Abstract
Airway hyperresponsiveness (AHR) often defines asthma. Murine allergic airway disease (AAD), like human eosinophilic asthma, is characterized by AHR, eosinophilia, goblet cell metaplasia (GCM), smooth muscle hypercontractility and increased production of IL-4 and IL-13—cytokines that induce these characteristics by binding to the IL-4Rα chain. We evaluated the epithelial and smooth muscle IL-4Rα-dependent contributions to AHR of BALB/c mice that possessed 0–2 functional IL-4Rα alleles and had airway disease induced by house dust mite extract (HDM) or exogenous IL-13. Two functional IL-4Rα alleles were required for maximal AHR, while only one functional allele was required for maximal GCM and systemic IL-4/IL-13 levels. Deletion of IL-4Rα from both smooth muscle and epithelial cells inhibited AHR >83% in mice with two functional IL-4Rα alleles. In mice with one functional IL-4Rα allele, selective epithelial cell IL-4Rα deletion maximally inhibited AHR, while selective smooth muscle IL-4Rα deletion decreased IL-13-induced, but not HDM-induced, AHR. Less IL-4Rα signaling is required to maximize the epithelial cell contribution to AHR compared to the smooth muscle contribution to AHR. Additionally, epithelial cell responses to IL-4/IL-13 can increase the IL-4Rα-dependent smooth muscle contribution to AHR. These findings carry increasing relevance as IL-4Rα-targeted therapy is administered to human asthmatics.
Introduction
Airway hyperresponsiveness (AHR) to methacholine can define asthma when symptoms are consistent with episodic bronchoconstriction.1 Although murine allergic airway disease (AAD) is an imperfect model of human asthma, mice reliably demonstrate AHR upon cholinergic stimulation when their pulmonary tissues have been pathologically modified by inflammation.2 While several cytokines are associated with the manifestation of AAD, IL-4 and IL-13 are considered the most central for several reasons.3–5 When binding the heterodimer of IL-4Rα and the common γ chain for type I cytokine receptors on lymphocytes, IL-4 provides for the differentiation of naïve T cells into Th2 cells, maintenance of the Th2 response through autocrine effects and IgE class switching by B cells.6, 7 When binding the heterodimer of IL-4Rα and IL-13Rα1 on structural cells of the lung, IL-13 and IL-4 induce chemokine secretion, goblet cell metaplasia (GCM), myofibroblast metaplasia and smooth muscle hyperresponsiveness.3, 8–13 Additionally, IL-4Rα is required for AAD in that IL-4Rα-deficient mice do not develop allergic disease and, when reconstituted with IL-4Rα-competent bone marrow, do not demonstrate AHR despite the presence of allergic inflammation.10, 14
Because allergic AHR depends on IL-4Rα so critically, IL-4Rα-directed studies have proven useful in dissecting the mechanism of AHR in experimental asthma. Several mouse studies have evaluated the importance of IL-4Rα signaling in airway structural cell types. While these studies have shown that expression and stimulation of IL-4Rα on epithelial cells (ECs) or smooth muscle cells (SMCs) can induce AHR, selective deletion of IL-4Rα from ECs or SMCs fails to fully suppress AHR.10–14 These observations leave several questions unanswered, particularly: what is the relative importance of IL-4Rα signaling in ECs and SMCs in AAD, how do ECs and SMCs cooperate to produce AHR through IL-4Rα signaling, do other airway structural cell types contribute substantially to AHR in AAD and do the contributions to AHR by structural cell types depend on the method of disease induction.
We have now addressed these uncertainties using mice that selectively lack IL-4Rα in airway ECs, SMCs or both cell types using a reductionistic model of asthma in which AAD is induced by recombinant mouse IL-13 delivered intratracheally and an active immunization model of asthma in which AAD is induced by house dust mite (HDM) delivered intratracheally. Our results demonstrate that IL-4Rα expression by ECs and IL-4Rα expression by SMCs are individually required for full manifestation of AHR in both models of disease. However, the contribution of ECs to AHR was larger than the contribution made by SMCs, sometimes by a striking degree in a manner that depended upon genomic IL-4Rα copy number. When IL-4Rα expression by SMC was impaired, the contribution made by SMCs to AHR depended upon IL-4Rα expression by ECs. Ultimately, we found that expression of IL-4Rα by both ECs and SMCs accounted for the great majority of AHR whether disease was induced by exogenous IL-13 or HDM.
Results
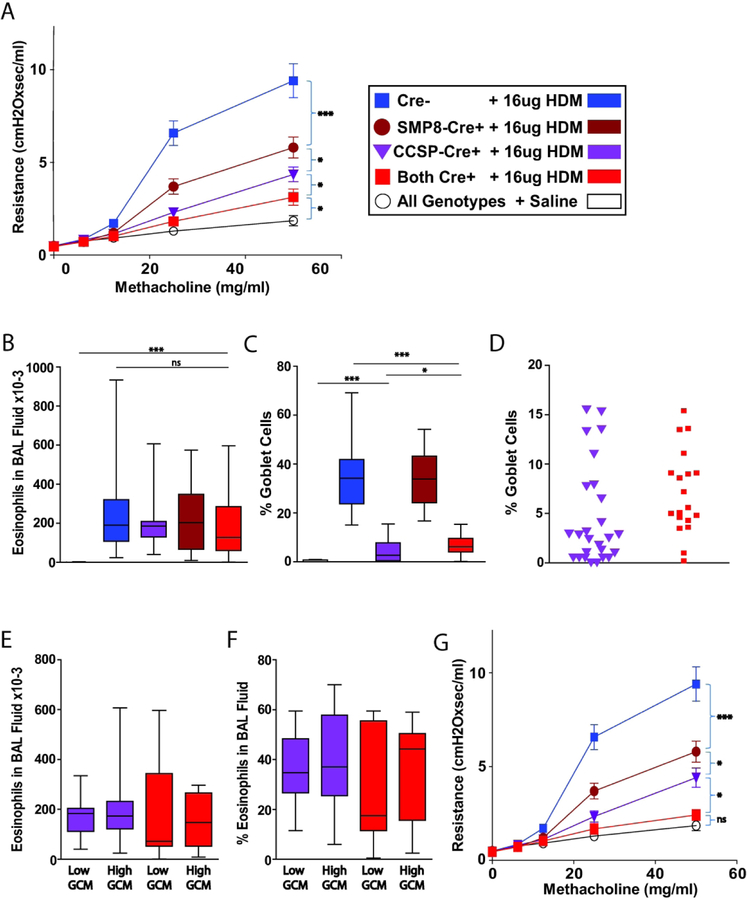
To increase efficiency of Cre-mediated disruption of IL-4Rα in targeted cell types, we initially used IL-4Rαflox/− mice that carried CC10-Cre, SMP8-Cre, both, or neither of these constructs. These mice were inoculated daily i.t. with 3 µg of IL-13 on 7 consecutive days. Severe AHR was induced in mice lacking Cre-constructs, and AHR was substantially attenuated in mice that had little or no expression of IL-4Rα by epithelial cells (ECs) or by smooth muscle cells (SMCs), as driven by the CC10-Cre or SMP8-Cre constructs, respectively. When IL-4Rα expression was reduced in both these cell types, AHR was reduced further, but not extinguished (Fig. 1A). Such treatment with IL-13 induced a modest pulmonary eosinophilia that did not consistently depend upon IL-4Rα expression by SMCs or ECs (Fig. 1B). IL-13 induced strong goblet cell metaplasia (GCM) that was impeded by the CC10-Cre construct (Fig. 1C). Because Cre-mediated gene targeting is often known to be incomplete, we compared the range and distribution of residual IL-13-induced GCM in CC10-Cre+/− and CC10-Cre+/−SMP8-Cre+/− groups (Fig. 1D). Finding their residual GCM to be very similar, we compared the AHR of these groups when there was good prevention of GCM (Fig. 1G). The value of this comparison is validated by the lack of a statistically significant difference in eosinophilia between the subgroups that demonstrated high or low GCM with either genotype (Fig. 1E and F). Fig. 1G demonstrates that AHR was still present in the CC10-Cre+/−SMP8-Cre+/− mice treated with IL-13 even when GCs were very well eliminated, but, importantly, the AHR of CC10-Cre+/−SMP8-Cre+/− mice was indistinguishable from CC10-Cre+/− mice. This demonstrates that the SMC-contribution to AHR in this model depends upon co-expression of IL-4Rα by ECs. Because the SMC-contribution to AHR was not apparent when mice carried the CC10-Cre construct and had <2% airway GCs, we wanted to evaluate whether the CC10-Cre construct was impairing SMC-function through its genomic insertion or non-specific effects of Cre. CC10-Cre+/−SMP8-Cre+/−IL-4Rα−/− mice were bred to wild-type mice to produce mice that were IL-4Rα+/− and carried one, both or neither of the Cre-constructs. Such mice would carry the putative damaging effects of the Cre-construct but the recombinase activity of Cre would not alter the native IL-4Rα allele. Administering 3 µg of the second lot of IL-13 i.t. daily 7 times to these mice generated AHR that was not impeded by either of the Cre-constructs (Fig. E4). Thus, Cre effects resulted from deletion of floxed IL-4Rα; not from a direct toxic effect.
Figure 1. IL-4Rα-expression by epithelial cells (ECs) and smooth muscle cells (SMCs) is required in IL-4Rαflox/− mice for maximal airway hyperresponsiveness (AHR) induced by exogenous IL-13.
IL-4Rαflox/− mice were treated i.t. with 3 µg of IL-13 daily for 7 days. A. Invasive measurement of AHR. B. Absolute eosinophil count of BAL fluid. C. Quantitation of goblet cell metaplasia (GCM). D. Distribution of residual GCM in club cell 10 kDa protein-Cre+ (CC10-Cre+) mice and CC10-Cre+ α-smooth muscle actin-Cre+ (SMP8-Cre+) mice. E. Absolute eosinophil count of BAL fluid in CC10-Cre+ and CC10-Cre+SMP8-Cre+ mice that had high or low residual GCM. F. Percentage of eosinophils in BAL fluid in CC10-Cre+ mice and CC10-Cre+SMP8-Cre+ that had high or low residual GCM. G, AHR when CC10-Cre+ and CC10-Cre+SMP8-Cre+ mice that had high residual GCM were excluded. The results represent 12 pooled experiments with panels A-D showing 17, 14, 26, 22 and 47 mice for groups: Cre− + IL-13, SMP8-Cre+ + IL-13, CC10-Cre+ + IL-13, Both Cre+ + IL-13, all genotypes + saline, respectively. Panel G represents 14 and 12 mice for groups: CC10-Cre+ + IL-13 and both Cre+ + IL-13, respectively. NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Because a substantial percent of CC10-Cre+ mice demonstrated excessive residual GCM upon disease induction, we sought a strain with higher efficiency for deleting IL-4Rα from epithelium. The CC10-Cre strain originally made by Dr. Brigid Hogan was generated by pseudorandom insertion of the CC10-Cre construct that very likely occurred at multiple sites within the genome. Such mice have been maintained on a non-BALB/c background by several labs, and we backcrossed the CC10-Cre strain that we received from Steven Shapiro onto the BALB/c background using a traditional approach. The same construct was independently backcrossed onto the BALB/c background by Andrew Lindsley’s lab using Taconic’s Speed Congenic system; the resulting strain was labelled CCSP-Cre. Because regions of the genome vary substantially with regard to transcription accessibility and because the process of backcrossing might have selected different insertion sites of the CC10-Cre construct, we thought it worthwhile to assess the CCSP-Cre strain’s efficacy for deletion of IL-4Rα. IL-4Rαflox/− mice that did, or did not, carry CCSP-Cre were treated with IL-13 or saline. AHR and GCM were substantially reduced, but not extinguished, in CCSP-Cre+/− mice (Fig. E5A–C) to a degree that matched the prior experiments using CC10-Cre mice. Greater, but still incomplete, reduction in AHR was observed when only CCSP-Cre+/− mice with low residual GCM were analyzed (Fig. E5D) as was observed in our studies with CC10-Cre mice (Fig. 1). Because the CCSP-Cre strain performed very similarly to the CC10-Cre strain, the CCSP-Cre stock was bred to IL-4Rαflox/flox mice for future use, while CC10-Cre+ mice were used for breeding and experimentation in the following figures.
We then compared the AHR produced by exogenous IL-13 to the AHR of allergic airway disease (AAD) produced by administering 16 µg of HDM from Greer i.t. every other day 7 times to mice that were IL-4Rαflox/− and carried at most one of the Cre-constructs. Fig. 2A–C, demonstrates very substantial reductions in both AHR and GCM when IL-4Rα-expression by respiratory epithelium is targeted by Cre, without a reduction in pulmonary eosinophilia. In contrast, Fig. 2D–F show very well preserved AHR despite Cre-mediated targeting of SMC-expression of IL-4Rα, which did not noticeably affect GCM or eosinophilia.
Figure 2. Cre-mediated deletion of IL-4Rα expression in ECs, but not SMCs, decreases AHR in house dust mite extract (HDM)-inoculated IL-4Rαflox/− mice.
IL-4Rαflox/− mice were treated i.t. with 16 µg of HDM extract (Greer) every other day for 14 days. A. Invasive measurement of AHR. B. Absolute eosinophil count of BAL fluid. C. Quantitation of GCM. D. Invasive measurement of AHR. E. Absolute eosinophil count of BAL fluid. F. Quantitation of GCM. Panels A-C, the results represent 3 pooled experiments showing 17, 19, and 34 mice for groups: Cre− + HDM, CC10-Cre+ + HDM and both genotypes + saline, respectively. Panels D-F, the results represent 5 pooled experiments showing 18, 20 and 36 mice for groups: Cre− + HDM, SMP8-Cre+ + HDM and both genotypes + saline, respectively. NS, not significant; **, p < 0.01; ***, p < 0.001.
Finding that IL-4Rα-expression by SMCs did not significantly impair AAD when ECs expressed IL-4Rα normally, we wanted to determine if such a contribution could be more readily identified when ECs had impaired expression of IL-4Rα and explore whether results varied with the preparation of HDM-extract. Consequently, we administered 16 µg of homemade HDM extract i.t. every other day over a 14 day period to mice that were IL-4Rαflox/− and carried one, both or neither of the Cre-constructs. The resultant AHR matched the prior Greer HDM experiments, and a substantial decrement in AHR was again present in CC10-Cre+/− mice but not in SMP8-Cre+/− mice (Fig. 3A). Pulmonary eosinophilia was substantial in those groups treated with HDM, irrespective of IL-4Rα expression by ECs and SMCs (Fig. 3B), while GCM depended on both HDM-administration and EC IL-4Rα expression (Fig. 3C). Analogous to the IL-13-based studies, CC10-Cre+/− mice and CC10-Cre+/−SMP8-Cre+/− mice with AAD demonstrated very similar residual GCM (Fig. 3D). When comparing the AHR of the subgroups that demonstrated very good prevention of GCM, almost all AHR was attributable to EC-expression of IL-4Rα (Fig. 3G), although there was an insignificant trend towards lower AHR in the group that expressed Cre in both SMCs and ECs. Again, analogous to the IL-13-based studies, eosinophilia did not consistently differ between the subgroups that demonstrated high or low residual GCM with either genotype (Fig. 3E and F).
Figure 3. SMC IL-4Rα expression has little effect on HDM extract-induced AHR in IL-4Rαflox/− mice.
IL-4Rαflox/− mice were treated i.t. with 16 µg of HDM extract (produced in our lab) every other day for 14 days. A. Invasive measurement of AHR. B. Absolute eosinophil count of BAL fluid. C. Quantitation of GCM. D. Distribution of residual GCM in CC10-Cre+ and CC10-Cre+SMP8-Cre+ mice. E. Absolute eosinophil count of BAL fluid in CC10-Cre+ and CC10-Cre+SMP8-Cre+ mice that had high or low residual GCM. F. Percentage of eosinophils in BAL fluid in CC10-Cre+ mice and CC10-Cre+SMP8-Cre+ that had high or low residual GCM. G. AHR when CC10-Cre+ and CC10-Cre+SMP8-Cre+ mice that had high residual GCM were excluded. H. In vivo cytokine responses. For panels A-G, the results represent 14 pooled experiments with panels A-D showing: 24, 19, 32, 20 and 52 mice for the groups: Cre− + HDM, SMP8-Cre+ + HDM, CC10-Cre+ + HDM, both Cre+ + HDM, all genotypes + saline, respectively. Panel G represents 14 and 13 mice for groups: CC10-Cre+ + HDM and Both Cre+ + HDM, respectively. Panel H represents 2 pooled experiments with 9, 9, 31, 26 mice per group: Cre- + saline, CC10-Cre+ + saline, Cre− + HDM and CC10-Cre+ + HDM, respectively. NS, not significant; *, p < 0.05; **, p = 0.01; ***, p < 0.001.
While the AHR of CC10-Cre+/−IL-4Rflox/− mice was substantially reduced in disease induced by exogenous IL-13, some of this reduction in the HDM-based model might have been due to reduced production of IL-13 and/or IL-4 in vivo. To address this, IL-4Rflox/− mice that did, or did not, carry CC10-Cre were treated i.t. with 16 µg of homemade HDM every other day for 14 days and secretion of IL-13, IL-4 and IFN-γ were measured by IVCCA. The substantial increase in IL-13 and IL-4 levels upon disease induction did not depend on the CC10-Cre genotype of the mouse (Fig 3, H). Thus, EC IL-4Rα expression does not appear to affect the type 2 cytokine response in AAD.
To address the potential significance of IL-4Rα heterozygosity in these experiments, we administered IL-13 to wild-type (WT = IL-4Rα+/+) and IL-4Rαflox/− mice. The WT mice developed substantially greater pulmonary eosinophilia and AHR than the IL-4Rαflox/− mice, although GCM appeared unaffected by IL-4Rα zygosity (Fig. 4A–C). This prompted us to also compare the responses of WT and IL-4Rαflox/− mice to HDM. Fig. 4D shows the AHR of WT mice was accentuated over that of IL-4Rαflox/− mice, while neither pulmonary eosinophilia nor GCM was affected by IL-4Rα zygosity (Fig. 4E and F). The similar GCM in IL-4Rα+/+, IL-4Rα+/− and IL-4Rαflox/− mice, but increased AHR in the IL-4Rα+/+ mice, suggested that non-epithelial airway cells contribute considerably more to AHR in IL-4Rα+/+ than in IL-4Rα+/− or IL-4Rαflox/− mice, while cytokine levels do not depend upon a second functional IL-4Rα allele. We evaluated this hypothesis with a subsequent experiment in which cytokine levels were measured in vivo when WT and IL-4Rα+/− mice were treated with HDM. IL-4Rα+/− mice actually demonstrated higher levels of IL-4 and IL-13 than WT mice (Fig. 4G). While several mechanisms might explain this finding, we favor the possibility that IL-4 and IL-13 usage (or neutralization by soluble receptors15) is higher in mice that have two, rather than one, functional IL-4Rα alleles.
Figure 4. A single functional IL-4Rα allele is sufficient for induction of maximal GCM and IL-4 and IL-13 responses, but not AHR.
A–C: mice were treated i.t. with 3 µg IL-13 daily for 7 days. A. Invasive measurement of AHR. B. Absolute eosinophil count of BAL fluid. C. Quantitation of GCM. D-G. Mice were treated i.t. with 16 µg HDM extract (produced in our lab) every other day over 14 days. D. Invasive measurement of AHR. E. Absolute eosinophil count of BAL fluid. F. Quantitation of GCM. G. Measurement of cytokine production in vivo for the ~24hrs following the seventh i.t. treatment with HDM or saline. A–C. 8, 17 and 18 mice for the experimental groups: WT + IL-13, IL-4Rαflox/− + IL-13 and both genotypes + saline, respectively. D–F. 17, 24 and 33 mice for the experimental groups: WT + HDM, IL-4Rαflox/− + HDM and both genotypes + saline, respectively. G. 5, 8, 7 and 8 mice for the groups: WT + saline, IL-4Rα+/− + saline, WT + HDM and IL-4Rα+/− + HDM, respectively. Because of breeding constraints, WT mice and IL-4Rα+/− mice were not littermates to IL-4Rαflox/− or to themselves. NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To determine if smooth muscle contributes more to AHR in IL-4Rαflox/flox than in IL-4Rαflox/− mice, we bred CCSP-Cre+/−IL-4Rαflox/flox mice to SMP8-Cre+/−IL-4Rαflox/flox mice and treated the offspring i.t. with 16 µg of Greer HDM every other day for 14 days. The AHR of HDM-inoculated IL-4Rαflox/flox mice exceeded that of IL-4Rαflox/− mice in our prior experiments, as shown by increased sensitivity to 25 mg/ml of methacholine (Compare Fig. 5A with Figs. 2A and 2D). This increase in AHR depended substantially on expression of IL-4Rα by both ECs and SMCs. Pulmonary eosinophilia depended on HDM-treatment but was independent of IL-4Rα expression by ECs and SMCs (Fig. 5B). GCM was induced by HDM treatment and depended upon IL-4Rα expression by ECs but not by SMCs (Fig. 5C and 5D). Given that Cre-mediated gene disruption is typically less than complete in any cell type, the AHR of BALB/c IL-4Rα+/+ mice with severe, HDM-induced AHR appears to be at least 67% attributable to EC-expression of IL-4Rα alone, at least 48% attributable to SMC-expression of IL-4Rα alone, and at least 83% attributable to simultaneous expression of IL-4Rα on ECs and SMCs. Indeed, when only those mice that had <5% GCM were considered, HDM-induced AHR appeared to be >92% attributable to simultaneous EC and SMC IL-4Rα expression (Fig. 5G). Table 1 summarizes the AHR, GCM and pulmonary eosinophilia produced by exogenous IL-13 or HDM in all of our experiments.
Figure 5. Both EC and SMC IL-4Rα expression contribute substantially to AAD AHR in IL-4Rαflox/flox mice.
IL-4Rαflox/flox mice were treated i.t. with 16 µg HDM extract (Greer) every other day for 14 days. A, Invasive measurement of AHR. B, Absolute eosinophil count of BAL fluid. C, Quantitation of GCM. D, Distribution of residual GCM in CCSP-Cre+ and CCSP-Cre+SMP8-Cre+ mice. E, Absolute eosinophil counts of BAL fluid in IL-4Rαflox/flox mice that possessed the CCSP-Cre construct and had high (>5%) or low (<5%) percentages of residual GCM. F, Eosinophil percentages of BAL fluid in IL-4Rαflox/flox mice that possessed the CCSP-Cre construct and had high or low percentages of residual GCM. G, Effect of CCSP-Cre and SMP8-Cre on AHR in HDM extract-immunized IL-4Rαflox/flox mice that had <5% goblet cells in their medium-sized airways. Results represent 6 pooled experiments with panels A-D showing 24, 15, 28, 20 and 23 mice for the experimental groups: Cre− + HDM, SMP8-Cre+ + HDM, CCSP-Cre+ + HDM, Both Cre+ + HDM, all genotypes + saline, respectively. For Panel G, the groups CCSP-Cre+ + HDM, Both Cre+ + HDM represent 18 and 8 mice respectively. NS, not significant; *, p < 0.05; ***, p < 0.001.
Table 1.
Summary of Results
| Stimulus | # of Functional IL-4Rα Alleles Per Cell in Smooth Muscle | # of Functional IL-4Rα Alleles Per Cell in Epithelium | # of Functional IL-4Rα Alleles In All Other Cell Types | AHR | GC Metaplasia | Eosinophilia | Figure # in Article |
|---|---|---|---|---|---|---|---|
| Exogenous IL-13 | 1 | 1 | 1 | ++++ | ++++++++ | + | 1 |
| Exogenous IL-13 | 0* | 1 | 1 | ++ | ++++++++ | + | 1 |
| Exogenous IL-13 | 1 | 0* | 1 | ++ | + | + | 1 |
| Exogenous IL-13 | 0 | 0 | 1 | + | + | + | 1 |
| Exogenous IL-13 | 2 | 2 | 2 | ++++++++ | ++++++++ | + | 4 |
| House Dust | 1 | 1 | 1 | +++ | ++++++++ | ++++++++ | 2,3 |
| Mite Extract | |||||||
| House Dust | 0 | 1 | 1 | +++ | ++++++++ | ++++++++ | 2,3 |
| Mite Extract | |||||||
| House Dust | 1 | 0 | 1 | + | + | ++++++++ | 2,3 |
| Mite Extract | |||||||
| House Dust | 0 | 0 | 1 | + | + | ++++++++ | 2,3 |
| Mite Extract | |||||||
| House Dust | 2 | 2 | 2 | ++++++ | ++++++++ | ++++++++ | 4,5 |
| Mite Extract | |||||||
| House Dust | 0 | 2 | 2 | +++ | ++++++++ | ++++++++ | 5 |
| Mite Extract | |||||||
| House Dust | 2 | 0 | 2 | +++ | + | ++++++++ | 5 |
| Mite Extract | |||||||
| House Dust | 0 | 0 | 2 | + | + | ++++++++ | 5 |
| Mite Extract |
“0” denotes complete genetic deletion of IL-4Rα in the great majority of the cells targeted by Cre, rather than complete genetic deletion in every Cre-targeted cell.
Discussion
AHR is the defining physiological characteristic of human asthma and animal models of this disorder.1, 16 The development and maintenance of AHR in most mouse models of AAD, which has several features in common with human asthma, completely depends on two cytokines, IL-4 and IL-13.4, 5 While IL-13 is produced in higher amounts than IL-4, both bind heterodimeric receptors that share the required subunit for signal-transduction, IL-4Rα.8, 17 This dependence has been demonstrated in studies utilizing molecular blockade of IL-4Rα function or genetic deletion of IL-4Rα.10, 18 The clinical relevance of these observations has been demonstrated by increased IL-4 and IL-13 production in the airways of patients who have eosinophilic asthma19 and by the therapeutic success of dupilumab, a mAb that blocks IL-4Rα.20–22
Previous studies with mice that entirely fail to express IL-4Rα or selectively express IL-4Rα on only a single cell type have implicated airway epithelial cells and smooth muscle cells as important effectors in IL-4Rα-dependent AHR.10–14 IL-13 administration and increased endogenous IL-13 production stimulate AHR in mice that only express IL-4Rα on smooth muscle or airway epithelium.10, 11 However, in contrast to mice that globally lack IL-4Rα expression, which totally fail to develop AHR and AAD, supraphysiological amounts of IL-13 induce AHR in mice that lack IL-4Rα on smooth muscle, but express IL-4Rα on airway epithelium, and vice versa.10, 11 These observations are compatible with at least 3 possibilities: (1) IL-4Rα-dependent effects on either smooth muscle and airway epithelial cells might be sufficient for IL-13 induction of AHR, regardless of IL-13 level; (2) IL-4Rα expression by both smooth muscle and airway epithelium might be more important for AHR induction when IL-13 levels are suboptimal than when supraphysiological levels of this cytokine are present; and (3) IL-4Rα-dependent stimulation of cell types other than smooth muscle and airway epithelium, such as endothelial cells, fibroblasts, eosinophils, mast cells, macrophages, dendritic cells, and neurons23–28 might be sufficient to induce AHR.
To distinguish among these possibilities, we studied the development of AHR and other features of AAD in mice using a Cre/loxp approach to selectively deplete IL-4Rα from smooth muscle, airway epithelium, or both cell types, and applied this approach to mice that expressed IL-4Rα through one or two functional IL-4Rα alleles (IL-4Rαflox/− and IL-4Rαflox/flox mice, respectively). AAD was induced in these mice by i.t. administration of either IL-13 or the clinically relevant allergen, HDM. All studies were performed in BALB/c background mice, which reliably develop severe AHR in response to both HDM and IL-13.
In studies with IL-4Rαflox/− mice, AHR produced by exogenous IL-13 was considerably attenuated by the absence of IL-4Rα on smooth muscle (SMP8-Cre+ mice) and by the absence of IL-4Rα on airway epithelium (CC10-Cre+ mice). Significantly greater suppression of HDM-induced AHR was observed when mice carried both SMP8-Cre and CC10-Cre than when mice carried only CC10-Cre, when all mice that expressed CC10-Cre were evaluated (Fig. 1A). However, this difference was not apparent when CC10-Cre+ mice demonstrating considerable goblet cell metaplasia were excluded based upon the high likelihood of incomplete deletion of IL-4Rα from their epithelium by CC10-Cre (Fig. 1G).
Different results were obtained when AHR was induced by i.t. HDM, which causes less severe AHR than IL-13 at the doses used (compare Figs. 1A and 3A). With HDM as the stimulus, AHR in IL-4Rαflox/− mice was strongly suppressed by CC10-Cre, while SMP8-Cre had no effect (Figs. 2A, 2D, and 3A). The combination of CC10-Cre and SMP8-Cre tended to suppress AHR induction more than CC10-Cre alone, but this tendency was not statistically significant whether all CC10-Cre-expressing mice or only those with the strongest suppression of goblet cell metaplasia were analyzed (Figs. 3A and G).
Because IL-13 and HDM induced more severe AHR in WT and IL-4Rαflox/flox mice than in IL-4Rαflox/− mice, it was possible that the effects of selective IL-4Rα deletion might also depend on IL-4Rα gene dose. Indeed, in experiments performed with HDM-inoculated IL-4Rαflox/flox mice, AHR was decreased by the presence of either CCSP-Cre or SMP8-Cre and further decreased by the carriage of both genetic constructs (Figs. 5A and 5G). Combined epithelial and smooth muscle expression of Cre decreased the maximal airway resistance induced by methacholine in HDM-inoculated IL-4Rαflox/flox mice by ~83%, as compared to HDM-inoculated IL-4Rαflox/flox Cre− mice and saline-inoculated mice when all mice carrying CCSP-Cre were included in our analysis. Notably, AHR appeared to be all but extinguished in HDM-treated CCSP-Cre+SMP8-Cre+ mice when only those mice with <5% GCM were included in analysis. It remains to be determined whether the remaining AHR resulted from IL-4/1L-13 effects on cell types other than smooth muscle and airway epithelium or from incomplete loss of IL-4Rα expression by these two cell types. Regardless, our observations indicate that both smooth muscle and airway epithelial responsiveness to IL-4/IL-13 contribute importantly to AHR in mice with two functional IL-4Rα genes that have been immunized via the airway with a clinically relevant allergen; and that the IL-4/IL-13 responsiveness of these two cell types accounts for the great majority of AHR induction by these cytokines. It remains, possible, however, that IL-4Rα expression by other cell types is necessary for AHR induced by HDM.
The likelihood that IL-4/IL-13-mediated effects on airway epithelium and smooth muscle account for most or all of the effects of these cytokines on AHR makes sense given that increases in airway resistance are caused by narrowing and occlusion of airways12, 29, 30. IL-4/IL-13 induction of goblet cell metaplasia and mucus hypersecretion narrows and likely occludes airways during methacholine challenge; such narrowing and occlusion is augmented by smooth muscle contraction that is enhanced by IL-4/IL-13 activity directly on smooth muscle. However, our observations also suggest that there are quantitative differences in AHR induction by IL-4/IL-13 effects on smooth muscle vs. airway epithelium. IL-4/IL-13 effects on airway epithelium contributed to AHR under all conditions tested, while their effects on smooth muscle were only observed in mice treated with HDM when two functional IL-4Rα alleles were present and in mice treated with supraphysiologic amounts of exogenous IL-13 when a single functional IL-4Rα allele was present. Because of this differential effect on smooth muscle and epithelium, it seems likely that greater IL-4Rα stimulation is required for smooth muscle than for airway epithelium to induce a physiologically significant effect on AHR. This is consistent with our previous observations that IL-4 and IL-13 have a much stronger effect on epithelial cell than smooth muscle gene expression.10
Experiments of the types that we have performed can be subject to artifacts; we have exerted considerable effort to investigate such possibilities. The concern that carriage of Cre-containing constructs might inhibit epithelial and smooth muscle cell function non-specifically was ruled out by comparing AHR in Cre− and Cre+ mice that had WT rather than floxed IL-4Rα genes. The concern that IL-4Rα deletion on airway epithelial cells might be incomplete was evaluated by counting airway GCs, inasmuch as GC metaplasia is driven by IL-4/IL-13 in our model, and mice with near-complete suppression of GC metaplasia were evaluated separately, when possible. Fortunately, depletion of IL-4Rα from SMCs was likely more extensive than in ECs in our experiments. Previously10, we found SMP8-Cre was highly effective at deleting IL-4Rα from SMCs in IL-4Rαflox/− mice. However, because efficacy was not confirmed in the current experiments, we cannot completely eliminate the possibility that some of the residual AHR seen in our experiments might be due to SMC expression of IL-4Rα.
The concern that near-complete suppression of GC metaplasia might merely reflect decreased IL-4/IL-13 stimulation was excluded by evaluating airway eosinophilia, which did not correlate with the percent of airway GCs in CC10-Cre+ IL-4Rαflox/− or IL-4Rαflox/flox mice. An additional concern, that IL-4Rα deletion from the airway epithelium might affect IL-4 and IL-13 levels, was negated by our measurement of these levels by IVCCA (Fig. 3H), showing higher levels of these cytokines in IL-4Rαflox/− mice compared to WT mice (Fig. 4G). The finding that IL-4Rα heterozygosity increased levels of IL-4 and IL-13 was unexpected. While several mechanisms for this are certainly possible and not mutually exclusive, an increased absorption of these cytokines in the WT mice because of higher expression of cytokine receptors has a good chance of contributing to this phenomenon. However, because we measured IL-4 and IL-13 systemically, we cannot exclude the possibility that a selective decrease in expression of IL-4Rα on airway smooth muscle or epithelium selectively increases local concentrations of IL-4/IL-13 by decreasing cellular receptor absorption of these cytokines. These putative increased IL-4/IL-13 concentrations could then stimulate the remaining IL-4Rα-expressing cell types to a greater extent. If so, this compensatory effect could lead to underestimation of the effects of IL-4Rα deletion from a single cell type.
There are, however, some remaining limitations of our study. As noted above, neither CC10-Cre nor CCSP-Cre reliably and completely deleted IL-4Rα from all airway epithelial cells, as judged by residual GC metaplasia. This may have led us to underestimate the importance of GC metaplasia and other possible effects of IL-4/IL-13 on airway epithelium in the pathogenesis of AHR. Secondly, SMP8 is not completely specific for smooth muscle cells, but is also expressed by myofibroblasts9. Consequently, our data are consistent with the possibility that IL-4Rα expression by this cell type contributes to AHR. Most importantly, there are considerable differences between murine and human airway anatomy, including the presence of submucosal mucus glands and a substantial layer of smooth muscle in humans, as well as obvious differences in lung size and the number of divisions of bronchioles.2 Although these differences may well affect the relative quantitative importance of smooth muscle vs. airway epithelial responsiveness to IL-4/IL-13 in AHR pathogenesis, it seems unlikely that the combined importance of these two cell types will differ substantially between the species.
The growing use of therapies that target IL-4Rα has increased the importance of identifying the cell types that are most involved in IL-4/IL-13 contributions to immune-mediated disease. Although IL-4Rα signaling drives allergic disorders, it also protects against multicellular parasites (arthropods and worms), contributes to wound healing, and may protect against immunologic disorders mediated predominantly by non-type 2 T cells.31–41 Consequently, the safety of targeting of IL-4Rα for therapeutic means would be optimal when such therapy is specific to the organ and/or cell types that manifest disease. Because our observations demonstrate that smooth muscle and airway epithelium mediate the great majority, if not all, AHR in experimental allergic asthma and do so through expression of IL-4Rα, future asthma therapeutics might achieve an optimal risk/benefit ratio by targeting IL-4Rα expression on just these two cell types.
Methods
Mice:
Both male and female mice on the BALB/c background were used at 7–10 weeks of age in all experiments. Littermates served as controls in all experiments except where noted. IL-4Rα−/− mice42 and IL-4Rαflox/flox mice43 were originally provided by Frank Brombacher and bred in our animal facility. Mice carrying either a club cell 10 kDa protein-Cre (CC10-Cre) construct44 or α-smooth muscle actin-Cre (SMP8-Cre) construct10 were provided on the C57BL/6 background by Steven Shapiro and James Fagin respectively, and were each backcrossed more than 10 generations onto the BALB/c background. Club cell secretory protein-Cre (CCSP-Cre)+/−45 mice were backcrossed onto BALB/c and provided by Andrew Lindsley. All 3 of these constitutively active Cre-constructs were then crossed onto the IL-4Rα−/− background. Additionally, the SMP8-Cre and CCSP-Cre constructs were crossed onto the IL-4Rαflox/flox background. For experiments on the IL-4Rαflox/− background, CC10-Cre+/−SMP8-Cre+/−IL-4Rα−/− mice, CC10-Cre+/−IL-4Rα−/− mice, and SMP8-Cre+/−IL-4Rα−/− mice were bred to IL-4Rαflox/flox mice. For experiments on the IL-4Rαflox/flox background, CCSP-Cre+/−IL-4Rαflox/flox mice were bred to SMP8-Cre+/−IL-4Rαflox/flox mice. The PCR primers for genotyping mice specifically for the SMP8-Cre construct were: GCC TGT GAC ACT CCC GCT and CCA GGC TAA GTG CCT TCT CTA CA, and for the CC10-Cre & CCSP-Cre constructs: GTG CAA TTT CTT GAG TGG AGG ACA AT and TTC TTG CGA ACC TCA TCA CTC. Animals were bred and used in an AAALAC-approved specific pathogen-free environment using barrier isolation with protocols approved by the Cincinnati Children’s Hospital Medical Center’s (CCHMC) Institutional Animal Care and Use Committee, allowed access to food and water ad libitum and monitored daily by the veterinary staff for health and well-being. All experimentation was performed in a manner to conscientiously minimize stress and suffering. Mice were sacrificed through intraperitoneal injection of pentobarbital and xylazine.
Reagents:
Lyophilized HDM extract from Greer (Lenoir,NC), XPB70D3A25, was resuspended in sterile saline and stored at −80° C. We also prepared an HDM extract from whole D. pteronyssinus mites (2.04 gms, ALK-abello Source Materials Inc. (Post Falls, ID), lot# 10–1289 and 5.03 gms Greer Source Materials (Lenoir,NC), RMB82F, lot#166421) by pulverizing them in phosphate buffered saline (PBS) in 50 ml conical tubes for 5 or 10 minutes using a tissue homogenizer (TissueMiser, FisherScientific) at maximum speed while on ice. Homogenate was progressively centrifuged up to 40 K rpm, and the supernatant was: passed through 40 µm pore strainer, brought to a pH of 7, dialyzed against normal saline using a MWCO 6000–8000 membrane (Spectra Por 1, FisherScientific), filtered through a 0.45 µm pore membrane by syringe and its protein content quantitated by Bradford / Coomassie blue. Lyophilized IL-13, from PeproTech (Rocky Hill, NJ), 210–13, was resuspended in molecular grade water and either: left at room temperature for a few minutes before storing at −80° C (Lot 1) or left at 4° C for 1 week before storing at −80° C (Lot 2).
Induction of airway disease:
For HDM-based experiments, 16 µg of HDM protein were administered intratracheally (i.t.) in 40 µl every other day for 7 treatments (Fig E1). For IL-13-based experiments, 2 or 3 µg IL-13 were administered i.t. in 40 µl daily for 7 treatments (Fig E2).
Determination of airway responsiveness to β-methacholine:
Barometric plethysmography was performed as described in some experiments46, being measured 1 day after the last treatment with HDM or approximately 8 hours after the last treatment of IL-13. Due to inherent imprecision and variability, this technique was abandoned and results not reported. Invasive measurement of airway resistance was performed as described by forced oscillation using the flexiVent system (Scireq, Montreal, Canada) and measured 2 days after the last treatment with HDM or 1 day after the last treatment with IL-13.46
Bronchoalveolar lavage (BAL) and quantitation of BAL cells:
This was performed as described.47
Goblet cell enumeration:
Periodic acid-Schiff (PAS)-stained histologic slides were prepared by the CCHMC research pathology core. The epithelial cells of all medium-sized airways in one lung section per mouse were counted in a blinded fashion and the percent of goblet cells was calculated. Central airways were also examined for their degree of goblet cell metaplasia (GCM). Mice were considered to have good prevention of GCM if less than 2% of the cells in medium sized airways were PAS+ and central airways lacked large patches of PAS+ cells.
Measurement of IL-4, IL-13 and IFN-γ production in vivo:
This was achieved using the In Vivo Cytokine Capture Assay (IVCCA) as previously described.48 Briefly: biotin-labeled anti-cytokine monoclonal IgG antibodies (mAb) specific for IL-4, IL-13, and/or IFN-γ were injected intravenously a few minutes before the last i.t. treatment with HDM, these mAbs bound the cytokines of interest with high affinity at an epitope that is required for cytokine binding to their respective cytokine receptors, blood was obtained ~24 hrs after injection and serum cytokine-IgG complexes were quantitated by ELISA (Fig E3).
Statistics:
The Shapiro-Wilk test was used to determine if data sets were normally distributed, which was generally not the case. For significance testing, the non-parametric Kruskall-Wallis test was used for multiple group comparisons, the non-parametric Mann-Whitney U test was used to compare 2 groups and a two-way ANOVA was used to analyze methacholine challenges. “Box and whiskers” plots depict the second and third quartiles of data within boxes which are additionally marked with the median value and depict the highest and lowest values with bars. A p value equal to or less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Frank Brombacher, Steven Shapiro and James Fagin for sharing their mice. This research was funded by: NIH R01 HL097360 (FDF), NIH/NIAID T32 AI060515 (CGM), and a University of Cincinnati Department of Internal Medicine Fellow to Faculty Award (CGM).
Disclosure
The authors have no disclosures for conflicts of interest regarding this work which was funded by: NIH R01 HL097360 (FDF), NIH/NIAID T32 AI060515 (CGM), and a University of Cincinnati Department of Internal Medicine Fellow to Faculty Award (CGM).
Footnotes
Supplementary Material is linked to the online version of the paper at: http://www.nature.com/mi.
Disclosure
All authors have no competing interests to report.
References
- 1.Steel MD, Holgate ST. Asthma In Samter’s Immunologic Diseases. . Sixth edition edn. Lippincott Williams & Wilkins: Philadelphia, 2001. [Google Scholar]
- 2.Finkelman FD, Wills-Karp M. Usefulness and optimization of mouse models of allergic airway disease. J Allergy Clin Immunol 2008; 121(3): 603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL et al. Interleukin-13: central mediator of allergic asthma. Science 1998; 282(5397): 2258–2261. [DOI] [PubMed] [Google Scholar]
- 4.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol 2006; 118(2): 410–419. [DOI] [PubMed] [Google Scholar]
- 5.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998; 282(5397): 2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med 1990; 172(3): 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol 1986; 136(12): 4538–4541. [PubMed] [Google Scholar]
- 8.Zurawski SM, Chomarat P, Djossou O, Bidaud C, McKenzie AN, Miossec P et al. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem 1995; 270(23): 13869–13878. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol 2001; 107(6): 1001–1008. [DOI] [PubMed] [Google Scholar]
- 10.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C et al. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 2011; 208(4): 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002; 8(8): 885–889. [DOI] [PubMed] [Google Scholar]
- 12.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? Journal of applied physiology (Bethesda, Md : 1985) 2004; 96(6): 2019–2027. [DOI] [PubMed] [Google Scholar]
- 13.Kuperman DA, Huang X, Nguyenvu L, Holscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol 2005; 175(6): 3746–3752. [DOI] [PubMed] [Google Scholar]
- 14.Kirstein F, Horsnell WG, Kuperman DA, Huang X, Erle DJ, Lopata AL et al. Expression of IL-4 receptor alpha on smooth muscle cells is not necessary for development of experimental allergic asthma. J Allergy Clin Immunol 2010; 126(2): 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodoun M, Lewis CC, Yang JQ, Orekov T, Potter C, Wynn T et al. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J Immunol 2007; 179(10): 6429–6438. [DOI] [PubMed] [Google Scholar]
- 16.Lewkowich IP, Wills-Karp M. Animal models of allergen-induced asthma In Middleton’s Allergy: Principles and Practice. Seventh edn. Elsevier: London, 2008. [Google Scholar]
- 17.Zurawski SM, Vega F Jr., Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J 1993; 12(7): 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomkinson A, Duez C, Cieslewicz G, Pratt JC, Joetham A, Shanafelt M-C et al. A Murine IL-4 Receptor Antagonist That Inhibits IL-4- and IL-13-Induced Responses Prevents Antigen-Induced Airway Eosinophilia and Airway Hyperresponsiveness. The Journal of Immunology 2001; 166(9): 5792–5800. [DOI] [PubMed] [Google Scholar]
- 19.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol 1995; 155(5): 2688–2694. [PubMed] [Google Scholar]
- 20.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368(26): 2455–2466. [DOI] [PubMed] [Google Scholar]
- 21.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med 2018; 378(26): 2486–2496. [DOI] [PubMed] [Google Scholar]
- 22.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med 2018; 378(26): 2475–2485. [DOI] [PubMed] [Google Scholar]
- 23.Barnes JC, Lumsden RV, Worrell J, Counihan IP, O’Beirne SL, Belperio JA et al. CXCR3 Requirement for the Interleukin-13-Mediated Up-Regulation of Interleukin-13Ralpha2 in Pulmonary Fibroblasts. Am J Respir Cell Mol Biol 2015; 53(2): 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011; 332(6035): 1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Bravo M, Minguito de la Escalera M, Dominguez PM, Gonzalez-Cintado L, del Fresno C, Martin P et al. IL-4 blocks TH1-polarizing/inflammatory cytokine gene expression during monocyte-derived dendritic cell differentiation through histone hypoacetylation. J Allergy Clin Immunol 2013; 132(6): 1409–1419. [DOI] [PubMed] [Google Scholar]
- 26.McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine 2015; 75(1): 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017; 171(1): 217–228 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamani A, Wu D, Waggoner L, Noah T, Koleske AJ, Finkelman F et al. The vascular endothelial specific IL-4 receptor alpha-ABL1 kinase signaling axis regulates the severity of IgE-mediated anaphylactic reactions. J Allergy Clin Immunol 2018; 142(4): 1159–1172 e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 2007; 175(8): 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy 2015; 45(4): 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen E, Xie K, Jwo K, Smith J, Mosaed S. Dupilumab-Induced Follicular Conjunctivitis. Ocul Immunol Inflamm 2018: 1–3. [DOI] [PubMed]
- 32.Tracey EH, Elston C, Feasel P, Piliang M, Michael M, Vij A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep 2018; 4(7): 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevray M, Dupre D, Misery L, Abasq-Thomas C. Hair regrowth and dissemination of molluscum contagiosum: two unexpected effects with dupilumab. J Eur Acad Dermatol Venereol 2019. [DOI] [PubMed]
- 34.Safa G, Paumier V. Psoriasis induced by dupilumab therapy. Clin Exp Dermatol 2019; 44(3): e49–e50. [DOI] [PubMed] [Google Scholar]
- 35.Yazdanyar S, Jemec GBE. Alopecia Areata After Treatment with Dupilumab. Dermatitis 2019; 30(2): 175–176. [DOI] [PubMed] [Google Scholar]
- 36.Maloney NJ, Worswick S, Cheng K. Development of alopecia in patients treated with dupilumab. Dermatol Ther 2019: e12869. [DOI] [PubMed]
- 37.Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep 2019; 5(1): 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A 2009; 106(1): 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog 2011; 7(5): e1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban JF Jr., Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ et al. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell-and T cell-dependent mechanism. J Immunol 2000; 164(4): 2046–2052. [DOI] [PubMed] [Google Scholar]
- 41.Herbert DR, Orekov T, Perkins C, Rothenberg ME, Finkelman FD. IL-4R alpha expression by bone marrow-derived cells is necessary and sufficient for host protection against acute schistosomiasis. J Immunol 2008; 180(7): 4948–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol 1999; 162(12): 7302–7308. [PubMed] [Google Scholar]
- 43.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 2004; 20(5): 623–635. [DOI] [PubMed] [Google Scholar]
- 44.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006; 25(14): 2105–2112. [DOI] [PubMed] [Google Scholar]
- 45.Zandvakili I, Davis AK, Hu G, Zheng Y. Loss of RhoA Exacerbates, Rather Than Dampens, Oncogenic K-Ras Induced Lung Adenoma Formation in Mice. PLoS One 2015; 10(6): e0127923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKnight CG, Jude JA, Zhu Z, Panettieri RA Jr., Finkelman FD. House Dust Mite-Induced Allergic Airway Disease Is Independent of IgE and FcepsilonRIalpha. Am J Respir Cell Mol Biol 2017; 57(6): 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKnight CG, Morris SC, Perkins C, Zhu Z, Hildeman DA, Bendelac A et al. NKT cells contribute to basal IL-4 production but are not required to induce experimental asthma. PLoS One 2017; 12(11): e0188221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr Protoc Immunol 2003; Chapter 6: Unit 6 28. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.