Supplemental Digital Content is available in the text.
Key Words: opioid use disorder, comparative cost analysis, treatment
Abstract
Background:
Relative costs of care among treatment options for opioid use disorder (OUD) are unknown.
Methods:
We identified a cohort of 40,885 individuals with a new diagnosis of OUD in a large national de-identified claims database covering commercially insured and Medicare Advantage enrollees. We assigned individuals to 1 of 6 mutually exclusive initial treatment pathways: (1) Inpatient Detox/Rehabilitation Treatment Center; (2) Behavioral Health Intensive, intensive outpatient or Partial Hospitalization Services; (3) Methadone or Buprenorphine; (4) Naltrexone; (5) Behavioral Health Outpatient Services, or; (6) No Treatment. We assessed total costs of care in the initial 90 day treatment period for each strategy using a differences in differences approach controlling for baseline costs.
Results:
Within 90 days of diagnosis, 94.8% of individuals received treatment, with the initial treatments being: 15.8% for Inpatient Detox/Rehabilitation Treatment Center, 4.8% for Behavioral Health Intensive, Intensive Outpatient or Partial Hospitalization Services, 12.5% for buprenorphine/methadone, 2.4% for naltrexone, and 59.3% for Behavioral Health Outpatient Services. Average unadjusted costs increased from $3250 per member per month (SD $7846) at baseline to $5047 per member per month (SD $11,856) in the 90 day follow-up period. Compared with no treatment, initial 90 day costs were lower for buprenorphine/methadone [Adjusted Difference in Differences Cost Ratio (ADIDCR) 0.65; 95% confidence interval (CI), 0.52–0.80], naltrexone (ADIDCR 0.53; 95% CI, 0.42–0.67), and behavioral health outpatient (ADIDCR 0.54; 95% CI, 0.44–0.66). Costs were higher for inpatient detox (ADIDCR 2.30; 95% CI, 1.88–2.83).
Conclusion:
Improving health system capacity and insurance coverage and incentives for outpatient management of OUD may reduce health care costs.
Opioid overdose deaths continue to surge in the United States, reaching nearly 50,000 fatalities in 2017.1 Although an understandable public health focus, the burden of opioid-related harms extends beyond these lives lost. The economic burden of opioid use disorder (OUD) from prescription opioids alone, exclusive of illicit opioids, was estimated to be $78.5 billion in 2013 due to a combination of direct health care, substance use treatment, and criminal justice costs, as well as lost productivity. In this study, annual health care insurance expenditures were ∼15 thousand dollars higher for individuals with OUD compared with controls matched for demographic characteristics and clinical comorbidities.2 One driver of OUD-related costs is acute care utilization, including medical complications of drug use such as injection-related infections. In 2012, there were >0.5 million inpatient hospitalizations for OUD accruing nearly $15 billion in charges—$700 million related to associated infections.3 The number of individuals with OUD is also growing, a recent analysis of Massachusetts public health data estimates the prevalence of OUD increased from 2.7% in 2011 to 4.6% in 2015.4
Despite these dire statistics, the availability of highly effective treatments for OUD are a reason for optimism. In particular, medications for opioid use disorder (MOUD), including methadone, buprenorphine, and naltrexone, have been shown to be effective in improving OUD outcomes.5–7 Observational studies have demonstrated that methadone and buprenorphine reduce mortality.8,9 The Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain and American Society of Addiction Medicine (ASAM) recommend buprenorphine or methadone for individuals with OUD.10,11 Psychosocial interventions are available in a range of settings including outpatient counseling, intensive outpatient (IOP) or partial hospitalization programs, or intensive inpatient detoxification or rehabilitation. Given increasing expense and limited capacity with inpatient settings, guidelines have been developed by ASAM to match patients to the least intensive setting needed based on patient presentation across several domains.12 Mismatch of patients’ care setting with ASAM criteria recommendations is associated with increased hospital utilization.13
Unfortunately, only 22% of individuals with OUD reported receiving any form of treatment in the United States from 2009 to 2013. On average individuals accessed treatment across >3 settings; self-help groups, outpatient, and inpatient settings were accessed by more than half of individuals.14 Health insurance coverage is key to OUD treatment access as evidenced by gains in OUD treatment following coverage expansion under the Affordable Care Act.15,16 However, coverage for OUD treatment varies and many plans limit coverage through mechanisms such as preauthorization or annual maximums.17
The relative cost of various OUD treatment pathways is unknown. These data are necessary to inform rational benefit design and development of provider networks and useful for policymakers, health plans, and health systems increasingly engaging in shared risk contracts. The goal of this analysis was to use claims data from a large national insurer to examine total costs of care among 6 common initial treatment pathways for individuals diagnosed with OUD: inpatient detox/rehabilitation (ASAM Levels 3 or 4); IOP or partial hospitalization services (ASAM Level 2); behavioral health outpatient services (ASAM Level 1); methadone or buprenorphine; naltrexone; and, no treatment. We examined whether total costs of care for each treatment group were different from no treatment. We defined total costs of care as inclusive of OUD-related and non–OUD-related services, and health plan and out-of-pocket costs.
METHODS
Study Design and Data Source
We conducted a retrospective cohort study using OptumLabs Data Warehouse (OLDW) data from January 2015 to December 2017. The OLDW contains de-identified, longitudinal medical, behavioral health, and pharmacy claims on commercial and Medicare Advantage enrollees from across the United States.18 Data from the OLDW has been determined to be de-identified and is thus considered not human subjects research and not subject to institutional review board review.
Cohort Selection
We included individuals aged 16 years and above with an incident diagnosis of OUD. As International Classification of Diseases diagnosis codes for opioid dependence, abuse, and misuse do not map directly to a Diagnostic and Statistical Manual of Mental Disorders-5 diagnosis of OUD, we developed the following algorithm to identify OUD. OUD was defined as meeting 1 of 2 criteria: (i) ≥1 inpatient opioid dependence claim or ≥2 outpatient opioid dependence claims that occurred within 90 days of each other not occurring during an episode of long-term opioid prescribing; or (ii) ≥1 opioid dependence, abuse or use claim plus a confirmatory diagnosis or event within a 90 day window. We excluded individuals with a diagnosis of opioid dependence during long-term opioid therapy to avoid capturing individuals with physiological dependence but not OUD. Confirmatory diagnoses or events included opioid overdose, hepatitis C, potentially injection-related infection, receipt of medication for OUD, or an inpatient opioid-related detoxification or rehabilitation stay. Hepatitis C, and bacterial infections such as endocarditis or abscess are often complications of injection drug use.19 A detailed description of the algorithm and codes used is included in Appendix A (Supplemental Digital Content 1, http://links.lww.com/MLR/C61).
We defined the OUD diagnosis date as the first opioid-related claim or event meeting above criteria. We defined the index date as the first date of OUD treatment (defined below) in the 90 days after OUD diagnosis. For individuals without OUD treatment within 90 days of OUD diagnosis, we randomly assigned an index date based on the distribution of index dates in those treated. We excluded individuals without 90 days of continuous enrollment before and after the index date to ensure ample observation time for identifying incident OUD, baseline characteristics, and to observe exposures and outcomes. To identify incident OUD, we required a 90 day washout period without evidence of OUD or receipt of MOUD or an inpatient opioid-related detoxification or rehabilitation stay. We also excluded individuals with diagnosis or treatment of cancer in the 90 days before index date (Appendix D, Supplemental Digital Content 1, http://links.lww.com/MLR/C61) and individuals who received a combined 90 days of Long-term Care from 90 days before to 90 days after the index date (Appendix E, Supplemental Digital Content 1, http://links.lww.com/MLR/C61). Finally, we excluded individuals without valid data for sex or age. A schematic of key study dates and covariate and exclusion assessment windows is included in Figure 1.
FIGURE 1.
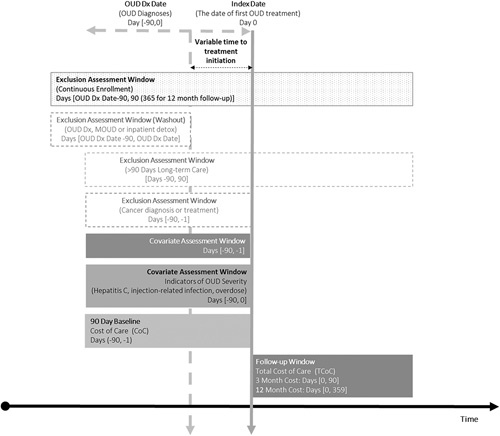
Graphical depiction of study design, including key study dates and exclusion and covariate assessment windows. DX indicates diagnosis; MOUD, medications for opioid use disorder; OUD, opioid use disorder.
Key Variables
The exposure of interest was initial treatment pathway. We assigned all individuals to 1 of 6 mutually exclusive initial treatment pathways (Appendices B and C, Supplemental Digital Content 1, http://links.lww.com/MLR/C61):
Inpatient Detox/Rehabilitation Treatment Center (RTC): corresponding to ASAM Levels 3 or 4.
Behavioral Health Intensive, IOP or Partial Hospitalization Services corresponding to ASAM Level 2.
Methadone or buprenorphine.
Naltrexone.
Behavioral Health Outpatient Services, corresponding to ASAM Level 1.
No treatment.
If individuals received treatment in categories 1–4 in the first 90 days following OUD diagnosis, we assigned individuals to the first of those treatments received. Individuals who received no treatment within 90 days were assigned to the no treatment pathway. We combined treatment with buprenorphine or methadone into a single pathway due to the small number of enrollees with methadone, recognizing they are both opioid agonists with similar treatment outcomes.7–9 If there was more than 1 treatment initiated on the same day we applied the following hierarchy: (a) Inpatient Detox/RTC; (b) Behavioral Health Intensive, IOP or Partial Hospitalization Services; (c) buprenorphine/methadone; (d) naltrexone; and (e) Behavioral Health Outpatient Services.
The outcome was total costs of care, comprehensive of OUD and non–OUD-related care, including both health plan and enrollee expenditures. We included adjudicated claims inclusive of medical, surgical, behavioral health, laboratory, durable medical equipment, and pharmacy claims in all inpatient and outpatient care settings. We excluded claims with negative cost values.
We examined participant characteristics, including age, sex, and race/ethnicity. We identified comorbidities in the 90 day baseline period before initial OUD diagnosis using a modified version of the Elixhauser index, excluding mental health and substance use subcomponents that were characterized separately (Appendix F, Supplemental Digital Content 1, http://links.lww.com/MLR/C61).20 Compared with other routes of administration such as oral or inhalation, injection drug use is associated with increasing OUD severity and worse outcomes, including overdose and infectious complications such as abscess, endocarditis, or hepatitis.19,21,22 As proxies of OUD severity, we identified individuals with a diagnosis for an injection-related infection, hepatitis C, or overdose on or in the 90 days before the index date (Appendix A2, Supplemental Digital Content 1, http://links.lww.com/MLR/C61).
Analyses
We assessed total costs of care over the 90 day periods before and after the index date. We modeled total costs of care using generalized linear models with a gamma distribution, and log link. We used a generalized estimating equation to account for within-subject correlation of cost before and after treatment initiation. We retained patients with zero total costs in all models. We report costs as per member per month (PMPM; month=30 d) in the 90 day baseline and follow-up periods as well as the ratio of follow-up to baseline costs for each treatment group. For descriptive purposes, we report the breakdown of health plan and out-of-pocket costs in the 90 day follow-up period. We used a difference-in-differences approach to compare these cost ratios for each treatment group, using no treatment as the reference group.23 In addition to providing an adjustment for baseline cost, difference in differences has the advantage of controlling for unobserved confounders that remain fixed over time. We estimated both empirical and robust SEs, which did not differ; empirical SEs are reported here. We examined the assumption of parallel trends in the baseline period by comparing costs among treatment pathways from days −90 to −46 to days −45 to −1 before the index date. We used a t test assuming unequal variances to compare trends between the no treatment group and active treatment groups in aggregate.
We examined costs of care for sequential 3 month periods out to 12 months. For these sequential periods, analyses were limited to individuals with continuous enrollment through the respective time period. We compared baseline characteristics of censored and retained individuals. To assess the impact of censoring, we conducted a sensitivity analysis calculating PMPM costs for the full cohort censoring follow-up at disenrollment, adjusting for inverse probability weights for the risk of censoring.24 To assess the impact of buprenorphine or methadone treatment duration, we examined mean total costs of care for individuals with continuous enrollment for 12 months following the index date that received buprenorphine or methadone for 0, 1–30, 31–180, or >180 days.
RESULTS
Of 74,895 individuals meeting inclusion criteria for having OUD during calendar years 2015–2017, 44,693 met the criteria for 3 months of continuous enrollment before and after the index date. We excluded 2044 individuals who did not have a 90 day washout period without evidence of prevalent OUD or treatment with MOUD or detox before OUD diagnosis. We also excluded 1526 with evidence of cancer, 36 individuals with 90 or more days of long-term care, 44 individuals without known age, sex, or commercial or Medicare Advantage insurance, and 158 individuals under age 16 years. The final cohort was 40,885 members. The cohort was 54% male, 74% White, 58% had commercial insurance, and 45% had a comorbid mental health diagnosis.
Within 90 days of diagnosis, 94.8% of individuals received some form of treatment for OUD. The distribution among initial treatments was: 15.8% for Inpatient Detox/RTC, 4.8% for Behavioral Health Intensive, IOP or Partial Hospitalization Services, 12.5% for buprenorphine/methadone, 2.4% for naltrexone, and 59.3% for Behavioral Health Outpatient Services. Higher proportions of individuals aged 16–25 years received inpatient treatment, IOP treatment, or naltrexone. Higher proportions of 26–34 and 35–44-year-old age groups received buprenorphine or methadone (Table 1). Regardless of whether a patient was covered by commercial or Medicare Advantage insurance coverage, behavioral health outpatient treatment was the most common treatment. However, this was particularly true among the under 65 Medicare Advantage (72.6%) and 65 plus Medicare Advantage populations (84.4%) relative to the commercially insured (46.2%). Among insurance types, commercially insured individuals had highest utilization of inpatient detox/RTC (21.4%) and buprenorphine or methadone (15.4%).
TABLE 1.
Baseline Characteristics by Initial Treatment Pathway
| Variables | Overall (n) | No Treatment [n (%)] | Inpatient Detox/RTC [n (%)] | Behavioral Health Intensive Outpatient/PH [n (%)] | Buprenorphine/Methadone [n (%)] | Naltrexone [n (%)] | Behavioral Health Outpatient [n (%)] |
|---|---|---|---|---|---|---|---|
| Total sample | 40,885 | 2116 (5.2) | 6455 (15.8) | 1970 (4.8) | 5123 (12.5) | 963 (2.36) | 24,258 (59.3) |
| Age groups (y) | |||||||
| 16–25 | 5978 | 437 (7.3) | 1837 (30.7) | 948 (15.9) | 578 (9.7) | 247 (4.1) | 1931 (32.3) |
| 26–34 | 5350 | 354 (6.6) | 1124 (21.0) | 404 (7.6) | 1194 (22.3) | 197 (3.7) | 2077 (38.8) |
| 35–44 | 6070 | 332 (5.5) | 1089 (17.9) | 290 (4.8) | 1172 (19.3) | 206 (3.4) | 2981 (49.1) |
| 45–54 | 7208 | 300 (4.2) | 1059 (14.7) | 188 (2.6) | 995 (13.8) | 167 (2.3) | 4499 (62.4) |
| 54–64 | 8897 | 318 (3.6) | 983 (11.0) | 117 (1.3) | 817 (9.2) | 108 (1.2) | 6554 (73.7) |
| ≥65 | 7382 | 375 (5.1) | 363 (4.9) | 23 (0.3) | 367 (5.0) | 38 (0.5) | 6216 (84.2) |
| Sex | |||||||
| Female | 18,713 | 797 (4.3) | 2482 (13.3) | 662 (3.5) | 1971 (10.5) | 387 (2.1) | 12,414 (66.3) |
| Male | 22,172 | 1319 (5.9) | 3973 (17.9) | 1308 (5.9) | 3152 (14.2) | 576 (2.6) | 11,844 (53.4) |
| Insurance type | |||||||
| Commercial | 23,636 | 1299 (5.5) | 5062 (21.4) | 1889 (8.0) | 3630 (15.4) | 841 (3.6) | 10,915 (46.2) |
| MA<65 | 10,322 | 457 (4.4) | 1067 (10.3) | 63 (0.6) | 1147 (11.1) | 91 (0.9) | 7497 (72.6) |
| MA≥65 | 6927 | 360 (5.2) | 326 (4.7) | 18 (0.3) | 346 (5.0) | 31 (0.4) | 5846 (84.4) |
| Race/ethnicity | |||||||
| White | 30,332 | 1485 (4.9) | 4976 (16.4) | 1552 (5.1) | 4044 (13.3) | 791 (2.6) | 17,484 (57.6) |
| Black | 4991 | 317 (6.4) | 628 (12.6) | 161 (3.2) | 468 (9.4) | 68 (1.4) | 3349 (67.1) |
| Hispanic | 3388 | 192 (5.7) | 511 (15.1) | 158 (4.7) | 338 (10.0) | 47 (1.4) | 2142 (63.2) |
| Other/unknown | 2174 | 122 (5.6) | 340 (15.6) | 99 (4.6) | 273 (12.6) | 57 (2.6) | 1283 (59.0) |
| Modified Elixhauser [mean (SD)] | 1.75 (2.35) | 1.25 (2.15) | 1.00 (1.67) | 0.51 (1.15) | 0.88 (1.49) | 0.94 (1.41) | 2.30 (2.60) |
| Any mental health diagnosis | 18,218 | 585 (3.2) | 3078 (16.9) | 933 (5.1) | 2060 (11.3) | 620 (3.4) | 10,942 (60.1) |
| Depression | 9733 | 270 (2.8) | 1670 (17.2) | 552 (5.7) | 965 (9.9) | 398 (4.1) | 5878 (60.4) |
| Anxiety | 10,704 | 274 (2.6) | 1921 (17.9) | 554 (5.2) | 1329 (12.4) | 391 (3.7) | 6235 (58.2) |
| ADHD | 1774 | 33 (1.9) | 402 (22.7) | 159 (9.0) | 272 (15.3) | 77 (4.3) | 831 (46.8) |
| PTSD | 1462 | 41 (2.8) | 245 (16.8) | 104 (7.1) | 153 (10.5) | 69 (4.7) | 850 (58.1) |
| Alcohol use disorder | 4166 | 174 (4.2) | 961 (23.1) | 471 (11.3) | 225 (5.4) | 496 (11.9) | 1839 (44.1) |
| Bipolar | 3138 | 102 (3.3) | 556 (17.7) | 183 (5.8) | 290 (9.2) | 146 (4.7) | 1861 (59.3) |
| Psychosis | 1526 | 76 (5.0) | 268 (17.6) | 76 (5.0) | 87 (5.7) | 40 (2.6) | 979 (64.2) |
| Injection-related infection | 5556 | 249 (4.5) | 330 (5.9) | 66 (1.2) | 151 (2.7) | 31 (0.6) | 4729 (85.1) |
| Hepatitis C | >2007 | 64 (3.2) | 181 (9.0) | 21 (1.0) | 121 (6.0) | <11 (<0.5) | 1623 (80.4) |
| Overdose | 2135 | 249 (11.7) | 267 (12.5) | 84 (3.9) | 86 (4.0) | 27 (1.3) | 1422 (66.6) |
ADHD indicates attention deficit hyperactivity disorder; MA, Medicare Advantage; PH, Partial Hospitalization; PTSD, post traumatic stress disorder; RTC, Rehabilitation Treatment Center.
For the entire cohort, average unadjusted costs increased from $3250 PMPM (SD $7846) in the baseline period to $5047 PMPM (SD $11,856) in the 90 day follow-up period. Out-of-pocket costs as a proportion of unadjusted total costs in the initial 90 day follow-up period were lowest for the no treatment (7.8%) and behavioral health outpatient (8.6%) groups and highest for buprenorphine/methadone (15.9%) and behavioral health intensive (16.3%) groups. Actual out-of-pocket amounts were highest for inpatient and behavioral health intensive groups at $1294 and $973 PMPM, respectively (Appendix Table 1, Supplemental Digital Content 1, http://links.lww.com/MLR/C61).
Costs increased from the baseline to follow-up 90 day periods for each initial treatment strategy (Table 2). Compared with no treatment, initial 90 day total costs were lower for buprenorphine/methadone [Adjusted Difference in Differences Cost Ratio (ADIDCR) 0.65; 95% confidence interval (CI), 0.52–0.80], naltrexone (ADIDCR 0.53; 95% CI, 0.42–0.67), and behavioral health outpatient (ADIDCR 0.54; 95% CI, 0.44–0.66). Costs were higher for inpatient detox (ADIDCR 2.30; 95% CI, 1.88–2.83; Table 2).
TABLE 2.
Unadjusted Costs, Within Group Adjusted* Cost Ratios, and Adjusted* Difference in Differences Cost Ratios at 3 and 12 Months After Initiation of Treatment by Treatment Category
| Unadjusted Mean Cost Per Member Per Month [Mean (SD)] ($) | |||||
|---|---|---|---|---|---|
| Initial Treatment Pathway | n | Baseline | Follow-up | Within Group Adjusted* Cost Ratio† | Adjusted* Difference in Differences Cost Ratio (ADIDCR)‡ |
| 3 mo follow-up (n=40,885) | |||||
| No treatment | 2116 | 2306 (6845) | 5250 (22,145) | 2.74 (2.26–3.32) | 1.00 (Reference) |
| Inpatient/Detox | 6455 | 2243 (5740) | 9222 (14,228) | 6.31 (5.89–6.76) | 2.30 (1.88–2.83) |
| Behavioral health intensive outpatient | 1970 | 3148 (5809) | 5943 (8936) | 2.48 (2.26–2.73) | 0.91 (0.73–1.12) |
| Buprenorphine/methadone | 5123 | 1494 (4065) | 2096 (5118) | 1.77 (1.63–1.92) | 0.65 (0.52–0.80) |
| Naltrexone | 963 | 4219 (7265) | 4960 (7458) | 1.46 (1.29–1.66) | 0.53 (0.42–0.67) |
| Behavioral health outpatient | 24,258 | 3940 (9004) | 4472 (10,918) | 1.48 (1.42–1.55) | 0.54 (0.44–0.66) |
| 12 mo follow-up (n=21,200) | |||||
| No treatment | 1017 | 2196 (6079) | 2747 (9018) | 1.58 (1.27–1.97) | 1.00 |
| Inpatient/Detox | 3138 | 2118 (5163) | 4534 (6580) | 2.92 (2.64–3.22) | 1.84 (1.45–2.34) |
| Behavioral health intensive outpatient | 1013 | 3066 (5494) | 3344 (5536) | 1.35 (1.18–1.55) | 0.85 (0.66–1.10) |
| Buprenorphine/methadone | 2368 | 1466 (3737) | 1972 (3375) | 1.62 (1.45–1.81) | 1.02 (0.80–1.31) |
| Naltrexone | 443 | 3966 (7025) | 3162 (5753) | 0.98 (0.81–1.20) | 0.62 (0.46–0.83) |
| Behavioral health outpatient | 13,221 | 3649 (8297) | 3158 (5687) | 1.14 (1.09–1.19) | 0.72 (0.58–0.90) |
*Adjusted for: age, sex, race, insurance type, baseline medical (modified Elixhauser) and mental health comorbidities (depression, anxiety, PTSD, ADHD), and evidence of overdose or infections related to injection drug use at baseline.
†Ratio of per member per month costs in follow-up to baseline within each treatment pathway.
‡Difference in differences estimate of total costs of care from baseline to follow-up for each treatment pathway compared with no treatment.
ADHD indicates attention deficit hyperactivity disorder; PTSD, post traumatic stress disorder.
We observed cost increases to a varying degree over the first and second half of the baseline period among treatment groups (Appendix Table 2, Supplemental Digital Content 1, http://links.lww.com/MLR/C61). We found no significant difference in the change in costs over the baseline period when comparing the no treatment group with the 5 active treatment groups in aggregate (P=0.16; Appendix Fig. 1, Supplemental Digital Content 1, http://links.lww.com/MLR/C61).
A total of 21,200 members had 12 months of continuous enrollment following the index date. The distribution among initial treatment pathways and baseline characteristics were similar between the subgroup of members with 12 months of continuous enrollment compared with the full cohort with 3 months of continuous enrollment (Appendix Tables 3 and 4, Supplemental Digital Content 1, http://links.lww.com/MLR/C61). For all treatment pathways, we observed highest costs in the first 3 months following the index date, followed by substantial drop and leveling off over the subsequent 9 months (Fig. 2). Compared with no treatment, 12-month costs were higher for inpatient detox (ADIDCR 1.84; 95% CI, 1.45–2.34; Table 2). We did not detect a difference in 12-month costs between no treatment and Behavioral Health Intensive (ADIDCR 0.85; 95% CI, 0.66–1.10), and buprenorphine/methadone (ADIDCR 1.02; 95% CI, 0.80–1.31). Twelve-month costs were lower than no treatment for naltrexone (ADIDCR 0.62; 95% CI, 0.46–0.83) and outpatient behavioral health (ARCCR 0.72; 95% CI, 0.58–0.90). Sensitivity analysis of PMPM costs among the full cohort censoring for disenrollment using inverse probability of censoring weights produced results consistent with the main approach (Appendix Table 5, Supplemental Digital Content 1, http://links.lww.com/MLR/C61).
FIGURE 2.
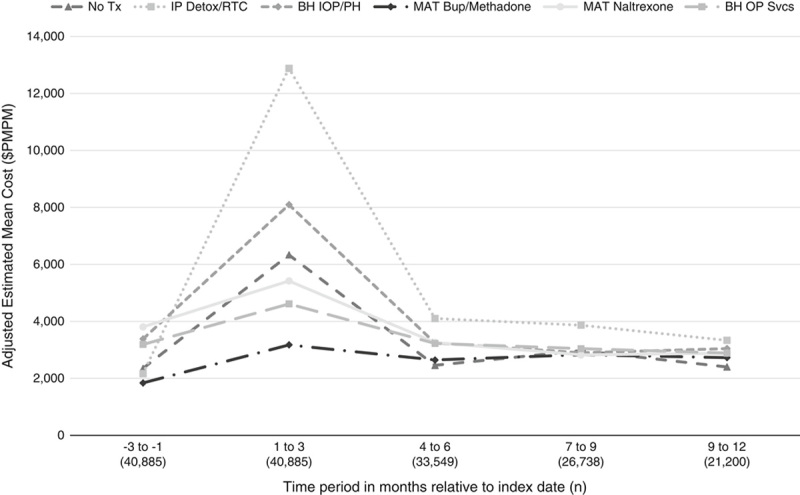
Estimated per member per month (PMPM) total cost for 3 month intervals from baseline through 12 months following the index date by treatment pathway. BH indicates behavioral health; BUP, buprenorphine; IOP, intensive outpatient; MAT, Medication for Addiction Treatment; OP, outpatient; PH, Partial Hospitalization; RTC Rehabilitation Treatment Center.
Among those with 12 months of continuous enrollment, 3594 (16.9%) received buprenorphine or methadone at least once. Of these 3594, 79% received buprenorphine or methadone for >1 month, and 48% for >6 months. 1300 (6.1%) received naltrexone at least once. Of these 1300, 56% received naltrexone for >1 month, and 12% for >6 months. Unadjusted PMPM cost ratios from the baseline to follow-up period were similar by buprenorphine/methadone and naltrexone treatment duration (Table 3).
TABLE 3.
Unadjusted Per Member Per Month Costs After Initiation of Treatment by Cumulative Duration of Buprenorphine/Methadone or Naltrexone Treatment Over 12 Months of Contiguous Enrollment
| Unadjusted Mean Cost Per Member Per Month [Mean (SD)] ($) | ||||
|---|---|---|---|---|
| Cumulative Duration on | n (%) | Baseline | Follow-up | Unadjusted Within Group Cost Ratio |
| Buprenorphine/methadone (d) | ||||
| 0 | 17,606 (83) | 3366 (7663) | 3360 (6132) | 1.00 |
| 1–30 | 738 (3.5) | 2094 (5363) | 3332 (5488) | 1.59 |
| 31–180 | 1127 (5.3) | 2037 (6608) | 2847 (5051) | 1.40 |
| >180 | 1729 (8.2) | 1356 (3256) | 1973 (2631) | 1.46 |
| Naltrexone (d) | ||||
| 0 | 19,900 (93.9) | 3104 (7407) | 3123 (5824) | 1.01 |
| 1–30 | 567 (2.7) | 2474 (4523) | 4361 (6136) | 1.76 |
| 31–180 | 574 (2.7) | 3116 (6321) | 4898 (6012) | 1.57 |
| >180 | 159 (0.8) | 3156 (5405) | 5023 (7129) | 1.59 |
DISCUSSION
We identified a large cohort of 40,885 individuals with a new diagnosis of OUD. The initial treatment strategy involved inpatient services or IOP services for 1 in 5 individuals compared with medications for OUD for 1 in 7 individuals. Compared with no treatment, costs of care over the first 3 months were higher for inpatient services and lower for buprenorphine/methadone, naltrexone, and outpatient behavioral health. Findings were similar over 12 months of follow-up with the exception of lack of persistence in lower costs for buprenorphine/methadone.
When combined with clinical outcomes, these data highlight the need for policymakers, health insurance plans, and care delivery systems to increase capacity, and reduce barriers to outpatient treatment for OUD, and in particular MOUD. A companion analysis of clinical outcomes with this same cohort demonstrated that compared with no treatment, buprenorphine/methadone and outpatient behavioral health were associated with reduced serious opioid-related acute care and buprenorphine and methadone was associated with reduced overdose.25 Reduction in acute care utilization may be a driver of reduced total costs observed for patients receiving MOUD or outpatient behavioral health in our study. MOUD should be covered as first line treatment for OUD without restriction such as prior authorization or step coverage. Out-of-pocket expenditures for a 30-day buprenorphine prescription have dropped by 52% between 2003 and 2015.26 Unfortunately, other barriers remain—an analysis of Medicare Part D plans found the proportion of plans covering buprenorphine without restriction dropped from 89% in 2007 to 35% in 2018.27 These restrictions have been found to be associated with reduced use of buprenorphine among addiction treatment programs.28
Despite higher costs associated with inpatient detoxification or rehabilitation, access to these treatments should be maintained when patients meet criteria based on ASAM Levels of Care. However, if the primary indication for inpatient level of care is medical management of withdrawal and a client is interested in initiating MOUD, there is ample evidence that MOUD could instead be initiated in outpatient settings. Several RCTs have demonstrated the ability to successfully initiate MOUD and refer patients for ongoing treatment in medical inpatient and emergency department settings.29,30 Outpatient clinics that offer transitional, low threshold access are becoming more common, and patients report positive experiences with care received from them compared with alternatives.31 Policy and health system efforts should recognize that more treatment “beds” may not be the answer but rather increased capacity for outpatient management of OUD and consider transforming detox units into treatment initiation centers.32
We found durable associations between initial OUD treatment approach and costs when extending follow-up from 3 to 12 months with the exception for lack of a continued finding of reduced cost for individuals receiving buprenorphine/methadone compared with no treatment. In a prior study of this cohort, we identified that transitions between treatments was uncommon.25 The reason for the lack of persistence in lower costs for buprenorphine/methadone is unclear. In our companion analysis of clinical outcomes, buprenorphine/methadone was associated with reduced overdose and opioid-related acute care compared with no treatment at 12 months, but there was no difference comparing naltrexone with no treatment.25 Collectively these cost and outcome data should not be used as the basis to favor naltrexone over buprenorphine or methadone in coverage policies. Notably, an analysis of a randomized clinical trial found buprenorphine to have superior cost-effectiveness compared with extended-release naltrexone.33 Furthermore, over 1 year of follow-up, only 12% of individuals receiving naltrexone received it for 6 months, compared with 48% of those receiving buprenorphine or methadone. Other studies have also found lower rates of retention with naltrexone compared with buprenorphine.34
Comparative data for costs of OUD treatment are limited, and we are not aware of other studies that compare costs based on the initial treatment for individuals diagnosed with OUD.35 One analysis using commercial insurance claims between 2005 and 2009 found MOUD was associated with lower health care utilization and costs of care compared with not receiving MOUD.36 Other analyses from the same timeframe, one using Veterans Health Administration data and another with Massachusetts Medicaid data similarly found buprenorphine was associated with lower costs compared with methadone.37,38
In our study, 95% of individuals received treatment, much higher than the 26% of individuals with OUD that self-report receipt of treatment in a national survey.39 Several points may explain this potential discrepancy. The individuals in this study are included based on a new diagnosis of OUD, and many individuals with OUD may be undiagnosed. There may be some misclassification of the 59% of individuals assigned to outpatient behavioral health as we are unable to disentangle whether or not those services were directly related to OUD or other behavioral health conditions. Notably, the individuals in the outpatient behavioral health group had both lower costs in this study and improved clinical outcomes in a companion analysis of this cohort.25
Our study has several strengths including the large number of individuals with OUD. We included the full complement of treatment options including MOUD and mapped treatment services to ASAM levels of care. Our study also has several limitations. First, the data are limited to a population with commercial insurance or Medicare advantage. Findings may not generalize to other populations such as those with Medicaid insurance. Second, there is potential for bias introduced by nonrandom assignment of individuals to treatment pathways. We attempted to address this limitation by adjusting for measured confounders. We also used a difference in differences design, controlling for baseline costs, which were substantially different by treatment pathway. Differences in differences also controls for unobserved confounders that remain constant over time. Finally, we recognize that International Classification of Diseases-10 codes do not map to Diagnostic and Statistical Manual of Mental Disorders-5 diagnosis of OUD. Two authors who are addiction medicine experts (M.R.L. and S.E.W.) worked to develop the algorithm used in this study that has face validity to address potential limitations of these codes.
In a large cohort of patients with OUD, initial treatment with MOUD or outpatient behavioral health were associated with lower total costs over the first 3 months. These cost data combined with previously published findings of improved clinical outcomes associated with these approaches provide motivation for policymakers, health insurers, and health systems to work together to reduce barriers, incentivize, and increase capacity to deliver outpatient management of OUD, particularly with MOUD.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.
ACKNOWLEDGMENT
The authors would like to acknowledge Pamela Hansen for her contributions to project administration and Erika Lozano for her contributions to formatting the manuscript and Supplemental Digital Content.
Footnotes
Preliminary results were presented at the Society of General Internal Medicine Annual Meeting in Washington, DC on May 9, 2019; and, Academy Health Annual Research Meeting in Washington, DC on June 3, 2019.
Funding for this work was provided by Optum—UnitedHealth Group.
In the past 36 months, S.E.W. has received research support through Massachusetts General Hospital from the National Institutes of Health National Institute of Drug Abuse (1R01DA044526-01A1 and 3UG1DA015831-17S2), the Substance Abuse and Mental Health Services Administration (1H79TI081442-01), the Health Resources and Services Administration (1T25HP37602-01), the Laura and John Arnold Foundation, and received salary support from OptumLabs, Celero Systems, Alosa Health, and UptoDate, and textbook royalties from Springer. In the past 36 months, O.A. has received research support from Boston University School of Public Health, Department of Veterans Affairs, Optum via UnitedHealthcare and the American Physical Therapy Association, and has been an employee of OptumLabs. In the past 36 months, C.E.C. has received research support through Boston School of Public Health from the National Institutes of Health (NIH) (P30 AG13846; 1U01AA021989; R01 AR055557), and from NIH via the National Institute of Neurological Disorders and Stroke (1U01NS093334-01; 1U01NS086659), and from NIH via the National Institute on Alcohol Abuse and Alcoholism (U24 AA020779; 1R01AA021335), and from the National Institute of Allergy and Infectious Diseases (U01 AA020776), and from NIH via the National Institute on Deafness and Other Communication Disorders (P50 DC013027), and from NIH via the National Center for Advancing Translational Sciences (1UL1TR001430), and from NIH via the National Institute on Minority Health and Health Disparities (U24 MD006964), and from NIH via the National Institute on Drug Abuse (R01DA032082; R01 DA037768), and from the Department of Defense (W81XWH-13-2-0072), and has been an employee of OptumLabs. In the past 36 months, J.T.M., W.H.C., and D.M.S. have been employees of OptumLabs. In the past 36 months, F.A. has been an employee of Optum—UnitedHealth Group. In the past 36 months, M.R.L. has received research support through Boston Medical Center from the National Institutes of Health via the National Institute on Drug Abuse (K23DA042168 and UM1DA049412) and the National Center for Advancing Translational Sciences (1UL1TR001430), Centers for Disease Control and Prevention (U01CE002780), Food and Drug Administration (HHSF2232009100006I), Office of National Drug Control Policy/University of Baltimore (G1799ONDCP06B), a Boston University School of Medicine Department of Medicine Career Investment Award, and served as a consultant for opioid-related health services research to the Harvard Medical School Department of Population Medicine.
REFERENCES
- 1.Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-2012. Health Aff (Millwood). 2016;35:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barocas JA, White LF, Wang J, et al. Estimated prevalence of opioid use disorder in Massachusetts, 2011–2015: a capture–recapture analysis. Am J Public Health. 2018;108:1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009:CD002209. [DOI] [PubMed] [Google Scholar]
- 7.Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014:CD002207. [DOI] [PubMed] [Google Scholar]
- 8.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort studymortality and medication for opioid use disorder after overdose. Ann Intern Med. 2018;169:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty K, Freedman KI, Kampman KM. Executive Summary of the Focused Update of the ASAM National Practice Guideline for the Treatment of Opioid Use Disorder. J Addict Med. 2020;14:99–112. [DOI] [PubMed] [Google Scholar]
- 12.Mee-Lee D, Shulman GD, Fishman MJ, et al. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions, 3rd ed Carson City, NV: The Change Companies; 2013. [Google Scholar]
- 13.Sharon E, Krebs C, Turner W, et al. Predictive validity of the ASAM Patient Placement Criteria for hospital utilization. J Addict Dis. 2003;22(suppl 1):79–93. [DOI] [PubMed] [Google Scholar]
- 14.Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004-2013. JAMA. 2015;314:1515–1517. [DOI] [PubMed] [Google Scholar]
- 15.Feder KA, Mojtabai R, Krawczyk N, et al. Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend. 2017;179:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinhofer A, Witman AE. The role of health insurance on treatment for opioid use disorders: evidence from the Affordable Care Act Medicaid expansion. J Health Econ. 2018;60:177–197. [DOI] [PubMed] [Google Scholar]
- 17.Grogan CM, Andrews C, Abraham A, et al. Survey highlights differences in medicaid coverage for substance use treatment and opioid use disorder medications. Health Aff (Millwood). 2016;35:2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Cambridge, MA. Reproduced with permission from OptumLabs; 2019.
- 19.Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353:1945–1954. [DOI] [PubMed] [Google Scholar]
- 20.Hude Q, Vijaya S, Patricia H, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 21.Riley ED, Evans JL, Hahn JA, et al. A longitudinal study of multiple drug use and overdose among young people who inject drugs. Am J Public Health. 2016;106:915–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83:S4–S7. [DOI] [PubMed] [Google Scholar]
- 23.Wooldridge JM. Econometric Analysis of Cross Section and Panel Data, 2nd ed Cambridge, MA: Massachusetts Institute of Technology; 2010. [Google Scholar]
- 24.Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1:35–47. [DOI] [PubMed] [Google Scholar]
- 25.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3:e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts AW, Saloner B, Dusetzina SB. Buprenorphine use and spending for opioid use disorder treatment: trends from 2003 to 2015. Psychiatr Serv. 2018;69:832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartung DM, Johnston K, Geddes J, et al. Buprenorphine Coverage in the Medicare Part D Program for 2007 to 2018 Buprenorphine Coverage in the Medicare Part D Program for 2007 to 2018 Letters. JAMA. 2019;321:607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews CM, Abraham AJ, Grogan CM, et al. Impact of Medicaid restrictions on availability of buprenorphine in addiction treatment programs. Am J Public Health. 2019;109:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow RL, Simon RE, Jack HE, et al. Patient experiences with a transitional, low-threshold clinic for the treatment of substance use disorder: a qualitative study of a bridge clinic. J Subst Abuse Treat. 2019;107:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Friedmann PD, Suzuki J. More beds are not the answer: transforming detoxification units into medication induction centers to address the opioid epidemic. Addict Sci Clin Pract. 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy SM, McCollister KE, Leff JA, et al. Cost-effectiveness of buprenorphine-naloxone versus extended-release naltrexone to prevent opioid relapse. Ann Intern Med. 2019;170:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan JR, Schackman BR, Leff JA, et al. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions. Pharmacoeconomics. 2016;34:863–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235–S248. [PubMed] [Google Scholar]
- 37.Barnett PG. Comparison of costs and utilization among buprenorphine and methadone patients. Addiction. 2009;104:982–992. [DOI] [PubMed] [Google Scholar]
- 38.Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff (Millwood). 2011;30:1425–1433. [DOI] [PubMed] [Google Scholar]
- 39.Wu LT, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.


