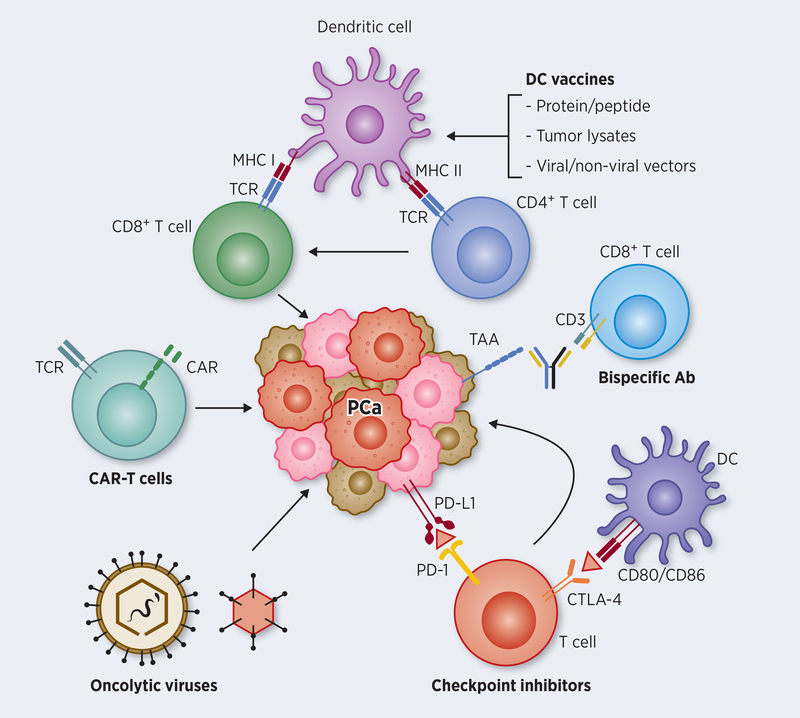
Once diagnosed as a localized disease, conventional interventional approach includes radical prostatectomy or radiation therapy, followed by a continuous monitoring of the levels of prostate-specific antigen (PSA) for biochemical recurrence. Development and progression of PCa is highly associated with chronic inflammation by prostatitis-induced cellular and genomic damage (7). Chronic inflammation in the prostate causes extracellular matrix remodeling and epithelial mesenchymal transition, which plays a key role in the disease development and progression (7). PCa is known as a slow-growing inflammatory disease compared to other malignancies, which allows PCa to be an ideal candidate for immunotherapy. Based on initial set of potential PCa antigens including PSA, different immunotherapy approaches have been attempted in patients with PCa (Figure 1). The following details provide an account of immunotherapy, including mechanistic aspects and updates on patient data from ongoing clinical trials with special emphasis on castration-resistant prostate cancer (CRPC).
Figure 1: Major immunotherapy pathways targeting PCa cells.
Attempts to activate tumor-specific CD8+ T cells against prostate cancer involved loading dendritic cells (DCs) with proteins and peptides of tumor antigens or transducing antigen genes into DCs using viral and non-viral vectors by ex vivo or in vivo approaches. Such antigen-loaded DCs, prompted by additional signals for maturation and APC function results in augmenting CTL effector function, both in number and in activity. Optimizing DC function further enables CD4+ T cells promote T helper function against growing tumor. Genetic approaches to harness tumor-specific CD8+ T cells directly involves harvesting T cells from prostate cancer, transfecting them with chimeric antigen receptor (CAR) genes directed against the patients’ tumor, expanding the modified T cells ex vivo, and reinfusing them back into the patients for antitumor activity. Bispecific antibody conjugates help direct tumor-specific CD8+ T cells to tumor target using an adapter molecule involving CD3 and a tumor surface antigen-specific antibodies. Whereas the above approaches help in the generation and activation of tumor-specific T cells, immune checkpoint inhibitors (ICIs) on the other hand help in blocking inhibitory pathways that dampen T cell function. The two major checkpoint molecules on T cells that block effector function are PD1 and CTLA-4 that interact with PD-L1 produced by tumor cells, and CD80/86 on APCs, respectively. Recent immunotherapy approaches using monoclonal antibody blockade of their inhibitory interaction are highly promising in the clinic to improve CTL function. Oncolytic viruses, which are engineered to selectively replicate and kill tumor cells further improve immunotherapy approaches for effective cross-presentation of tumor antigens to the immune system, either as standalone treatment or in combination with other immunotherapy approaches.
PASSIVE AND ACTIVE IMMUNOTHERAPIES
Passive approaches
Cancer immunotherapy can be largely classified into two categories; passive and active immunotherapies. Dating back to the work of Dr. William Coley in late 1800s, passive immunotherapy adopts a short-term innate immune boost or adoptive immune restoration of T-helper cell (Th)-1 response by providing exogenous pro-inflammatory cytokines and monoclonal antibodies to cancer patients. With steady improvements in our understanding of key immune mechanisms that fight invading microbes and prompt cellular transformation or aberrant cells in the host system, recombinant protein technologies started taking over therapeutic approaches applying recombinant cytokines interleukin (IL)-2 and IL-12 in multiple solid tumor models including lung, metastatic melanoma, and disseminated renal carcinoma (8–12) to activate immune responses. It is noteworthy that in such immune inductions by applying specific cytokines with high-purity, early clinical studies have shown modest response in extending patient survival (13). This initial response paved the way for utilizing/testing other pro-inflammatory cytokines and growth factors activating immune response against the tumor.
An example of passive immunotherapy is a recombinant tumor necrosis factor (TNF)-alpha (TNFerade) therapy. Although clinical use of TNFerade as a cancer immunotherapy agent was limited only for locally advanced tumors, metastatic melanoma and soft tissue sarcoma, due to uncontrolled systemic innate immune response that caused toxicity in patients (14), its application has been discontinued in recent times. In a similar manner, recombinant interferon (IFN)-gamma therapy triggered uncontrolled adaptive immune boosting, Th-1 response, which unleashed cytotoxic T cells that resulted in autoimmune-like organ damage (9).
Chimeric antigen receptor (CAR)-T cell therapy is another example of well-established passive immunotherapy approach in which T cells from cancer patients are genetically modified ex vivo, to express a specific CAR gene, targeting a tumor-specific antigen, and culture-expanded CAR-T cells infused back into the patient. Recent studies have shown promising results from CAR-T cell therapy in solid tumors, including CAR-T strategy targeting a cancer cell surface antigen, mesothelin, in malignant pleural disease, which has shown a favorable response in an ongoing phase I clinical trial ( NCT02414269) (15). In addition, an ongoing phase I clinical trial ( NCT03159819) of CAR-T cell therapy targeting claudin 18.2, a protein highly expressed on gastric and pancreatic adenocarcinomas, has shown anti-tumor activity in patients with advanced gastric and pancreatic adenocarcinomas (16). Despite these potentials, CAR-T cell therapy has shown a better clinical response in hematological malignancies than in solid tumors. (17–22) For targeting PCa, CAR-T cells were generated against prostate-specific membrane antigen (PSMA) and embedding CD28 as a costimulator (23). The CAR-T cell strategy targeting PSMA has shown improved anti-tumor effects in vivo, compared to IgCD28TCR T cells, suggesting a translational potential for targeting CRPC. In line with other cell-based immunotherapies, CAR-T cell therapy also faces difficulties in treating solid tumors including PCa. One of the major limitations in CAR-T therapy is the immunosuppressive tumor microenvironment (TME). In addition to immunosuppressive cytokines and growth factors, the TME is generally replete with protumorigenic tumor-associated macrophages (TAM), regulatory T cells, and myeloid-derived suppressor cells (MDSC), as encountered in lung cancer and renal cell carcinoma (24–27). Well-characterized T cell inhibitory factors in the TME are programmed cell death ligand-1 (PD-L1), which is expressed on cancer cells and interacts with PD-1 on T cells inducing CD8+ T cell anergy, and transforming growth factor-beta (TGF-beta), which suppresses effector immune cell function (28,29). In addition, the emergence of resistant clones with neoantigens further warrants next generation CAR-T cell technology with multiple single-chain variable fragment (scFv) targeting multiple tumor-specific antigens.
Another strategy of passive immunotherapy currently being studied is radiolabeled monoclonal antibodies targeting PSMA, which is highly expressed specifically on PCa cells. The PSMA strategy has advantage of effective local delivery of the agent because of its high specificity and internalization into PCa cells upon PSMA binding the agent. A phase II clinical trial testing anti-PSMA labeled with lutetium-177 (177Lu-J591) demonstrated PSA decline after receiving single treatment in 59.6% of 47 patients with metastatic CRPC (mCRPC) (30). Also, a recent phase I/II study with 177Lu-J591 showed promising therapeutic efficacy in patients with mCRPC, when combined with higher cumulative radiation therapy (31). In addition, the most updated outcome of a study with alternative PSMA ligand, PSMA-617 radiolabeled with lutetium-177 (177Lu-PSMA-617), described that 50% or greater decrease in PSA was observed in 32 of 50 patients with mCRPC (32). Besides anti-PSMA monoclonal antibody conjugates being tested as promising immunotherpeutic agent in PCa, they serve as a useful tool for CRPC diagnosis and imaging by identifying metastatic sites (33,34).
In addition to PSMA, prostate stem cell antigen (PSCA) has emerged as an ideal immunotherapeutic target because of its overexpression in PCa including metastatic and hormone refractory tumors, but not in normal prostate tissue. A phase I/II clinical trial with PSCA is currently ongoing to evaluate safety and clinical activity of PSCA-Specific CAR-T cells (BPX-601), where T cells were engineered to recognize PSCA-expressing PCa cells, in patients with previously treated for PSCA (NCT02744298). Also, a recently initiated clinical trial of anti-PSCA adopted PSCA-targeting CAR-T cell strategy in patients with PSCA positive mCRPC ( NCT03873805). This phase I clinical trial has not yet reported primary outcomes. Along with anti-PSMA antibody, properties of iodine labeled anti-PSCA, [124I] PSCA-minibody, as a useful drug for positron emission tomography (PET) imaging have been evaluated which resulted in positive outcomes ( NCT02092948). Collectively, these clinical outcomes point to the potential of PCa antigens targeting in co-adjuvant setting to improve survival.
Active approaches
Active immunotherapy stimulates a patient’s own immune response, resulting in the activation of immune cells, natural killer cells or cytotoxic T cells, or antibody production targeting tumor-specific antigens. This approach is intended to provoke adaptive immune response, particularly, to establish a long-term T cell memory that actively and specifically targets tumor-specific antigens. Various tumor-specific and tumor-associated antigens have been identified, cancer antigen (CA)-125 in ovarian cancer (35), human epidermal growth receptor (HER) 2 in breast cancer and carcinoembryogenic antigen (CEA) in breast and colon cancers (36), melanoma antigen gene (MAGE) in melanoma, and alpha-fetoprotein (AFP) in hepatocellular carcinoma, and tested in clinical trials with moderate success (37,38).
Sipuleucel-T (Provenge) is an example of active immunotherapy targeting prostatic acid phosphatase (PAP), one of PSAs (39). This FDA-approved autologous active cellular therapy is designed to induce T cell-mediated immune response via ex vivo stimulation of patient’s immature antigen-presenting cells (APCs) in combination with recombinant PAP and costimulatory granulocyte-macrophage colony-stimulating factor (GM-CSF). A completed phase III clinical trial of Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT: NCT00065442) indicated that the Sipuleucel-T improved overall survival (OS) by 4.1 months and a 22% reduction of relative mortality risk in patients diagnosed with mCRPC (40). The IMPACT study further indicated that the patients with lower disease burden demonstrated the greatest benefit (41,42), suggesting a higher efficacy of the therapy in early stages of PCa. However, only minimal anti-tumor responses were observed, in spite of OS benefit, which is possibly due to that the concept of Sipuleucel-T is to achieve remission of advanced PCa, not regression, by exerting antigen presentation of antigen-presenting cells. Although phase II trial of sipuleucel-T in combination with pidilizumab (anti-programmed cell death-1; anti-PD-1) and cyclophosphamide (chemotherapy) in patients with mCRPC was initiated in 2012 ( NCT01420965), unfortunately it was terminated due to drug supply issues. Another clinical trial to investigate the effect of combination therapy of sipuleucel-T and ipilimumab and anti-cytotoxic T-lymphocyte antigen (CTLA)-4, ( NCT01832870) in mCRPC was also terminated without any result reported at phase I (43).
An example of active immunotherapy agent that was clinically tested involved use of viral vectors. A poxvirus-based cancer vaccine, composed of rilimogene galvacirepvec (V-PSA-TRICOM; PROSTVAC-V), a recombinant vaccinia virus, and rilimogene glafolivec (F-PSA-TRICOM; PROSTVAC-F), a recombinant fowlpox virus, with potential immunostimulatory and antineoplastic activities have been tested as immunotherapeutic agents (44). Both viruses encoded modified forms of human PSA and the three co-stimulatory molecules (TRIad of Costimulatory Molecules; TRICOM), B7–1 (CD80), intercellular adhesion molecule-1 (ICAM-1), and lymphocyte function-associated antigen-3 (LFA-3). In spite of positive outcomes in patients who received PROSTVAC-VF resulting in 8.5 months prolongation of median OS in phase II trial (45), a large phase III confirmatory trial (PROSPECT: NCT01322490), where 1,200 asymptomatic patients with mCRPC were randomly assigned to PROSTVAC-VF with or without GM-CSF, failed to confirm previous results with no significant differences in OS between the treatment arms (46). A currently ongoing clinical trial ( NCT03315871) of PROSTVAC investigating if the combination therapy (PROSTVAC + M7824: monoclonal antibody targeting PD-L1 and TGF-beta R II + CV301: recombinant vaccine of Avipoxvirus) exerts anti-tumorigenic effect on PCa patients with biochemical recurrence, defined as the state which PSA level is increased.
In spite of development and clinical application of active and passive immunotherapies, clinical outcomes have only been modest due to the limitations, including low levels of targeting molecules, side effects, and short half-life of the agents (46–49). Moreover, TME-induced immunosuppression hindered the efficacy of these immunotherapies (50). Such limitations promoted the development of alternative approaches in cancer immunotherapy. Recent clinical trial reports and studies have shown that the application of immune checkpoint inhibitors has been successful in the treatment of various malignancies (51,52), indicating a promising cancer therapy that overcomes the limitations of conventional therapies.
CHECKPOINT BLOCKADE THERAPY TO IMPROVE EFFECTOR T CELL FUNCTION
Therapy targeting programmed cell death protein (PD)-1 and PD ligand (PD-L)1
One of the important mechanisms by which cancer cells evade immune surveillance is the activation of immune checkpoint pathways, which suppress anti-tumor responses by causing T cell exhaustion or anergy as seen in different types of solide tumors (53–59). Immune checkpoint inhibitors sustain anti-tumor activities by interfering T cell co-inhibitory signaling pathways, thus enhancing immune-mediated tumoricidal effect (60). Examples of immune checkpoint inhibitors are nivolumab (Opdivo) and pembrolizumab (Keytruda) that block an immune checkpoint protein PD-1, resulting in the restoration of T cells to target cancer cells. These drugs have shown to inhibit the progression of certain types of solid tumors (61–65). Currently, two phase II clinical trials of pembrolizumab targeting PD-1 are ongoing to investigate its effects against progression of the disease ( NCT02787005: KEYNOTE-199) in mCRPC after androgen-deprivation therapy (ADT) and in mCRPC patients treated with enzalutamide ( NCT02312557). A recent update on the KEYNOTE-199 trial stated that pembrolizumab responses are durable, and the observed OS benefit is promising (66).
In addition to agents targeting PD-1, anti-PD-L1 immunotherapies are currently being studied using avelumab (Bavencio) and atezolizumab (Tecentriq). Recent updates on ongoing phase I clinical trial of avelumab ( NCT01772004) showed that only 3 out of 17 patients with mCRPC resulted in prolonged PSA doubling time (67). A phase III clinical trial of atezolizumab ( NCT03016312) in combination with enzalutamide for mCRPC patients, which is designed to measure OS with the time frame of 42 months, has been ongoing since January 2017. It is noteworthy to highlight that there are currently two distinct PDLs, PD-L1 and PD-L2, have been identified. Given the fact that T cells interact with APCs expressing both PDLs during initial priming phase, it has been suggested that PD-1 blockade, which inhibits interaction with both PD-L1 and PD-L2 is more effective immunotherapeutic strategy in exerting T cell priming than targeting PD-L1 alone (68).
Cytotoxic T-lymphocyte antigen (CTLA)-4 as a potential immune checkpoint inhibitor
Ipilimumab (Yervoy) is an immune checkpoint inhibitor that blocks CTLA-4, expressed on the surface of cytotoxic T cells, preventing T cell-mediated anti-tumor immune responses (53). Administration of this monoclonal antibody has already been approved by the FDA as a cancer immunotherapy agents (69). Initial clinical trial with ipilimumab monotherapy was discontinued at phase III due to only a marginal improvement of patient OS when compared to the placebo arm (70). As an alternative strategy, ongoing clinical trials for mCRPC adopt combinations of immune checkpoint inhibitors. For instance, a phase II clinical trial, CheckMate 650, was initiated to study a combination of ipilimumab and nivolumab in mCRPC patients who developed resistance to androgen receptor (AR)-targeted therapies (71,72). However, recently Cancer Discovery 2019 reported that the combination of the two drugs resulted in only 25% of objective response rate (73). In addition, discontinuation of the therapy in the study population was reported due to the disease progression and increased side effects (72,73). Another phase III trial, in which patients with mCRPC that had progressed after Taxol chemotherapy ( NCT00861614) received radiation therapy targeting bone metastasis followed by ipilimumab treatment, resulted in prolonged median OS (74). Furthermore, the result showed that OS rate at one year in patients received ipilimumab therapy was 46.5%, compared to 40.8% in the placebo group.
BISPECIFIC ANTIBODY CONJUGATES FOR THERAPEUTIC TARGETING OF PROSTATE CANCER
Bispecific antibodies, conjugated to tumor antigens expressed on PCa cells and CD3 molecule on T cells have emerged recently as a promising new approach to treat hormone-refractory disease. In this context, various combinations of bispecific conjugates have been tested with encouraging results. A site-specific, bispecific antibody, containing moieties of PSMA and anti-CD3 Fab has shown excellent potency and activity in vitro and in vivo xenograft models (75–77). Translation of this approach using a bispecific conjugate targeting CD3 and Her2 on tumor cells in a phase I clinical study demonstrated encouraging results with no dose-limiting toxicities, and with partial respond as well as significant decreases PSA levels and pain scores in a few patients. Immune evaluations of responders showed increases in IFN-gamma and Th1 serum cytokines (78), indicating a strong rationale for future application of this approach. Further, this antibody conjugate approach was recently tested in PCa tissue specimens using oncolytic viral platform, fibroblast activation protein-bispecific T-cell engager (FAP-BiTE), combining virolysis with endogenous T-cell activation signals. Interestingly, this approach has also shown efficacy of targeting cancer associated fibroblasts in addition to PCa cells prompting a multimodal treatment strategy within a single therapeutic agent (79). Based on encouraging data from preclinical studies, a phase I study using PSMA-targeted bispecific T cell agent pasotuxizumab in mCRPC recently reported antitumor activity in a dose-dependent manner, with two patients showing durable response for over one year (80).
THE EFFECT OF ANDROGEN-DEPRIVATION THERAPY IN IMMUNE MODULATION
Androgen deprivation by surgical castration or antiandrogens is a mainstay therapy to target AR signaling in treating PCa. Short-term increase in the number of naïve T cells and Th-1 cells, and decrease in the number of Tregs after the initiation of ADT have been reported (81,82). In addition, increased number of tumor-infiltrating T cells, transiently Th-1 biased, has been observed in animal models, supporting anti-tumor immune response of ADT (83). Although studies have shown immunostimulatory benefits of ADT in PCa treatment, patients who have undergone standard ADT eventually result in relapse. This maybe due to short-term Th-1 response caused by ADT, which eventually fails to establish immunostimulatory response, resulting in tumor infiltrated immune cells polarizing toward immunosuppressive cells. Therefore, combination therapy of ADT with immunotherapies blocking such protumorigenic events will be beneficial in treating PCa.
There are emerging evidences that, in fact, ADT exerts immunosuppressive responses. A recent study has reported that T cell suppressive activity of AR antagonists, including flutamide and enzalutamide, resulting in decreased IFN-gamma production by T cells and/or APCs in vivo (84). Furthermore, the study revealed that immunosuppression induced by AR antagonists occurs during initial T cell priming phase rather than at later stages of T cell stimulation (84), suggesting that the accurate timing of ADT when treating PCa in combination with other immunotherapies is crucial to avoid unintended immunosuppressive effect of AR antagonists. Indeed, a follow up study of a combination therapy with Prostvac and nilutamide showed that patients who received Prostvac followed by nilutamide resulted in significantly increased survival rate compared to population that received nilutamide followed by Prostvac (85), suggesting vaccine followed by antiandoregen sequence may be a preferred approach to increase the efficacy of combination therapy.
EXISTING LIMITATIONS OF CURRENT IMMUNOTHERAPY IN GENERAL AND PROSTATE CANCER IN PARTICULAR
While immunotherapies (e.g. immune checkpoint inhibitors) have shown encouraging clinical responses in certain types of cancer including melanoma, their application to other cancers needs further optimization. Unpredictable efficacy and toxicity of the therapy often become hindrances of successful immunotherapy in many cancers. Various patient responses to the same immunotherapy in patients with different types and stages of cancers have been observed (86). In addition, the patient response depends on multiple factors including intratumor heterogeneity and previous treatment history, which suggests the need of personalized and combination therapy as important future direction for successful immunotherapy.
Prostate cancer grows slowly compared to other types of malignancies, which allows it to be an ideal candidate where immunotherapy can be effective. However, various clinical trials by active immunotherapy, passive immunotherapy, adoptive T cell therapy and immune checkpoint inhibitors in combination with chemotherapy thus far have only shown modest clinical outcomes in mCRPC when compared to other genitourinary cancers. There are proposed hypotheses why immunotherapy trials were not successful in particular for prostatic malignancies. Particularly, TME in prostate lesions is known for establishing a niche unsuitable for tumor infiltrating immune cells with anti-tumor activities, leading to limited efficacy of immunotherapy (87). In fact, a study has revealed a significantly smaller number of tumor infiltrating CD8+ T cells in primary prostate tumors in patients who underwent abiraterone treatment, an antiandrogen agent inhibiting biosynthesis of androgen, when compared to other types of malignancies (46). Generally, blocking the interaction between PD-1 and PD-L1 is expected to restore anti-tumor responses induced by tumor infiltrating CD8+ T cells. However, there are many other immunosuppressive characteristics associated with prostate TME, which possibly renders immunotherapeutic strategies using immune checkpoint inhibitors ineffective. For example, increased level of plasma TGF-beta that directly suppresses CD8+ T cells was observed in bladder cancer (88). Also, increased number of immunosuppressive cells including TAM, regulatory T cells and MDSC affecting the anti-tumor response of CD8+ T cells (89–92). Another explanation of different responses to immunotherapies, especially immune checkpoint inhibitors, can be supported by different types of tumors with various levels of tumor mutation burdens. Types of cancer with higher response rate to anti-PD-1/PD-L1 immunotherapy are melanoma and non-small-cell lung carcinoma, which are known to have higher tumor mutation burden (93). These tumors are prone to be recognized by T cells because they express more number of neoantigens. On the other hand, tumors with low tumor mutation burden, less somatic mutations, such as PCa will less likely to respond to these immune checkpoint inhibitors (93), which explains why immunotherapies in PCa have been relatively unsuccessful than in high mutation burden tumors (93). Another clinical speculation suggests that low levels of PD-L1 expression is associated with PCa progression. Studies have demonstrated, surprisingly, a downregulation of PD-L1 expression in primary PCa (46–48,94), which may explain why early clinical trials of anti-PD-1 monotherapy in mCRPC was not successful. For example, PD-L1 expression was rarely observed in PCa patient specimens, whereas the level of PD-L1 expression increased in response to proinflammatory signals, IFN-gamma in vitro (48). In addition, gene analysis study revealed that PD-L1 expression was low, while the level of PD-L2 (another ligand for PD-1) expression remained significantly high in PCa (95). Given that the number of effector T cells is low in the immune-privileged prostate lesions, downregulation of PD-L1 expression can be explained by relatively low levels of proinflammatory cytokines, secreted from CD8+ T cells. However, other reports have suggested an increase in PD-L1 expression in CRPC (96). A study has shown that PCa that has progressed after receiving enzalutamide resulted in upregulated PD-L1 expression in PCa and circulating dendritic cells in patients and preclinical model (97). Increased number of circulating PD-1+ T cells has also been observed in preclinical model ( NCT02312557) (97). Interestingly, it is noteworthy that PCa under abiraterone acetate therapy in combination with prednisone showed a downregulation of PD-L1 expression in the tumors (46). The variations in PD-L1 expression in prostate tumors partly suggest that the levels of immune checkpoint molecule expression vary in different stages of PCa progression, in response to ADT (96). Since clinical trials have been performed with patients only in advanced stages of cancer, PD-L1 expression levels may also vary depending on the types of previous therapies received before the progression of the disease.
Unlike some of the high-responsive tumors for immunotherapies, such as melanoma and non-small-cell lung carcinoma, characterized by increased tumor-infiltrating lymphocytes, PCa is considered as a “cold tumor” not only from the perspective of limited number of tumor-associated antigens and neoantigens available for immune targeting, but also existence of a complex TME, resisting T cell infiltration, even in the combat of blockade with immune chekpoint inhibiors. Hence, adjusting strategies to overcome histologic barriers, including tissue hypoxia and dense stromal network would complement immunotherapy approaches for effective cytotoxic T lymphocyte infiltration. A recent preclinical study demonstrated that reducing hypoxia using a hypoxia-activated prodrug, TH-302, significantly reduced hypoxia in PCa-TME and improved efficacy of immune checkpoint inhibitors (98).
PRECISION IMMUNOTHERAPY FOR PROSTATE CANCER THERAPY
Tumor is composed of subpopulations of cancer cells with distinct phenotypic and genotypic profiles, defined as tumor heterogeneity. Tumor heterogeneity allows subpopulations of cells to present different behaviors and response rates to cancer immunotherapies. Different types of mutated proteins exist in a tumor, including KRAS and TP53 (99). The number of mutations in certain tumors can be used as a tool to predict their response to immunotherapies, anti-PD-1/PD-L1 and anti-CTLA4 monoclonal antibodies (99). For example, tumors with high mutational burden show high response rates to immune checkpoint inhibitors, anti-PD-1 immunotherapy (99). A study has revealed that PCa bears 35 mutated peptides, whereas lung adenocarcinoma and melanoma resulted in 197 and 276 mutated proteins, respectively, describing relatively low tumor mutation burden in PCa through the cancer genome atlas profiles (99). Recent studies have identified the existence of a large heterogeneity in mutation types in different foci within the same patients with PCa (100). Further, existence of clonal evolution of genetically distinct mutations in multifoci PCa, even in younger patients (101) suggests the importance of identifying patients-specific molecular signatures to design rational immunotherapy strategies. The complex nature of cancer with genomic heterogeneity and immunosuppressive TME highlights a need for personalized genomic therapy, which possibly will benefit clinical outcomes in CRPC. For a successful cure for PCa, a combinatorial approach with individualized medicine, for instance, cancer genomics targeting newly identified gene components in the TME as part of therapeutic regimen, is essential. Cancer genomics compares the genomes of tumors with non-cancerous cells to identify the specific mutations, especially in heterogeneous tumors. A recent next-generation genome sequencing (NGS) analysis has identified that the PCa patients who have undergone a course of ipilimumab therapy have increased expression of v-domain Ig suppressor of T cell activation (VISTA), a newly discovered immune checkpoint on macrophages (71,102), suggesting a new potential immunotherapy target in prostate TME. Although VISTA-mediated signaling pathways are yet to be determined, VISTA expression on immunosuppressive subpopulation (e.g. MDSC, TAM, and Treg) is known to induce T cell anergy/ exhaustion through its interaction with potential binding counterparts (e.g. VSIG3 and PSGL-1), expressed on T cell surface (102,103). More recently, it has been reported that VISTA-VISTA trans-interaction also directly induces naïve T cell quiescence (104,105), demonstrating a complex mechanism of VISTA biology in suppressing effector T cell function in TME. These new findings pave way to further investigate additional modulators on VISTA expression in T cells. Given the fact that AR activation is reported to regulate adaptive immunity, including effector T cells (106), it is conceivable whether if AR target genes include VISTA, along with others like PD-L1. Further studies in this angle may underscore the effectiveness of AR-directed therapies in combination with current immunotherapy regimens.
Further discovery of new genes to target heterogeneous TME through genomics/deep sequencing will play a significant role in the successful personalized treatment. Furthermore, a combination therapy of Kristen rat sarcoma viral oncogene (KRAS) inhibitor and existing immunotherapies (e.g. anti-PD-1/PD-L1) to target KRAS mutation-induced neoantigens in mutant KRAS tumors will be another multimodal therapeutic regimen. Hence, combination of multimodal immunotherapy that is personalized based on cancer genomics would lead to more effective interventions.
IMMUNOTHERAPY TARGETING CANCER STEM CELLS
Cancer stem cells are defined as subpopulations of heterogeneous cancer cells with self-renewing capability for continuous tumorigenesis. Different types of cancer stem cells are distinguished depending on types of cell surface proteins they express. Common cell surface markers to identify cancer stem cells in solid tumors include CD133, CD44, CD24, and epithelial cell adhesion molecule (EpCaM). Subpopulations of PCa cells with stem cell-like properties are known to coexpress cell surface markers, CD44, α2β1 integrin, CD133, CD49f, and CD176 (107). High expression of aldehyde dehydrogenase (ALDH) was observed more in stem-like cells in metastatic PCa compared to tumors without metastasis (108). The expression of ALDH was positively associated with expression of other PCa cancer stem cell markers including EpCaM, CD44 and integrin (108). Cancer stem cell-targeting immunotherapy has recently been attempted in preclinical models of PCa with CAR T-cells engineered against EpCaM expressing cancer stem cell population and results indicate promising outcome with murine PCa model (109). Conventional PCa therapies targeting differentiated or differentiating cancer cells with non-stem cell-like characteristics can cause tumor relapse by allowing tumorigenesis of cancer stem cells, while combinational therapy of traditional PCa therapy and cancer stem cell specific immunotherapy will provide a better clinical outcome by targeting different cell populations in heterogeneous tumor.
FUTURE DIRECTIONS
Collective analysis of existing limitations and renewed promise in immunotherapy clinical trials lends more optimism to further refine different aspects of this treatment paradigm improved clinical outcomes in PCa. Some of the key directions the field of cancer immunotherapy, in general, is geared towards identifying molecular immune mechanisms in non-responders and developing combination therapies targeting engineered biomolecules in the TME (110,111), improving the potential of systemic oncolytic virotherapy (112), adaptations to improve tumors, refractory to T cell infiltration (113), and improving preclinical animal models that recapitulate the human immune mechanisms (114). Prostate cancer progression in particular, adopts immune evasion, involving multi-layered cellular alterations, where cancer cells interact with and regulate immune and various stromal components, eventually polarizing them to form an immunosuppressive TME. Such complex events mediated by various molecular signaling pathways, including immune checkpoint expression patterns, may also differ depending on the microenvironment of metastatic sites or organs. Thus, immunotherapy targeting prostate tumors in the early stage before acquiring phenotypic heterogeneity during disease progression may be critical for therapeutic success.
Protumorigenic immunoediting events begin at the very first step of immune infiltration to primary tumors of prostate, where PCa cells hijack danger signals to recruit innate immune cells. Indeed, high levels of antimicrobial peptides (but far less than the levels found in pathogenic infection) are expressed by PCa epithelia with a pattern of gradual increase with tumor growth (115,116). A study of cabiralizumab (cabira: FPA-008), a monoclonal antibody targeting TAM expressing macrophage colony-stimulating factor receptor (M-CSFR), is currently in a phase II clinical trial ( NCT02471716). Even within the context of immune checkpoint targeting strategy, a phase II clinical trial of cabiralizumab in combination with nivolumab is also under investigation in advanced pancreatic cancer (117).
Regarding immunotherapy combination with AR-directed therapy, it is also interesting to note that a specific AR-targeting agent enzalutamide and ipilimumab combination is so far the only ongoing phase III clinical trial in patients with mCRPC. As noted, androgen-AR axis is still a mainstay of targeting CRPC (118,119), however, AR-directed therapies appear to induce cross-resistance when combined with immune checkpoint inhibitors. For instance, PCa under abiraterone acetate therapy, which inhibits biosynthesis of androgens, in combination with prednisone and leuprolide showed a downregulation of PD-L1 expression in the tumors (46), rendering checkpoint immunotherapy ineffective. However, it is also noteworthy that PD-L1 expression is highly upregulated in enzalutamide-resistant clones and circulating dendritic cells with no classical AR activation, suggesting that AR in hormone-naïve setting may downregulate PD-L1 expression in PCa (97). These variations in PD-L1 expression in prostate tumors partly suggest that the expression levels of immune checkpoint molecule differ based on clinical grade of the disease (96). These results also suggest, in part, that PD-1/PD-L1 expression is differentially regulated depending on ligand availability and AR activation status (including AR gene mutations) in CRPC. Therefore, it may be of great interest to analyze AR regulation of immune checkpoint expression in CRPC, where nuclear AR levels may be a potential indicator of checkpoint immunotherapy success towards tumors progressing over AR-targeted therapies.
ACKNOWLEDGEMENTS
This work was supported by NIH grant R01CA184770 and Mike Slive Prostate Cancer Foundation Award to S.P.
Footnotes
The authors declare no potential conflicts of interest
REFERENCES
- 1.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature reviews Cancer 2019;19:133–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, et al. Advances in cancer immunotherapy 2019 - latest trends. Journal of experimental & clinical cancer research : CR 2019;38:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tewari AK, Stockert JA, Yadav SS, Yadav KK, Khan I. Inflammation and Prostate Cancer. Advances in experimental medicine and biology 2018;1095:41–65 [DOI] [PubMed] [Google Scholar]
- 4.Gurel B, Lucia MS, Thompson IM Jr., Goodman PJ, Tangen CM, Kristal AR, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014;23:847–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012;60:199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. American journal of clinical and experimental urology 2013;1:3–11 [PMC free article] [PubMed] [Google Scholar]
- 7.Cai T, Santi R, Tamanini I, Galli IC, Perletti G, Bjerklund Johansen TE, et al. Current Knowledge of the Potential Links between Inflammation and Prostate Cancer. International journal of molecular sciences 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott DF, Cheng SC, Signoretti S, Margolin KA, Clark JI, Sosman JA, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21:561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016;5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Li X, Wang J, Gao D, Li Y, Li H, et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nature communications 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, et al. T-cell Homing Therapy for Reducing Regulatory T Cells and Preserving Effector T-cell Function in Large Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24:2920–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. Journal of immunology 2014;192:5451–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldmann TA. Cytokines in Cancer Immunotherapy. Cold Spring Harbor perspectives in biology 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. The oncologist 2006;11:397–408 [DOI] [PubMed] [Google Scholar]
- 15.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science translational medicine 2014;6:261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan XWB, Li Z, Li J, Wang H, Chen L, Jiang H, Wu M. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:2509 [Google Scholar]
- 17.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine 2014;371:1507–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine 2011;365:725–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine 2013;368:1509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends in molecular medicine 2012;18:377–84 [DOI] [PubMed] [Google Scholar]
- 23.Ma Q, Gomes EM, Lo AS, Junghans RP. Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy. The Prostate 2014;74:286–96 [DOI] [PubMed] [Google Scholar]
- 24.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinion in immunology 2013;25:268–76 [DOI] [PubMed] [Google Scholar]
- 25.Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer cell 2017;31:311–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017;169:750–65 e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017;169:736–49 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017;8:2171–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Molecular cancer 2019;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19:5182–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tagawa ST, Vallabhajosula S, Christos PJ, Jhanwar YS, Batra JS, Lam L, et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177) Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 2019;125:2561–9 [DOI] [PubMed] [Google Scholar]
- 32.Vapiwala N, Hofman MS, Murphy DG, Williams S, Sweeney C. Strategies for Evaluation of Novel Imaging in Prostate Cancer: Putting the Horse Back Before the Cart. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:765–9 [DOI] [PubMed] [Google Scholar]
- 33.Cimadamore A, Cheng M, Santoni M, Lopez-Beltran A, Battelli N, Massari F, et al. New Prostate Cancer Targets for Diagnosis, Imaging, and Therapy: Focus on Prostate-Specific Membrane Antigen. Frontiers in oncology 2018;8:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grubmuller B, Senn D, Kramer G, Baltzer P, D’Andrea D, Grubmuller KH, et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. European journal of nuclear medicine and molecular imaging 2019;46:1063–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Killock D Gynaecological cancer: Biomarker potential of CA-125 enhanced. Nature reviews Clinical oncology 2015;12:437. [DOI] [PubMed] [Google Scholar]
- 36.Criscitiello C Tumor-associated antigens in breast cancer. Breast care 2012;7:262–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryder SD, British Society of G. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003;52 Suppl 3:iii1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weon JL, Potts PR. The MAGE protein family and cancer. Current opinion in cell biology 2015;37:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P & T : a peer-reviewed journal for formulary management 2011;36:197–202 [PMC free article] [PubMed] [Google Scholar]
- 40.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine 2010;363:411–22 [DOI] [PubMed] [Google Scholar]
- 41.Silvestri I, Cattarino S, Giantulli S, Nazzari C, Collalti G, Sciarra A. A Perspective of Immunotherapy for Prostate Cancer. Cancers 2016;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013;81:1297–302 [DOI] [PubMed] [Google Scholar]
- 43.Scholz M, Yep S, Chancey M, Kelly C, Chau K, Turner J, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. ImmunoTargets and therapy 2017;6:11–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulley JBM, Vogelzang NJ, Ng S, Agarwal N, Parker C, Pook DW. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:1051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:1099–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calagua C, Russo J, Sun Y, Schaefer R, Lis R, Zhang Z, et al. Expression of PD-L1 in Hormone-naive and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23:6812–22 [DOI] [PubMed] [Google Scholar]
- 47.Patel A, Fong L. Immunotherapy for Prostate Cancer: Where Do We Go From Here?-PART 2: Checkpoint Inhibitors, Immunotherapy Combinations, Tumor Microenvironment Modulation, and Cellular Therapies. Oncology 2018;32:e65–e73 [PubMed] [Google Scholar]
- 48.Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate cancer and prostatic diseases 2015;18:325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Buanec H, Bensussan A, Bagot M, Gallo RC, Zagury D. Active and passive anticytokine immune therapies: current status and development. Advances in immunology 2012;115:187–227 [DOI] [PubMed] [Google Scholar]
- 50.Yu Y, Cui J. Present and future of cancer immunotherapy: A tumor microenvironmental perspective. Oncology letters 2018;16:4105–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Experimental & molecular medicine 2018;50:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363:711–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine 2011;364:2517–26 [DOI] [PubMed] [Google Scholar]
- 55.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 2015;373:1627–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine 2015;372:2018–28 [DOI] [PubMed] [Google Scholar]
- 57.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. The Lancet Oncology 2016;17:e542–e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine 2015;373:1270–1 [DOI] [PubMed] [Google Scholar]
- 59.Cheng W, Fu D, Xu F, Zhang Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis 2018;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. Journal of hematology & oncology 2018;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janiczek M, Szylberg L, Kasperska A, Kowalewski A, Parol M, Antosik P, et al. Immunotherapy as a Promising Treatment for Prostate Cancer: A Systematic Review. Journal of immunology research 2017;2017:4861570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishio M, Takahashi T, Yoshioka H, Nakagawa K, Fukuhara T, Yamada K, et al. KEYNOTE-025: Phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1-positive advanced non-small-cell lung cancer. Cancer science 2019;110:1012–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecologic oncology 2019;152:243–50 [DOI] [PubMed] [Google Scholar]
- 64.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 2018;378:2078–92 [DOI] [PubMed] [Google Scholar]
- 65.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. The New England journal of medicine 2018;378:1789–801 [DOI] [PubMed] [Google Scholar]
- 66.Emmanuel S, Antonarakis JCG, Marine Gross-Goupil, Vaishampayan Ulka N., Piulats Josep M., De Wit Ronald, Tuomo Alanko, Satoshi Fukasawa, Kenichi Tabata, Susan Feyerabend, Raanan Berger, Haiyan Wu, Jeri Kim, Christian Heinrich Poehlein, and De Bono Johann S. Pembrolizumab for metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel: Updated analysis of KEYNOTE-199. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;27:216 [Google Scholar]
- 67.Fakhrejahani FMR, Dahut WL, Karzai F, Cordes LM, Schlom J, Gulley JL. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:159 [Google Scholar]
- 68.De Sousa Linhares A, Battin C, Jutz S, Leitner J, Hafner C, Tobias J, et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Scientific reports 2019;9:11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell research 2015;25:208–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:40–7 [DOI] [PubMed] [Google Scholar]
- 71.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nature medicine 2017;23:551–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma PPR, Narayan V, Flechon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, Duan T, Saci A, Hu S, Han GC, Fizazi K. Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castration-resistant prostate cancer (mCRPC; CheckMate 650). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:142 [Google Scholar]
- 73.Anti-PD-1-CTLA4 Combo Hits Prostate Cancer. Cancer discovery 2019;9:569–70 [DOI] [PubMed] [Google Scholar]
- 74.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology 2014;15:700–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim CH, Axup JY, Lawson BR, Yun H, Tardif V, Choi SH, et al. Bispecific small molecule-antibody conjugate targeting prostate cancer. Proceedings of the National Academy of Sciences of the United States of America 2013;110:17796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patterson JT, Isaacson J, Kerwin L, Atassi G, Duggal R, Bresson D, et al. PSMA-targeted bispecific Fab conjugates that engage T cells. Bioorganic & medicinal chemistry letters 2017;27:5490–5 [DOI] [PubMed] [Google Scholar]
- 77.Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, et al. MOR209/ES414, a Novel Bispecific Antibody Targeting PSMA for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Molecular cancer therapeutics 2016;15:2155–65 [DOI] [PubMed] [Google Scholar]
- 78.Vaishampayan U, Thakur A, Rathore R, Kouttab N, Lum LG. Phase I Study of Anti-CD3 x Anti-Her2 Bispecific Antibody in Metastatic Castrate Resistant Prostate Cancer Patients. Prostate cancer 2015;2015:285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freedman JD, Duffy MR, Lei-Rossmann J, Muntzer A, Scott EM, Hagel J, et al. An Oncolytic Virus Expressing a T-cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer research 2018;78:6852–65 [DOI] [PubMed] [Google Scholar]
- 80.Hummel H-DKP, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler M-E, et al. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting Bispecific T cell Engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2019;37:5034 [Google Scholar]
- 81.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. American journal of physiology Endocrinology and metabolism 2006;290:E856–63 [DOI] [PubMed] [Google Scholar]
- 82.Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocrine-related cancer 2017;24:T297–T310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proceedings of the National Academy of Sciences of the United States of America 2014;111:9887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pu Y, Xu M, Liang Y, Yang K, Guo Y, Yang X, et al. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Science translational medicine 2016;8:333ra47. [DOI] [PubMed] [Google Scholar]
- 85.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14:4526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, et al. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. European journal of cancer 2017;81:116–29 [DOI] [PubMed] [Google Scholar]
- 87.Madan RA, Gulley JL. Finding an Immunologic Beachhead in the Prostate Cancer Microenvironment. Journal of the National Cancer Institute 2019;111:219–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shariat SF, Kim JH, Andrews B, Kattan MW, Wheeler TM, Kim IY, et al. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer 2001;92:2985–92 [DOI] [PubMed] [Google Scholar]
- 89.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. Journal of immunology 2006;177:7398–405 [DOI] [PubMed] [Google Scholar]
- 90.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14:1032–40 [DOI] [PubMed] [Google Scholar]
- 91.Getnet D, Maris CH, Hipkiss EL, Grosso JF, Harris TJ, Yen HR, et al. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. Journal of immunology 2009;182:4675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology 2009;182:4499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maleki Vareki S High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. Journal for immunotherapy of cancer 2018;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baas W, Gershburg S, Dynda D, Delfino K, Robinson K, Nie D, et al. Immune Characterization of the Programmed Death Receptor Pathway in High Risk Prostate Cancer. Clinical genitourinary cancer 2017;15:577–81 [DOI] [PubMed] [Google Scholar]
- 95.Zhao SG, Lehrer J, Chang SL, Das R, Erho N, Liu Y, et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. Journal of the National Cancer Institute 2019;111:301–10 [DOI] [PubMed] [Google Scholar]
- 96.Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. The American journal of pathology 2018;188:1478–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015;6:234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. The Journal of clinical investigation 2018;128:5137–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castle JC, Uduman M, Pabla S, Stein RB, Buell JS. Mutation-Derived Neoantigens for Cancer Immunotherapy. Frontiers in immunology 2019;10:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lovf M, Zhao S, Axcrona U, Johannessen B, Bakken AC, Carm KT, et al. Multifocal Primary Prostate Cancer Exhibits High Degree of Genomic Heterogeneity. European urology 2019;75:498–505 [DOI] [PubMed] [Google Scholar]
- 101.Lu Z, Williamson SR, Carskadon S, Arachchige PD, Dhamdhere G, Schultz DS, et al. Clonal evaluation of early onset prostate cancer by expression profiling of ERG, SPINK1, ETV1, and ETV4 on whole-mount radical prostatectomy tissue. The Prostate 2020;80:38–50 [DOI] [PubMed] [Google Scholar]
- 102.Mehta N, Maddineni S, Mathews II, Andres Parra Sperberg R, Huang PS, Cochran JR. Structure and Functional Binding Epitope of V-domain Ig Suppressor of T Cell Activation. Cell reports 2019;28:2509–16 e5 [DOI] [PubMed] [Google Scholar]
- 103.Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 2019;574:565–70 [DOI] [PubMed] [Google Scholar]
- 104.ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, et al. VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science 2020;367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slater BT, Han X, Chen L, Xiong Y. Structural insight into T cell coinhibition by PD-1H (VISTA). Proceedings of the National Academy of Sciences of the United States of America 2020;117:1648–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. The American journal of pathology 2012;181:1504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li F, Glinskii OV, Mooney BP, Rittenhouse-Olson K, Pienta KJ, Glinsky VV. Cell surface Thomsen-Friedenreich proteome profiling of metastatic prostate cancer cells reveals potential link with cancer stem cell-like phenotype. Oncotarget 2017;8:98598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzman-Ramirez N, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer research 2010;70:5163–73 [DOI] [PubMed] [Google Scholar]
- 109.Codd AS, Kanaseki T, Torigo T, Tabi Z. Cancer stem cells as targets for immunotherapy. Immunology 2018;153:304–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21:4286–93 [DOI] [PubMed] [Google Scholar]
- 111.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20:1747–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:2780–8 [DOI] [PubMed] [Google Scholar]
- 113.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231–5 [DOI] [PubMed] [Google Scholar]
- 114.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2−/−IL2Rgammanull Immunodeficient Mice. Cancer research 2015;75:3466–78 [DOI] [PubMed] [Google Scholar]
- 115.Cha HR, Lee JH, Hensel JA, Sawant AB, Davis BH, Lee CM, et al. Prostate cancer-derived cathelicidin-related antimicrobial peptide facilitates macrophage differentiation and polarization of immature myeloid progenitors to protumorigenic macrophages. The Prostate 2016;76:624–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, Siegal GP, et al. LL-37 as a therapeutic target for late stage prostate cancer. The Prostate 2011;71:659–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang-Gillam AORE, Bendell JC, Wainberg ZA, Borazanci EH, Bahary N. A randomized phase II study of cabiralizumab (cabira) + nivolumab (nivo) ± chemotherapy (chemo) in advanced pancreatic ductal adenocarcinoma (PDAC). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:465 [Google Scholar]
- 118.Lee JH, Isayeva T, Larson MR, Sawant A, Cha HR, Chanda D, et al. Endostatin: A novel inhibitor of androgen receptor function in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America 2015;112:1392–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee JH, Kang M, Wang H, Naik G, Mobley JA, Sonpavde G, et al. Endostatin inhibits androgen-independent prostate cancer growth by suppressing nuclear receptor-mediated oxidative stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2017;31:1608–19 [DOI] [PMC free article] [PubMed] [Google Scholar]