Abstract
We investigated the prognostic value of a range of histologic parameters in medullary thyroid carcinoma (MTC) to design a grading system to predict overall survival. We assessed 76 patients with MTCs undergoing primary tumor resection for age, sex, tumor size, vascular space invasion, lymph node metastasis, multiple endocrine neoplasia type 2 (MEN2) status, mitotic count, Ki-67 proliferative index, spindled morphology, sheet-like growth pattern, coagulative necrosis, incipient necrosis, nuclear grade, multinucleation, prominent nucleoli, fibrosis, and amyloid deposition. In addition to the clinical features of age and the diagnosis of MEN2, the only histologic features that significantly predicted reduced overall survival were Ki-67 proliferative index, mitotic count, and the presence of coagulative necrosis. Using a combination of these 3 variables, we propose a 3-tiered grading system based solely on proliferative activity (Ki-67 proliferative index and mitotic count) and necrosis. There were 62 (82%) low-grade MTCs (low proliferative activity, no necrosis), 9 (12%) intermediate grade (low proliferative activity and necrosis present, or intermediate proliferative activity and no necrosis), and 5 (7%) high grade (intermediate proliferative activity and necrosis present, or high proliferative activity with or without necrosis). The mean overall survival was 193, 146, and 45 months, respectively (P=0.0001) for the 3 grades. The grading system remained prognostic when controlled for other factors associated with survival including age and known MEN2 syndrome. We conclude that this proposed grading system, which uses only a combination of proliferative activity (Ki-67 index, mitotic count) and coagulative necrosis, is a strong predictor of overall survival in MTC.
Key Words: medullary thyroid carcinoma, Ki-67 proliferative index, mitotic count, coagulative necrosis, grading system
Despite accounting for only 2% to 3% of all thyroid malignancies,1 medullary thyroid carcinoma (MTC) is responsible for a disproportionately high number of deaths compared with other thyroid carcinomas due to its relatively aggressive biological behavior.2 Among patients presenting with a palpable thyroid nodule, the incidence of clinical cervical lymph node involvement at the time of diagnosis has been reported to be as high as 75%.3
Approximately 25% of MTCs are hereditary and occur in the setting of the autosomal dominant hereditary cancer syndrome multiple endocrine neoplasia type 2 (MEN2), which is caused by activating germline RET mutations.4 In these cases, early detection of thyroid-confined disease through biochemical or genetic screening studies has resulted in significant improvements in prognosis.5 However, little is known about the etiology of sporadic MTC. Unlike other thyroid malignancies, there is no apparent association with external ionizing irradiation.1 Moreover, no histologic grading system has been established for this neoplasm, and only patient age and TNM stage have been found to have prognostic value in a number of studies in the literature.6–8
Although clinical and pathologic variables alone may suffice to predict patient outcome in most cases, the variable and unpredictable behavior of MTC in some cases suggests that other biological elements may also influence disease progression and survival.9–11 Several studies using molecular techniques have identified various genetic factors that may be useful in the risk stratification of MTC patients.12–14 In particular, somatic RET mutations, which occur in 30% to 50% of sporadic MTCs, have been shown to be a negative predictor of cancer remission and survival.15–20 However, the specific types of RET mutations and their associations with clinical and pathologic features varied considerably across these reports, suggesting that alternative biological markers could also be useful for determining prognosis in MTC.11
Several studies have demonstrated the potential for Ki-67 immunohistochemistry, both independently and in combination with RET mutation testing, to predict cancer progression and survival in patients with MTC.11,21–23 In addition, 2 studies in relatively small cohorts of MTC patients have reported vascular invasion as an adverse predictor of disease-free status,24,25 while an isolated report suggested that stromal desmoplasia may be a predictor of metastatic potential.26 Various other histologic and immunohistochemical parameters, including cellular composition (spindled vs. epithelioid), nuclear pleomorphism, extent of amyloid deposition, and extent of calcitonin staining, have also been investigated as potential prognostic markers, but none has proven to be significant in multivariate analyses.1
We therefore sought to investigate the potential prognostic value of a range of histologic parameters, and to design a grading system that can be used to risk stratify patients with MTC.
MATERIALS AND METHODS
Patients
The computerized database of the Department of Anatomical Pathology at the Royal North Shore Hospital in Sydney, NSW, Australia was searched for cases of MTC undergoing surgical resection from June 1, 1998, to December 31, 2016. Inclusion criteria included the presence of sufficient tumor in archived formalin-fixed paraffin-embedded blocks to permit tissue microarray (TMA) construction. Data on TNM stage, patient demographics, all-cause survival, and serum calcitonin levels were obtained from the pathology reports and electronic medical records. All-cause survival data were current as of January 22, 2020. Data on adjuvant chemotherapy were not available. The study was approved by the Northern Sydney Local Health District Human Research Ethics Committee.
Evaluation of Morphologic Parameters
A single hematoxylin and eosin–stained slide containing a representative section of tumor was evaluated by 1 pathologist who was blinded to all clinical and pathologic data. A number of histologic observations were recorded for each tumor and illustrated in Figure 1. These features comprised: mitotic count per 2 mm2 (equivalent to 10 contiguous HPFs on many microscopes, assessed in the most mitotically active part [hotspot] of the tumor); proportion of cells with spindled morphology; the presence of a sheet-like growth pattern in >10% of the tumor (illustrated in Fig. 1D); the presence of coagulative necrosis; the presence of incipient necrosis (defined as clusters or zones of cells showing nuclear pyknosis, karryhorexis, and cytoplasmic condensation suggesting impending ischemic infarction); nuclear grade (assessed semiquantitatively as low, moderate, or high grade based on the degree of nuclear pleomorphism); the presence of multinucleated cells; prominent nucleoli (defined as nucleoli visible at ×100 magnification similar to Fuhrman nuclear grade 3 in renal cell carcinoma); and the proportion of tumor fibrosis/amyloid deposition. Fibrosis and amyloid deposition were assessed together as in specific areas of individual cases hyalinised fibrosis was difficult to distinguish from amyloid. The pattern of growth that we termed “sheet-like” (illustrated in Fig. 1D) bore a superficial resemblance to small cell carcinoma and an alternative term for this pattern could be “small cell like” as it was composed of smaller cells with diffuse growth and little cytoplasm (illustrated in Fig. 1D). However it is emphasized that this sheet-like growth pattern differed from true small cell carcinoma by the absence of the classic features of nuclear molding, intense mitotic activity, prominent apoptotic debris, and extremely high (usually >50%) Ki-67 proliferative index. Necrosis thought to be attributable to preoperative fine needle aspiration or core biopsy was disregarded (that is not classified as coagulative necrosis for the purposes of grading). Clues used to distinguish fine needle aspiration–associated necrosis from tumor type coagulative necrosis included localization to certain areas or needle tracts, and association with linear scar tissue.
FIGURE 1.
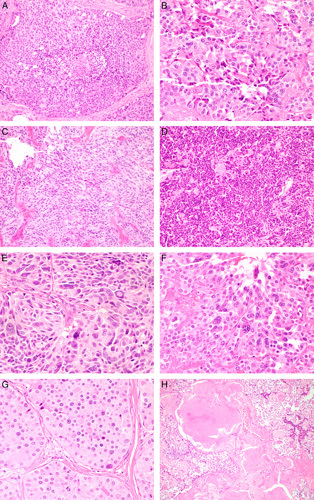
Histologic features of MTC. A, Coagulative necrosis. B, Incipient necrosis defined as clusters of cells showing nuclear pyknosis, karryhorexis, and cytoplasmic condensation. C, Spindle cell morphology. D, Sheet-like growth. E, High nuclear grade. F, Multinucleation. G, Prominent nucleoli defined as nucleoli visible at ×100 magnification. H, Fibrosis/amyloid deposition >50% of tumor area (hematoxylin and eosin).
Ki-67 Immunohistochemistry and Scoring
A TMA was constructed containing two 1 mm diameter cores of formalin-fixed paraffin-embedded tumor tissue. The TMA blocks were sectioned at 4 µm onto positively charged slides and the slides were stained for Ki-67 with a commercially-available mouse monoclonal antibody (dilution 1:50, clone M7240; Dako, Carpineteria, CA) on the automated BOND III platform (Leica Biosystems, Mount Waverley, Vic., Australia) using a polymer detection system after heat-induced epitope retrieval in the manufacturer’s alkaline retrieval solution (ER2). For assessment of Ki-67 staining, nuclei were considered positive when the intensity of immunostaining ranged from weak to strong and when positivity was confined to the nuclei. Nuclei with no immunostaining were considered negative. The Ki-67 score was defined as the percentage of positively staining cells among the total number of malignant cells in the two 1 mm cores. All cases were scored by 1 pathologist who was blinded to all clinical and pathologic data. A minimum of 100 neoplastic cells were required for Ki-67 assessment and if not present in the TMA slides, staining was repeated on whole sections. When performed on whole sections, our intention was to assess the Ki-67 proliferative index in “hotspots” (areas of highest proliferative index) although we subsequently found that Ki-67 was uniform across the whole sections (ie, hotspots effectively did not exist). We subsequently performed Ki-67 on additional whole slides from 71 cases to determine the concordance with the TMA sections.
Statistical Analysis
All statistical analyses were performed using SPSS, version 25.0 (IBM Inc., Armonk, NY). Overall survival was defined as the time from surgery until any-cause death (date of census: January 22, 2020). The continuous Ki-67 scores were categorized based on the cutoffs used for the current World Health Organization (WHO) classification of neuroendocrine neoplasms27,28 into low (<3%), intermediate (3% to 20%) and high (>20%) groups. To determine the most prognostically significant cutoff, the continuous mitotic count variable was dichotomized first at a cutoff of 2 mitoses per 2 mm2 (identical to that used to indicate a grade 1 low-grade neuroendocrine tumor in the gastrointestinal tract) and then at a cutoff of 3 mitotic figures per 2 mm2. The other continuous variables were the proportion of amyloid/fibrosis and the proportion of tumor showing spindled morphology. These were each arbitrarily dichotomized at a cutoff of 50%. Kaplan-Meier curves were constructed for each morphologic parameter. Univariate Cox proportional hazard regression analyses were used to evaluate the association between age, sex, MEN2 syndrome, tumor size, vascular space invasion, lymph node metastasis, Ki-67 proliferative index, mitotic count, coagulative necrosis, spindled morphology, sheet-like growth, incipient necrosis, nuclear grade, multinucleated cells, prominent nucleoli, and fibrosis/amyloid deposition and overall survival. A multivariate analysis was performed using the variables that were found to be significant in univariate analyses. Serum calcitonin doubling time was calculated using the American Thyroid Association online calculator (available at: www.thyroid.org/professionals/calculators/thyroid-cancer-carcinoma/). For the purposes of survival analyses, the continuous calcitonin doubling time variable was dichotomized into long and short categories using a cutoff of the median value. The first calcitonin level was also categorized as high or low using a cutoff at the median value. Kaplan-Meier survival analyses were performed using the categorical calcitonin doubling time and the first serum calcitonin variables. All statistical tests were 2 sided and P-values ≤0.05 were considered significant.
RESULTS
Patient Characteristics
The cohort included 76 patients with resected primary MTC. The median age at surgery was 60 years (range: 22 to 85 y). Overall, 41 (54%) patients were female. Eleven (14%) patients had MEN2 syndrome. Tumor size ranged from 1 to 65 mm (median: 17 mm). During the follow-up period, 19 (25%) had death recorded. The mean duration of follow-up was 6.7 years. Patient characteristics and associations with overall survival are summarized in Table 1.
TABLE 1.
Univariate and Multivariate Analyses of Prognostic Factors for Overall Survival
| Univariate Analysis† | Multivariate Analysis† | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n (%) | Mean Survival (mo)* | HR | 95% CI | P | HR | 95% CI | P |
| Age at diagnosis (y) | ||||||||
| Median (range): 60 (22-85) | ||||||||
| ≤60 | 40 (53) | 253 | 1 | 1 | ||||
| >60 | 36 (47) | 135 | 4.336 | 1.438-13.073 | 0.004 | 4.243 | 1.351-13.327 | 0.013 |
| MEN2 syndrome | ||||||||
| Yes | 11 (14) | 253 | 1 | 1 | ||||
| No | 65 (86) | 154 | 7.862 | 1.026-60.258 | 0.020 | 9.075 | 1.072-76.830 | 0.043 |
| Ki-67 proliferative index (%) | ||||||||
| <3 | 63 (83) | 195 | 1 | 1 | ||||
| ≥3 | 13 (17) | 94 | 3.781 | 1.375-10.398 | 0.006 | 1.556 | 0.298-8.122 | 0.600 |
| Mitotic count (/2 mm2) | ||||||||
| <3 | 68 (89) | 178 | 1 | 1 | ||||
| ≥3 | 8 (11) | 105 | 3.855 | 1.307-11.372 | 0.010 | 5.795 | 1.281-26.220 | 0.023 |
| Coagulative necrosis | ||||||||
| Present | 5 (7) | 58 | 6.567 | 1.793-24.058 | 0.001 | 2.110 | 0.410-10.860 | 0.372 |
| Absent | 71 (93) | 190 | 1 | 1 | ||||
| Sex (male) | ||||||||
| Female | 41 (54) | 188 | 1 | — | — | — | ||
| Male | 35 (46) | 167 | 2.016 | 0.808-5.030 | 0.125 | |||
| Tumor size (n=70) (mm) | ||||||||
| Median (range): 17 (1-65) | ||||||||
| ≤17 | 35 (50) | 179 | 1 | — | — | — | ||
| >17 | 35 (50) | 157 | 0.873 | 0.316-2.411 | 0.793 | |||
| Vascular space invasion | ||||||||
| Present | 36 (47) | 181 | 1.249 | 0.495-3.152 | 0.638 | — | — | — |
| Absent | 40 (53) | 157 | 1 | |||||
| Lymph node metastasis | ||||||||
| Present | 45 (59) | 186 | 0.796 | 0.306-2.070 | 0.639 | — | — | — |
| Absent | 31 (41) | 169 | 1 | |||||
| Spindled morphology | ||||||||
| ≥50% | 14 (18) | 193 | 0.730 | 0.207-2.566 | 0.622 | — | — | — |
| <50% | 62 (82) | 187 | 1 | |||||
| Sheet-like growth | ||||||||
| Present | 8 (11) | 49 | 2.604 | 0.559-12.132 | 0.206 | — | — | — |
| Absent | 68 (89) | 185 | 1 | |||||
| Incipient necrosis | ||||||||
| Present | 57 (75) | 176 | 1.797 | 0.589-5.486 | 0.296 | — | — | — |
| Absent | 19 (25) | 182 | 1 | |||||
| Nuclear grade | ||||||||
| High | 14 (18) | 179 | 0.990 | 0.321-3.048 | 0.985 | — | — | — |
| Low | 62 (82) | 189 | 1 | |||||
| Multinucleated cells | ||||||||
| Present | 39 (51) | 190 | 0.811 | 0.311-2.113 | 0.668 | — | — | — |
| Absent | 37 (49) | 131 | 1 | |||||
| Prominent nucleoli | ||||||||
| Present | 41 (54) | 180 | 1.257 | 0.486-3.254 | 0.637 | — | — | — |
| Absent | 35 (46) | 158 | 1 | |||||
| Fibrosis/amyloid deposition | ||||||||
| ≥50% | 16 (21) | 175 | 0.492 | 0.113-2.144 | 0.335 | — | — | — |
| <50% | 60 (79) | 174 | 1 | |||||
*Mean survival calculated using the Kaplan-Meier method.
†Cox regression model.
P-values are obtained using a χ2 test.
Bold values indicate statistically significant.
CI indicates confidence interval; HR, hazard ratio.
Survival Analyses
Advanced age, absence of MEN2 syndrome, high Ki-67 proliferative index, high mitotic count, and coagulative necrosis were all significantly associated with worse overall survival in univariate analyses. There was no significant association between overall survival and sex, tumor size, vascular invasion, lymph node metastasis, spindled morphology, sheet-like growth pattern, incipient necrosis, nuclear grade, multinucleated cells, prominent nucleoli, or fibrosis/amyloid deposition. A multivariate analysis was performed using the variables that were found to have a statistically significant association with survival in the univariate analyses. In multivariate analysis, only age, MEN2 syndrome, and mitotic count were found to be associated with overall survival. The results of the univariate and multivariate analyses are summarized in Table 1.
Ki-67 Proliferative Index
Ki-67 was scored as a continuous variable for all cases. After categorizing the continuous Ki-67 variable, 63 cases had a low Ki-67 proliferative index (<3%), 12 cases were intermediate (3% to 20%), and 1 case was high (>20%). There was no significant association between Ki-67 proliferative index and sex, age, or tumor size. Overall survival was best in the low Ki-67 group (mean survival: 195 mo), followed by the intermediate Ki-67 group (mean survival: 99 mo), and then the high Ki-67 group (24 mo) (P=0.0001) (Fig. 2A). In the 71 cases in which the Ki-67 was assessed on both whole slides and TMA sections, Cohen κ analysis showed good concordance (κ=0.732, 95% confidence interval: 0.549-0.915, P=0.0001) (n=71).
FIGURE 2.
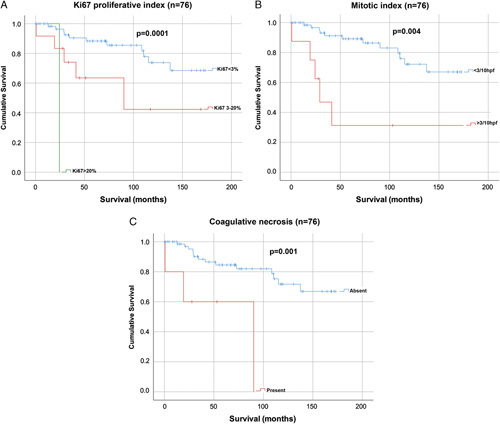
Kaplan-Meier survival curves for Ki-67 proliferative index (n=76) (A); mitotic count (n=76) (B); and coagulative necrosis (n=76) (C).
Mitotic Index
A mitotic index was calculated for all cases by assessing one representative whole tumor section and determining the number of mitotic figures per 2 mm2 (10 contiguous HPFs). Mitotic counts ranged from 0 to 14 per 2 mm2. When dichotomized cases into low (<2 mitoses/2 mm2; n=64) and high (≥2 mitoses/2 mm2; n=12) mitotic groups, no significant survival difference was observed between the 2 groups (mean survival: 175 vs. 170 mo, P=0.163). However, when using a cutoff of 3 mitoses per 10 HPF, that is, low <3 per 2 mm2 (n=68) and high ≥3 per 2 mm2 (n=8), the survival benefit did reach statistical significance (mean survival: 178 vs. 105 mo) (P=0.010) (Fig. 2B).
Coagulative Necrosis
The presence or absence of coagulative tumor necrosis was recorded for all cases. In total, 5 cases had coagulative necrosis. There was no significant association between the presence of coagulative necrosis and sex, age, or tumor size. Overall survival was significantly better in cases without coagulative necrosis (mean survival: 190 mo) compared with those with coagulative necrosis (mean survival: 58 mo) (P=0.001) (Fig. 2C).
Serum Calcitonin
In total, 19 (25%) patients had serum calcitonin levels recorded. The median first calcitonin level was 102 ng/L (range: 40 to 7900 ng/L). The median calcitonin doubling time was 13.86 months (range: 0.86 to 80.77 mo). Survival analyses using the categorical calcitonin variables showed no association between overall survival and first serum calcitonin (P=0.425) or calcitonin doubling time (P=0.264).
Calcitonin and CEA Immunohistochemistry
Immunohistochemistry for calcitonin and carcinoembryonic antigen (CEA) was performed on the TMA sections as well as whole sections for 5 cases that did not have tumor present in the TMA. All but 1 case were positive for calcitonin and all but 4 cases were positive for CEA. The case that was negative for calcitonin had a significantly shorter survival than that of the positive cases (24 vs. 185 mo) (P=0.001). The mean survival of the CEA negative cases was also significantly shorter than the CEA positive cases (52 vs. 186 mo) (P=0.042).
Grading System
Using a combination of Ki-67 proliferative index, mitotic count, and the presence or absence of coagulative necrosis, cases were divided into low-grade, intermediate-grade, and high-grade groups. Using this system, cases with low proliferative activity (defined as <3 mitoses/2 mm2 and a Ki-67 proliferative index of <3%), and no coagulative necrosis were considered low grade. Cases with intermediate proliferative index (defined as 3 to 20 mitoses/2 mm2 or a Ki-67 index of 3% to 20%) without coagulative necrosis were defined as intermediate grade. Cases with a low proliferative index (defined as <3 mitoses/2 mm2 and a Ki-67 proliferative index of <3%) but with coagulative necrosis were also considered intermediate grade. Cases with an intermediate proliferative index (defined as 3 to 20 mitoses/2 mm2 or a Ki-67 index of 3% to 20%) and coagulative necrosis were considered high grade. Cases with a high proliferative index (>20 mitoses/2 mm2 or a Ki-67 index of >20%) were also considered high grade regardless of whether or not there was coagulative necrosis. The proposed grading system is summarized in Table 2. In total, 62 cases were found to be low grade, 9 cases were intermediate grade, and 5 cases were high grade.
TABLE 2.
Proposed Histopathologic Grading System for MTC
| Mitotic Count/2 mm2 | Ki-67 Proliferative Index (%) | Coagulative Necrosis | Grade |
|---|---|---|---|
| <3 | <3 | Absent | Low |
| <3 | <3 | Present | Intermediate |
| 3-20 | 3-20 | Absent | Intermediate |
| 3-20 | 3-20 | Present | High |
| >20 | >20 | Present or absent | High |
Survival Analyses Using the Grading System
Overall survival was best for the low-grade tumors (mean survival: 195 mo), followed by the intermediate-grade tumors (mean survival: 137 mo), and then the high-grade tumors (mean survival: 45 mo) (P=0.0001) (Fig. 3B).
FIGURE 3.
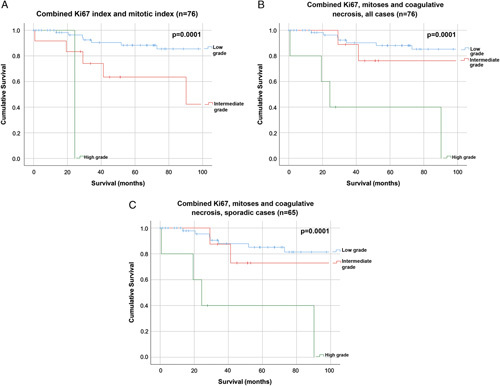
Kaplan-Meier survival curves for combined Ki-67 index and mitotic count (n=76) (A); combined Ki-67 index, mitotic count, and coagulative necrosis in all cases (n=76) (B); and combined Ki-67 index, mitotic count, and coagulative necrosis in sporadic MTC cases only (n=65) (C).
The grading system remained prognostic when analyses were restricted to sporadic cases only (P=0.0001) (Fig. 3C). There were insufficient cases of familial MTC (n=11) to conduct survival analyses within this subgroup.
When graded using only Ki-67 index and mitotic count, without the addition of coagulative necrosis, only 1 case was identified as high grade, 15 as intermediate grade, and 60 as low grade. With the addition of coagulative necrosis, 5 cases were high grade, 9 cases were intermediate grade, and 62 were low grade, and the prognostic significance of the grading system remained the same (P=0.0001) (Figs. 3A, B).
The grading system also remained prognostically significant when controlling for age and MEN2 status (hazard ratio: 4.368, 95% confidence interval: 1.490-12.804, P=0.007).
DISCUSSION
Despite close surveillance with imaging, serum calcitonin, and CEA testing, the clinical behavior of MTC remains unpredictable. In some patients who are not biochemically cured, the disease may pursue a very slow clinical course with prolonged patient survival, even in the presence of distant metastases. In contrast, some tumors exhibit much more aggressive biological behavior, with these patients rapidly succumbing to their disease. Clearly, the currently used prognostic factors are inadequate for predicting outcome in all cases of MTC.
In the present study, we propose a novel grading system that uses a combination of proliferative activity (that is the combination of mitotic count and Ki-67 proliferative index), and coagulative necrosis to predict overall survival in MTC. Several studies have shown that the Ki-67 index, mitotic count, and the presence of coagulative necrosis are of prognostic relevance in human cancers, especially in neuroendocrine tumors.29–31 Indeed, a few studies have demonstrated the value of proliferation markers as prognostic tools in MTC.11,21–23 We therefore hypothesized that a grading scheme using these factors would be of prognostic use in MTC.
In the thyroid, it has been shown that anaplastic carcinomas have higher Ki-67 indices than well-differentiated thyroid tumors,32 and that Hurthle cell carcinomas have higher Ki-67 indices than benign Hurthle cell adenomas.33 In MTC, it has been shown that Ki-67 indices are significantly higher in primary tumors that have metastasized than in those that did not metastasize.22 The same authors also found a significant association between higher Ki-67 indices and reduced survival.22 Similarly, Mian et al11 demonstrated the prognostic value of combining somatic RET mutation analysis with Ki-67 assessment in a cohort of 60 sporadic MTC patients. In an interesting study by Faggiano et al,21 it was shown that a Ki-67 score >2% could predict fluorodeoxyglucose-positron emission tomography avid tumor recurrences in a cohort of 35 MTC patients. Nevertheless, there remains some controversy surrounding the routine use of Ki-67 immunohistochemistry in MTC, as other authors have not found prognostic significance for this variable.24
Many morphologic parameters have been investigated in MTC. However there is considerable lack of consistency with regard to which of these has prognostic significance.8–10,34–37 For example, while most studies have not found an association between different cytologic and architectural patterns in MTC and prognosis,38,39 others suggest that spindle cell morphology is associated with a worse prognosis.34,40 The presence of amyloid is another controversial subject, with some authors reporting that it lacks prognostic significance,24,38–41 whereas others hold the opposite opinion.42 Other histologic variables that have been reported to have prognostic significance include vascular invasion,24,43 necrosis,34,38–40 mitotic activity,44 and desmoplasia.26 In our experience, desmoplasia is a particularly subjective feature and can be difficult to assess, especially in cases with a large amount of fibrosis or amyloid deposition. We therefore did not include this variable in our study separate to fibrosis/amyloid deposition. In the present study, the only histologic variables found to have prognostic significance were proliferative activity and the presence of coagulative necrosis. Ultimately, this variability across different studies could be explained by the low incidence of this tumor, and the variability in study design, with a large number of studies that each examines only a very small number of variables. Future cross-institutional studies with larger patient cohorts would be needed to confirm the findings in the present study and justify the implementation of this grading scheme into routine clinical practice.
The present study demonstrated a significant survival benefit for familial MTC when compared with sporadic MTC. This is a well-recognized phenomenon and is likely an effect of early diagnosis through routine genetic testing of family members of known MEN2 patients, facilitating younger age and lower disease stage at diagnosis.24,45 Although the number of familial cases in our cohort was too small to permit survival analyses within this subgroup, many authors have reported similar prognoses for familial and sporadic MTC when matched for stage.46–48 While we were able to confirm the prognostic value of our proposed grading scheme within a subgroup analysis of sporadic cases, future studies would be required to definitively confirm its prognostic value in cases of familial MTC.
It is important to note that although only the mitotic count was found to be prognostic in multivariate analyses, we have included Ki-67 index and coagulative necrosis in our grading scheme as these were prognostically significant in univariate analyses. This approach is similar to that taken by McCall et al29 in the grading of well-differentiated pancreatic neuroendocrine tumors, which is justified because the combination of these variables contributed to more accurate grading than either of the variables in isolation. In total, 9 cases in our cohort had discordant Ki-67 and mitotic grades. Unfortunately, the small number of these cases did not permit individual survival analyses within these subgroups. Similarly, although only 5 cases had coagulative necrosis, the addition of this variable to our grading scheme identified patients with a significantly shorter survival than did a grading scheme that used Ki-67 and mitotic count alone. Moreover, the robustness of the grading system is evident in its ability to predict overall survival even after controlling for age and MEN2 status.
This study is not without its weaknesses. It is a single institution study of only 76 cases, and before the grading scheme could be considered for routine practice it should be validated in independent cohorts. Although we primarily assessed the Ki-67 proliferative index in TMA sections and have used these results in our analyses, we subsequently performed Ki-67 on whole slides for 71 cases, and found good concordance for the Ki-67 scores between the whole slides and TMA sections. We also noted that Ki-67 expression and mitotic rate was quite uniform on whole sections (ie, there were no true “hotspots”). This concordance between Ki-67 index as assessed on TMAs and whole sections is in keeping with other studies in different neuroendocrine tumors.49
The study is also limited by the incomplete data on serum calcitonin levels, which were only available in 19 cases. Although no association was found between overall survival and first serum calcitonin or calcitonin doubling time, the small number of cases makes it difficult to draw conclusions from these findings. Similarly, immunohistochemistry for calcitonin and CEA was performed on all cases, and identified only 1 calcitonin negative case and 4 CEA negative cases. While these patients did have significantly shorter overall survival than those with positive staining, and it is likely that loss of expression of one or both of these markers portends a worse prognosis, the significance of these results should be interpreted with caution due to the very small number of cases. Nevertheless, this is an area that would merit further investigation in future studies with larger cohorts. Finally, we do not have data on treatment modalities, and it may be possible that in the era of targeted therapy including RET inhibition,50 the molecular findings which are not assessed in this model may take on further significance.
To the best of our knowledge, this is the first study to examine a large number of clinicopathologic variables and to propose a histopathologic grading system that can predict overall survival in MTC without the use of molecular testing. The grading system is easy to use as it includes 3 parameters that are already in widespread use for grading other neuroendocrine tumors. If our findings are confirmed in other multi-institutional cohorts, a strong argument could be made to include this simple and inexpensive grading system in routine clinical practice.
Footnotes
Conflicts of Interest and Source of Funding: Supported by the Sydney Vital Translational Cancer Research Centre, through a Cancer Institute NSW competitive grant. The views expressed herein are those of the authors and are not necessarily those of the Cancer Institute NSW. B.G.R. reports personal fees from Loxo Oncology, during the conduct of the study. B.G.R., R.J.C.-B., and V.T. report personal fees from Eisai, outside the submitted work. The remaining authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.DeLellis RA, Ghuzlan Al, Saavedra AJ, et al. Lloyd RV, Osamura RY, Kloppel G, Rosai J. Medullary thyroid carcinoma. World Health Organization (WHO) Classification of Tumours of Endocrine Organs, Vol 10, 4th edition Lyon, France: WHO, International Agency for Research on Cancer (IARC) Press; 2017:108–116. [Google Scholar]
- 2.Reagh J, Bullock M, Andrici J, et al. NRASQ61R mutation-specific immunohistochemistry also identifies the HRASQ61R mutation in medullary thyroid cancer and may have a role in triaging genetic testing for MEN2. Am J Surg Pathol. 2017;41:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Rowland KJ, Moley JF. Hereditary thyroid cancer syndromes and genetic testing. J Surg Oncol. 2015;111:51–60. [DOI] [PubMed] [Google Scholar]
- 4.LiVolsi V, DeLellis R, Komminoth P, et al. Lloyd RV, Osamura RY, Kloppel G, Rosai J. Multiple endocrine neoplasia type 2. World Health Organization (WHO) Classification of Tumours of Endocrine Organs, Vol 10, 4th edition Lyon, France: WHO, International Agency for Research on Cancer (IARC) Press; 2017:108–116. [Google Scholar]
- 5.Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharib H, McConahey WM, Tiegs RD, et al. Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin Proc. 1992;67:934–940. [DOI] [PubMed] [Google Scholar]
- 7.Girelli ME, Nacamulli D, Pelizzo MR, et al. Medullary thyroid carcinoma: clinical features and long-term follow-up of seventy-eight patients treated between 1969 and 1986. Thyroid. 1998;8:517–523. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–1148. [DOI] [PubMed] [Google Scholar]
- 9.Dottorini ME, Assi A, Sironi M, et al. Multivariate analysis of patients with medullary thyroid carcinoma. Prognostic significance and impact on treatment of clinical and pathologic variables. Cancer. 1996;77:1556–1565. [DOI] [PubMed] [Google Scholar]
- 10.Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’e´tude des tumeurs a‘ calcitonine. Clin Endocrinol (Oxf). 1998;48:265–273. [DOI] [PubMed] [Google Scholar]
- 11.Mian C, Pennelli G, Barollo S, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol. 2011;164:971–976. [DOI] [PubMed] [Google Scholar]
- 12.Joshi PP, Kulkarni MV, Yu BK, et al. Simultaneous downregulation of CDK inhibitors p18(Ink4c) and p27(Kip1) is required for MEN2ARET-mediated mitogenesis. Oncogene. 2007;26:554–570. [DOI] [PubMed] [Google Scholar]
- 13.Cavalheiro BG, Rodrigues JC. Expression of MMP-2 and TIMP-2 in medullary thyroid carcinoma. Thyroid. 2008;18:865–871. [DOI] [PubMed] [Google Scholar]
- 14.Buergy D, Weber T, Maurer GD, et al. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int J Cancer. 2009;125:894–901. [DOI] [PubMed] [Google Scholar]
- 15.Uchino S, Noguchi S, Yamashita H, et al. Somatic mutations in RET exons 12 and 15 in sporadic medullary thyroid carcinomas: different spectrum of mutations in sporadic type from hereditary type. Jpn J Cancer Res. 1999;90:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilling T, Bürck J, Sinn HP, et al. Prognostic value of codon 918 (ATG/ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. Int J Cancer. 2001;95:62–66. [DOI] [PubMed] [Google Scholar]
- 17.Fugazzola L, Muzza M, Mian C, et al. RET genotypes in sporadic medullary thyroid cancer: studies in a large Italian series. Clin Endocrinol (Oxf). 2008;69:418–425. [DOI] [PubMed] [Google Scholar]
- 18.Dvorakova S, Vaclavikova E, Sykorova V, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol Cell Endocrinol. 2008;284:21–27. [DOI] [PubMed] [Google Scholar]
- 19.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. [DOI] [PubMed] [Google Scholar]
- 20.Moura MM, Cavaco BM, Pinto AE, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;100:1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggiano A, Grimaldi F, Pezzullo L, et al. Secretive and proliferative tumor profile helps to select the best imaging technique to identify postoperative persistent or relapsing medullary thyroid cancer. Endocr Relat Cancer. 2009;16:225–231. [DOI] [PubMed] [Google Scholar]
- 22.Tisell LE, Oden A, Muth A, et al. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer. 2003;89:2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank-Raue K, Machens A, Leidig-Bruckner G, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid. 2013;23:294–300. [DOI] [PubMed] [Google Scholar]
- 24.Rios A, Rodriguez JM, Acosta JM, et al. Prognostic value of histological and immunohistochemical characteristics for predicting the recurrence of medullary thyroid carcinoma. Ann Surg Oncol. 2010;17:2444–2451. [DOI] [PubMed] [Google Scholar]
- 25.Abraham DT, Low TH, Messina M, et al. Medullary thyroid carcinoma: long-term outcomes of surgical treatment. Ann Surg Oncol. 2011;18:219–225. [DOI] [PubMed] [Google Scholar]
- 26.Koperek O, Scheuba C, Cherenko M, et al. Desmoplasia in medullary thyroid carcinoma: a reliable indicator of metastatic potential. Histopathology. 2008;52:623–630. [DOI] [PubMed] [Google Scholar]
- 27.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosai J, WHO Classification of Tumours of Endocrine Organs, 4th ed Lyon, France: IARC Press; 2017:248–250. [Google Scholar]
- 29.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. [DOI] [PubMed] [Google Scholar]
- 31.Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Tranl Lung Cancer Res. 2017;6:513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr K, Heffes C, Jin L, et al. Immunohistochemical analysis of thyroid carcinomas utilizing antibodies to p53 and Ki67. Appl Immunohistochem. 1993;1:201–207. [Google Scholar]
- 33.Erickson LA, Jin L, Goellner JR, et al. Pathologic features, proliferative activity, and cyclin D1 expression in Hurthle cell neoplasms of the thyroid. Mod Pathol. 2000;13:186–192. [DOI] [PubMed] [Google Scholar]
- 34.Franc B, Rosenberg-Bourgin M, Caillou B, et al. Medullary thyroid carcinoma: Search for histological predictors of survival (109 proband cases analysis). Hum Pathol. 1998;29:1078–1084. [DOI] [PubMed] [Google Scholar]
- 35.Brierley J, Tsang R, Simpson M, et al. Medullary thyroid cancer: analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid. 2009;6:305–310. [DOI] [PubMed] [Google Scholar]
- 36.Gulben K, Berberogu U, Boyabatli M. Prognostic factors for sporadic medullary thyroid carcinoma. World J Surg. 2006;30:84–90. [DOI] [PubMed] [Google Scholar]
- 37.Fuchshuber PR, Loree TR, Hicks WL, et al. Medullary carcinoma of the thyroid: prognostic factors and treatment recommendations. Ann Surg Oncol. 1998;5:81–86. [DOI] [PubMed] [Google Scholar]
- 38.Saad MF, Ordonez NG, Rashid RK, et al. Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine (Baltimore). 1984;63:319–342. [PubMed] [Google Scholar]
- 39.Schroder S, Bocker W, Baisch H, et al. Prognostic factors in medullary thyroid carcinoma. Survival in relation to age, sex, stage, histology, immunocytochemistry and DNA content. Cancer. 1988;61:806–816. [DOI] [PubMed] [Google Scholar]
- 40.Williams ED, Brown CL, Doniach I. Pathological and clinical findings in a series of 67 medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holm R, Sobrinho M, Nesland JM, et al. Medullary carcinoma of the thyroid gland: an immunocytochemical study. Ultrastruct Pathol. 1985;8:25–41. [DOI] [PubMed] [Google Scholar]
- 42.Pyke CM, Hay ID, Goellner JR, et al. Prognostic significance of calcitonin immunoreactivity, amyloid stainind, and flow cytometric DNA measurements in medullary thyroid carcinoma. Surgery. 1991;110:964–970. [PubMed] [Google Scholar]
- 43.Fontanini G, Vignati S, Pacini F, et al. Microvessel count: an indicator of poor outcome in medullary thyroid carcinoma but not in other types of thyroid carcinoma. Mod Pathol. 1996;9:634–641. [PubMed] [Google Scholar]
- 44.Bigner SH, Cox EB, Mendelsohn G, et al. Medullary carcinoma of the thyroid in the multiple endocrine neoplasia 2A syndrome. Am J Surg Pathol. 1981;5:459–472. [DOI] [PubMed] [Google Scholar]
- 45.Samaan NA, Schultz PN, Hickey RC. Medullary thyroid carcinoma. Prognosis of familial versus sporadic disease and the role of radiotherapy. J Clin Endocrinol Metab. 1988;7:801–805. [DOI] [PubMed] [Google Scholar]
- 46.Ukkat J, Gimm O, Brauckhoff M, et al. Single center experience in primary surgery for medullary thyroid carcinoma. World J Surg. 2004;28:1271–1274. [DOI] [PubMed] [Google Scholar]
- 47.Brauckhoff M, Lorenz K, Ukkat J, et al. Medullary thyroid carcinoma. Scand J Surg. 2004;93:249–260. [DOI] [PubMed] [Google Scholar]
- 48.Gimm O, Ukkat J, Niederle BE, et al. Timing and extent of surgery in patients with familial medullary thyroid carcinoma/multiple endocrine neoplasia 2A-related RET mutations not affecting codon 634. World J Surg. 2004;28:1312–1316. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–860. [DOI] [PubMed] [Google Scholar]
- 50.Chou A, Brown IS, Kumarasinghe MP, et al. RET gene rearrangements occur in a subset of pancreatic acinar cell carcinomas. Mod Pathol. 2020;33:657–664. [DOI] [PubMed] [Google Scholar]


