Abstract
Objective
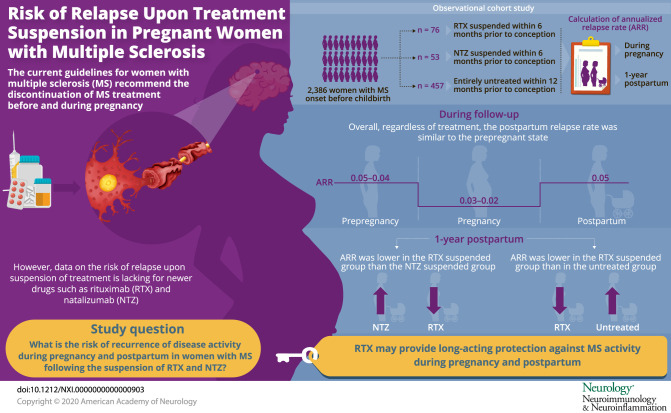
To evaluate risks of disease reactivity during pregnancy and postpartum following rituximab (RTX) and natalizumab (NTZ) suspension in women with MS.
Methods
An observational cohort study of all women with MS disease onset before childbirth between 2006 and 2017. Women were identified through the Swedish MS Registry, a nationwide clinical register, with substratification into 3 groups: women who suspended RTX and NTZ within 6 months before conception and women who were not treated with any disease-modifying treatment (DMT) within 1 year of conception. The primary outcome was the annualized relapse rate (ARR) during pregnancy and 1 year postpartum.
Results
We identified 2,386 women with MS onset before a live birth; of these, 76 women suspended RTX and 53 suspended NTZ, and 457 were untreated within 1 year before conception. In all women, regardless of the treatment type, the ARR declined from 0.05–0.04 prepregnancy to 0.03–0.02 during pregnancy, returning to prepregnancy rates at 3–6 months (0.05) postpartum. In the suspended cohort, 76% (98/129) of women resumed a DMT after delivery. The relapse rate 1 year postpartum was significantly higher in the suspended NTZ women compared with the suspended RTX women (adjusted rate ratio [aRR] 7.65, 95% CI 2.47–23.6) and was lower in the suspended RTX women compared with the untreated women (aRR 0.21, 95% CI 0.08–0.61).
Conclusion
Disease reactivity during the postpartum period was lower among women with MS who suspended RTX before pregnancy, relative to those who suspended NTZ and untreated women. These findings suggest that RTX may exert long-acting effects on MS disease activity that encompass pregnancy and postpartum periods.
Classification of evidence
This study provides Class IV evidence that in patients with MS who were on treatment before pregnancy, RTX reduces clinical disease activity compared with NTZ in the postpartum period.
MS predominantly affects women, and the clinical onset of MS most often occurs during child-bearing age. Until just a few decades ago, women with MS were often advised to avoid pregnancy. In parallel with the introduction of more effective disease-modifying treatments (DMTs), earlier start of therapy due to revised diagnostic criteria using MRI (MRI),1 and accumulated body of knowledge demonstrating no adverse effects in newborns of mothers with MS, pregnancy rates have been increasing in women with MS.2 Still, the use of DMTs in pregnancy remains controversial, and the current guidelines are to discontinue DMTs before and during pregnancy unless the risk of disease worsening outweighs the risk to the fetus.3 However, in practice, this is difficult to implement, given that comparative risk-benefit information concerning pregnancy for newer DMTs is lacking.
Earlier clinical observations4–8 suggest that the frequency of MS relapses decreases during pregnancy, particularly during the third trimester, relative to prepregnancy rates. In contrast, the relapse rate significantly increases in the first 3 months postpartum relative to prepregnancy rates, particularly in those who did not resume DMTs.9 Nevertheless, this appears to average out such that over the whole pre- to post-pregnancy period, the risk of a relapse is similar to the nonpregnant state.10
The introduction of high-efficacy DMTs such as natalizumab (NTZ) represents a major clinical challenge in women of child-bearing age, given that heightened risk of disease reactivity during and after pregnancy has been reported after withdrawal of NTZ.11–13 Rituximab (RTX), a chimeric monoclonal B cell–depleting anti-CD20 antibody, is frequently used off-label for the treatment of MS in Sweden.14,15 Given the extended biological effects of RTX, preliminary analyses have shown that administration of RTX before conception may potentially diminish the risk of disease reactivity, without major adverse effects during pregnancy.16 Nevertheless, contemporary data are limited for RTX, with only a handful of small studies having examined disease activity in women with MS who suspended RTX before conception.16 Therefore, the objective of our study was to evaluate the risk of disease reactivation during pregnancy and postpartum following RTX and NTZ suspension in women with MS.
Methods
Data sources
Using the person-unique national registration numbers assigned to each Swedish resident, data from the Swedish MS register (SMSreg) were linked to the Swedish Medical Birth Register.17 The SMSreg was established in 2000 to capture clinical data on patients with MS. It covers approximately 80% of all patients with MS in Sweden, and all clinical data are recorded by physicians or nurses through an electronic interface.18 Data include demographic information and MS-specific clinical information such as DMT exposure (dosage and start and stop date), Expanded Disability Status Scale [EDSS] score), relapses, and MRI results. A large-scale national validation study was recently completed, which demonstrated a very high accuracy for DMT use and moderate underreporting of relapses (i.e., sensitivity just below 80% and specificity above 99%) while MRI data were mainly underreported (71% added).19 The Medical Birth Register includes information on nearly all births in Sweden since 1973.17 Using standardized prenatal, obstetrical, and neonatal records, information is prospectively collected during pregnancy, delivery, and the neonatal period.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Research Ethics Committee at Karolinska Institutet, Stockholm, Sweden (No. 2017/700-31/4).
Study population
All women with MS disease onset before a live birth from January 1, 2006, to December 12, 2017, were identified through the SMSreg and the Medical Birth Register. Inclusion criteria were suspended RTX or NTZ within 6 months before conception or not treated with any DMT within 1 year of conception and continuous follow-up 1 year before conception and 1 year after the live birth. In the suspended cohort, only 1 pregnancy per woman was included, and for the untreated group, we included only the first live birth. Women who were treated with an MS DMT during pregnancy were excluded from the study. The date of conception was obtained by adding 2 weeks to the estimated first day of the last menstrual cycle, which was determined by subtracting the gestational age.
Study covariates and outcomes
Maternal age was calculated in completed years. Clinical characteristics evaluated were disease duration (from onset of MS to the child's birthdate); MS clinical course (relapsing-remitting, secondary progressive, and primary progressive); EDSS score within 6 months of conception; and the presence of gadolinium-enhancing (Gd+) lesions occurring within 12 months of conception. Relapses were recorded by the treating neurologist and defined as new or recurrent neurologic symptoms not associated with fever or infection that lasted for at least 24 hours and were accompanied by new neurologic signs. Annualized relapse rates (ARRs) for each trimester in the year before pregnancy, during pregnancy, and in the year after delivery were examined. The ARR was calculated by dividing the number of relapses for each woman by the total number of women in each treatment group for each time interval. MRI scans were performed according to standard follow-up guidelines and MRI protocols,20 using 1.5-T or 3-T MRI scanners (GE Healthcare, Siemens Healthcare, and Philips Medical Systems). The primary outcome was the relapse rate during pregnancy and in the year after delivery, and the secondary outcome was postpartum Gd+ lesions on the first MRI within 1 year after delivery.
Study analyses
The primary purpose of analyses was to describe the risk of disease reactivation during pregnancy and postpartum following RTX and NTZ suspension in women with MS. Categorical variables were described as frequencies (%), and continuous variables were described using mean (SD) or median (interquartile range). Missing data were reported but not imputed. The ARR before, during (in each trimester), and after pregnancy was examined in all women regardless of the treatment type and then by the DMT type (RTX and NTZ women compared with untreated women). The association between the treatment type and the relapse rate during pregnancy and 1 year postpartum was examined using negative binomial regressions for each time period, adjusting for maternal age at delivery and relapse (any vs none) in the year before pregnancy. Confounders were included in the final models based on the literature11 or statistical significance (p value <0.10). There was no evidence of overdispersion as assessed by the deviance statistic divided by its degrees of freedom. We reported rate ratios (RRs) and 95% CIs for the associations. Last, the relative odds of experiencing Gd+ lesions during the 12-month postpartum period were assessed using logistic regression models, adjusting for maternal age and relapse in the year before pregnancy. We also conducted post hoc analysis for the suspended NTZ cohort to examine the association between the timing of treatment initiation after delivery and the rate of postpartum relapse. This analysis could not be performed for RTX, as there were only 4 relapses postpartum in this group. Data were analyzed with the use of SAS software, version 9.4 (SAS Institute). Two-sided p values of less than 0.05 were considered to indicate statistical significance.
Data availability
Data related to the current article are available from Fredrik Piehl, Karolinska Institutet. To be able to share data from the Swedish MS Registry, a data transfer agreement needs to be completed between Karolinska Institutet and the institution requesting data access. This is in accordance with the data protection legislation in Europe (General Data Protection Regulation). Persons interested in obtaining access to the data should contact Thomas Frisell (Thomas.Frisell@ki.se).
Results
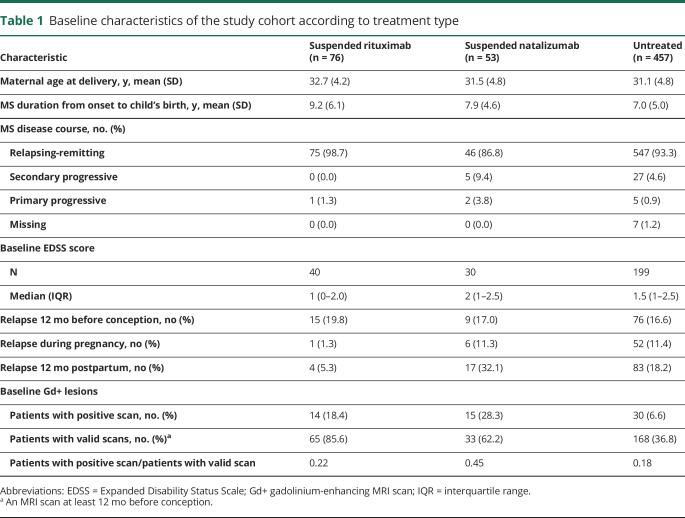
Between January 1, 2006, and December 12, 2017, we identified 2,386 women with MS disease onset before a live birth. Among those, there were 76 women who suspended RTX and 53 who suspended NTZ within 6 months before conception and were compared with 457 nulliparous women untreated within 1 year before conception. Table 1 shows the main demographic and clinical characteristics of the study cohort. Compared with the untreated women, women who suspended RTX and NTZ had longer disease duration, whereas the suspended NTZ women had a slightly higher baseline EDSS score, on average. Suspended NTZ women had a higher relapse activity in the year preceding pregnancy and during and after pregnancy compared with the suspended RTX women and the untreated women. The proportion of women with Gd+ on MRI within 12 months before conception was higher for the suspended NTZ women (28.3%) compared with both the suspended RTX (18.4%) and the untreated women (6.6%).
Table 1.
Baseline characteristics of the study cohort according to treatment type
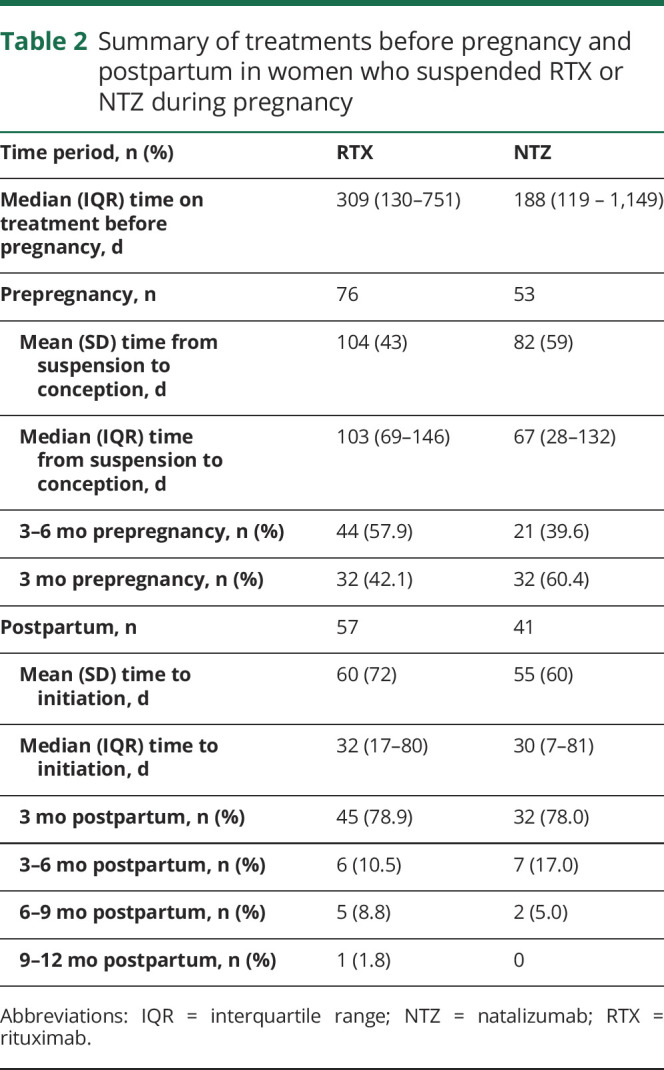
In the suspended cohort, 76% of women resumed a DMT within 1 year after delivery (57 RTX, 41 NTZ). For women initiating a DMT within 1 year, the mean (SD) time from live birth to first RTX treatment was 60 (72) days, and for NTZ, it was 55 (60) days (table 2). Approximately 78% of women initiated NTZ and RTX, within the first 3 months after birth. In the untreated group, 33% (151/457) initiated treatment after pregnancy, and the mean (SD) time from birth to treatment initiation was 170 (106) days.
Table 2.
Summary of treatments before pregnancy and postpartum in women who suspended RTX or NTZ during pregnancy
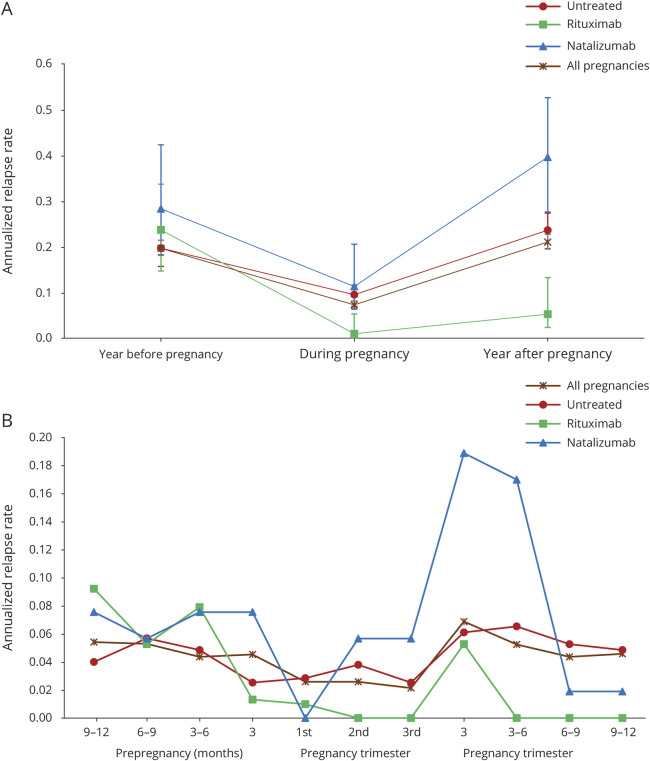
The frequency of relapse 1 year postpartum was 5.3% in the suspended RTX, 32.1% in the suspended NTZ, and 18.2% in the untreated women. During the 1-year follow-up period postpartum, 82.3% (482/586) of women experienced no relapse. Of those with a relapse over the 1-year follow-up, most had 1 (80/104 [77%]), and the remaining had 2 (20/104 [19%]) or ≥3 (4/145 [4%]) relapses. The ARR in the year before conception, during pregnancy, and after delivery in RTX, NTZ, and untreated women is illustrated in the figure. The ARR for all women, regardless of treatment, declined from 0.05–0.04 prepregnancy to 0.03–0.02 during pregnancy, returning to prepregnancy rates at 3–6 months (0.05) postpartum (figure). Although in the suspended NTZ women, the expected temporal profile of disease activity (decrease of relapse during pregnancy and increase of relapse rate after delivery) was observed, in the suspended RTX women, the relapse rate postpartum was lower than the prepregnancy rate (figure, A). Compared with the suspended NTZ women, the ARR in the prepregnancy period was lower in the suspended RTX women, remained lower during pregnancy, and ended at zero during the last 3 postpartum quarters (with a peak in the first trimester [figure, B]).
Figure. Annualized relapse rate in the year before, during, and after pregnancy in rituximab, natalizumab, and untreated women (A) overall and (B) in each trimester.
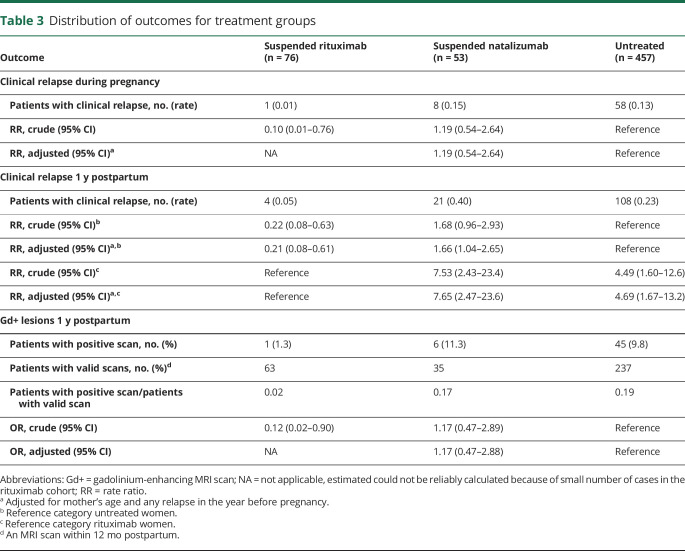
The relapse rate 1 year postpartum was significantly higher in the suspended NTZ women compared with the suspended RTX women, when adjusting for baseline covariates (adjusted RR 7.65, 95% CI 2.47–23.6; table 3) and was also higher in the suspended NTZ women compared with the untreated women (adjusted RR 1.66, 95% CI 1.04–2.65). Among the suspended NTZ women, the ARR was 67% lower in those who resumed treatment within the first 3 months postpartum (0.30) compared with those who did not initiate treatment in the first 3 months postpartum (0.60, RR 0.33, 95% CI 0.13–0.81).
Table 3.
Distribution of outcomes for treatment groups
MRI scans performed demonstrated 52 women with Gd+ lesions within 12 months postpartum, 1 (1.3%) in the RTX group, 6 (11.3%) in the NTZ group, and 45 (9.8%) in the untreated women (table 3). The mean time to the first MRI scan postpartum was similar in the RTX and NTZ groups (82 days vs 97 days, respectively) and slightly longer in the untreated group (120 days). The proportions of patients with positive scans among patients with valid scans were 0.02, 0.17, and 0.19 for RTX, NTZ, and the untreated women, respectively. In the logistic regression, compared with the untreated women, the odds of having Gd+ lesions postpartum were not significantly higher in the suspended NTZ women (adjusted OR, 1.17, 95% CI 0.47–2.88; table 3).
Discussion
In this observational study, we observed that, overall, women with MS did not show an increase in disease reactivity during the postpartum period, as the relapse rates remained the same as prepregnancy rates regardless of treatment type. Nevertheless, women with MS who suspended RTX within 6 months of conception had significantly fewer clinical relapses and enhancing lesions relative to the suspended NTZ and the untreated women. Last, in the suspended NTZ women, early initiation of DMT within 3 months after birth reduced the risk of relapse postpartum.
In our study, the postpartum relapse rate was similar to the prepregnant state, which is in contrast to the majority of previous research, demonstrating a significant rebound disease activity in the early postpartum period.10,11,21 However, 1 recent study also reported an absence of increased disease activity in the postpartum period, with the ARR returning to prepregnancy rates at 4–6 months.22 The relatively low ARR and the lack of rebound disease activity in the early postpartum period in our cohort are likely due to the inclusion of women from a population-based setting with milder disease activity and the widespread use of high-efficacy therapies in Sweden.
In Sweden, RTX has been used as a therapeutic alternative and is now the most frequent DMT prescribed nationwide.14 Overall, treatment choice (i.e., RTX vs NTX) is primarily based on patient and physician preference. However, RTX has shown to be efficacious in suppressing neuroradiologic disease activity for relapsing-remitting MS and reducing the disability progression rate in young patients and even in those with active primary progressive MS.23,24 Our findings revealed that women who suspended RTX before conception had significantly lower risk of disease reactivity during pregnancy and the postpartum period than those who suspended NTZ. In the suspended RTX women, only 1 maternal relapse occurred during pregnancy and among the 4 cases of relapse that occurred in the first quarter after delivery, only one experienced new Gd+ lesions. These findings suggest that the new era of immune reconstitution therapy, including B cell–depleting treatments such as RTX, exerts prolonged protective effects on MS disease activity that can encompass the pregnancy and postpartum periods. In planned pregnancies, RTX could be given before conception and after delivery, which will help reduce the chance of neonatal B-cell depletion, given that the active transport of RTX across the placenta begins after 20 weeks' gestation.25 Thus, our study provides evidence and supports that RTX may be the preferred choice of therapy for women with MS considering pregnancy. A few studies provide further assurance, as they concluded that there have been no major safety concerns associated with RTX use during pregnancy16,26; however, these were based on a small number of women, and future studies focused on safety, such as risk of malformation or stillbirth, should be conducted to confirm findings.
In line with other finding,11,21,27 our results further demonstrated that disease reactivity is expected among the suspended NTZ women, and early resumption of NTZ infusion after delivery (within 3 months) could lower the frequency of postpartum relapses. For women on NTZ with high disease activity, proactive clinical considerations could be either a) to switch to anti–B-cell therapy, which protects against rebound of disease activity associated with NTZ withdrawal,28 or b) shorten the washout period, continue NTZ until conception and even into pregnancy, or c) resume treatment early postpartum. The latter approach requires a discussion with the mother regarding the benefits of resuming DMT vs the risks of relapse with breastfeeding.
This study represents one of the largest population-based studies to date examining disease activity in women treated with RTX during the pregnancy/postpartum period. In Sweden, all citizens have access to universal, publicly funded health care, and all DMTs are covered by the national health insurance, including off-label medications, suggesting that there was limited bias in terms of access to treatment. Limitations of the present study include the observational design, which is inherently sensitive to confounding by indication, as all factors affecting choice of therapy cannot be accounted for. In this study, there were differences in prepregnancy disease activity levels between the suspended and untreated groups, with the untreated cohort displaying milder activity levels. Guidelines for follow-up differ to some degree between different DMTs. Thus, until 2014, 2 yearly visits were recommended for RTX, and NTZ, whereas 1 visit per year was recommended for injectable DMTs, which may have affected the sensitivity to detect adverse outcomes in the untreated cohort. Nevertheless, since 2015, guidelines regarding follow-up have been identical across all therapies. Expanding Disability Status Scale ratings were not included as an outcome, as ratings are less reliable when performed in clinical routine, and the relatively limited follow-up time diminished the possibility to analyze long-term disability outcomes. The vast majority of patients in the Swedish MS Registry are of Northern European descent, and therefore, these findings are mostly generalizable to this group of patients. Last, we did not have information on lactation status that could potentially influence therapy resumption after delivery.
In conclusion, this observational study of women of child-bearing age indicates a superior effectiveness of RTX compared with NTZ in mitigating disease activity during pregnancy and postpartum. In the absence of a formal randomized clinical trial, RTX appears to be the superior choice, compared with NTZ, for women considering pregnancy. Further studies are needed to shed light on the safety of RTX exposures during pregnancy and a potential adverse effect on the developing fetus.
Glossary
- ARR
annualized relapse rate
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- Gd+
gadolinium-enhancing
- NTZ
natalizumab
- RR
rate ratio
- RTX
rituximab
- SMSreg
Swedish MS register
Appendix. Author
Study funding
Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (MS-1511–33196).
Disclosure
N. Razaz receives funding from the Swedish Research Council for Health, Working Life and Welfare (No. 4-2702/2019). F. Piehl has received research grants from Genzyme, Merck KGaA, and Novartis and fees for serving as Chair of DMC in clinical trials with Parexel. T. Frisell reports no disclosures relevant to the manuscript. K.A. McKay receives funding from the Canadian Institutes of Health Research and the European Committee for Treatment and Research in Multiple Sclerosis. K. Fink served on scientific advisory boards for Merck, Roche, and Johnson & Johnson. Go to Neurology.org/NN for full disclosures.
References
- 1.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houtchens MK, Edwards NC, Schneider G, Stern K, Phillips AL. Pregnancy rates and outcomes in women with and without MS in the United States. Neurology 2018;91:e1559–e1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bove R, Alwan S, Friedman JM, et al. Management of multiple sclerosis during pregnancy and the reproductive years: a systematic review. Obstet Gynecol 2014;124:1157–1168. [DOI] [PubMed] [Google Scholar]
- 4.Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol 1990;47:738–742. [DOI] [PubMed] [Google Scholar]
- 5.Bernardi S, Grasso MG, Bertollini R, Orzi F, Fieschi C. The influence of pregnancy on relapses in multiple sclerosis: a cohort study. Acta Neurol Scand 1991;84:403–406. [DOI] [PubMed] [Google Scholar]
- 6.Sadovnick AD, Eisen K, Hashimoto SA, et al. Pregnancy and multiple sclerosis. A prospective study. Arch Neurol 1994;51:1120–1124. [DOI] [PubMed] [Google Scholar]
- 7.Worthington J, Jones R, Crawford M, Forti A. Pregnancy and multiple sclerosis—a 3-year prospective study. J Neurol 1994;241:228–233. [DOI] [PubMed] [Google Scholar]
- 8.Roullet E, Verdier-Taillefer MH, Amarenco P, Gharbi G, Alperovitch A, Marteau R. Pregnancy and multiple sclerosis: a longitudinal study of 125 remittent patients. J Neurol Neurosurg Psychiatry 1993;56:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 1998;339:285–291. [DOI] [PubMed] [Google Scholar]
- 10.Houtchens MK, Edwards NC, Phillips AL. Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology 2018;91:e1570–e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: fetal risks. Neurology 2018;90:e823–e831. [DOI] [PubMed] [Google Scholar]
- 12.Verhaeghe A, Deryck OM, Vanopdenbosch LJ. Pseudotumoral rebound of multiple sclerosis in a pregnant patient after stopping natalizumab. Mult Scler Relat Disord 2014;3:279–281. [DOI] [PubMed] [Google Scholar]
- 13.Rasenack M, Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert Rev Neurother 2016;16:587–594. [DOI] [PubMed] [Google Scholar]
- 14.Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016;87:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple SclerosisRituximab and other initial treatment choices for multiple SclerosisRituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 2018;75:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das G, Damotte V, Gelfand JM, et al. Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurology-Neuroimmunology Neuroinflammation 2018;5:e453 doi: 10.1212/NXI.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swedish National Board of Health and Welfare. The Swedish Medical Birth Register: a summary of content and quality. 2003. Available at: wwwsocialstyrelsense/Lists/Artikelkatalog/Attachments/10655/2003-112-3_20031123pdf. Accessed February 15, 2016. [Google Scholar]
- 18.Hillert J, Stawiarz L. The Swedish MS registry–clinical support tool and scientific resource. Acta Neurol Scand 2015;132:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alping P, Piehl F, Langer-Gould A, Frisell T. Validation of the Swedish multiple sclerosis register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology 2019;30:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vågberg M, Axelsson M, Birgander R, et al. Guidelines for the use of magnetic resonance imaging in diagnosing and monitoring the treatment of multiple sclerosis: recommendations of the Swedish Multiple Sclerosis Association and the Swedish Neuroradiological Society. Acta Neurol Scand 2017;135:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology 2018;90:e840–e846. [DOI] [PubMed] [Google Scholar]
- 22.Langer-Gould A, Smith JB, Albers KB, et al. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology 2020;94:e1939–e1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 24.Boremalm M, Juto A, Axelsson M, et al. Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol 2019;26:1060–1067. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011;117:1499–1506. [DOI] [PubMed] [Google Scholar]
- 26.Smith JB, Hellwig K, Fink K, Lyell DJ, Piehl F, Langer-Gould A. Rituximab, MS, and pregnancy. Neurol Neuroimmunol Neuroinflamm 2020;7:e734 doi: 10.1212/NXI.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli V, Colombo B, Dalla Costa G, et al. Recurrent disease-activity rebound in a patient with multiple sclerosis after natalizumab discontinuations for pregnancy planning. Mult Scler 2016;22:1506–1508. [DOI] [PubMed] [Google Scholar]
- 28.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 2016;79:950–958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to the current article are available from Fredrik Piehl, Karolinska Institutet. To be able to share data from the Swedish MS Registry, a data transfer agreement needs to be completed between Karolinska Institutet and the institution requesting data access. This is in accordance with the data protection legislation in Europe (General Data Protection Regulation). Persons interested in obtaining access to the data should contact Thomas Frisell (Thomas.Frisell@ki.se).