Abstract
Pain is a percept of critical importance to our daily survival. In most cases, it serves both an adaptive function by helping us respond appropriately in a potentially hostile environment and also a protective role by alerting us to tissue damage. Normally, it is evoked by the activation of peripheral nociceptive nerve endings and the subsequent relay of information to distinct cortical and sub-cortical regions, but under pathological conditions that result in chronic pain, it can become spontaneous. Given that one in three chronic pain patients do not respond to the treatments currently available, the need for more effective analgesics is evident. Two principal obstacles to the development of novel analgesic therapies are our limited understanding of how neuronal circuits that comprise these pain pathways transmit and modulate sensory information under normal circumstances and how these circuits change under pathological conditions leading to chronic pain states. In this review, we focus on the role of inhibitory interneurons in setting pain thresholds and, in particular, how disinhibition in the spinal dorsal horn can lead to aberrant sensory processing associated with chronic pain states.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00936-0) contains supplementary material, which is available to authorized users.
Key Words: GABA, glycine, spinal cord, chronic pain, allodynia.
Inhibitory Interneurons in the Spinal Dorsal Horn
The dorsal horn of the spinal cord is the principal termination site of primary afferents that innervate the skin and deeper tissues of the trunk and limbs and is composed of several distinct classes of neurons. These afferent fibers engage discrete, modality-specific circuits comprised of spinal interneurons that play important roles in modulating and gating afferent input, and projection neurons that relay the processed information to higher brain centers [1]. Nociceptive afferents of various types terminate primarily in laminae I, II, and V, with the central terminals of thinly myelinated Aδ fibers terminating in lamina I and V [2], peptidergic C-fibers arborizing in lamina I and the outer part of lamina II (IIo), and non-peptidergic C-fibers that express the mas-related G protein-coupled receptor MrgD (CMrgD afferents) and bind isolectin B4 (IB4) terminating in mid-lamina II [3, 4]. Low-threshold mechanoreceptor afferents (LTMRs) terminate in deeper dorsal horn laminae, with unmyelinated C-LTMRs arborizing in the ventral part of lamina IIi, Aδ-LTMRs in lamina IIi and III, and Aβ-LTMRs in lamina IIi and III [5]. To allow the barrage to sensory input into the spinal cord to be perceived in context, afferent input into the central nervous system must be gated and prioritized—this process is achieved by the action of spinal interneurons. Local interneurons are thought to account for 99% of all neurons in the spinal dorsal horn [6] and can be subdivided into two principal classes based on their neurotransmitter content: excitatory interneurons that release glutamate and inhibitory interneurons that use GABA and/or glycine (Fig. 1). In both the rat and mouse, inhibitory interneurons account for approximately 25% of neurons in laminae I and II and 40% of those in lamina III [7–9]. These cells can be subdivided further into distinct subclasses based on their neurochemical, electrophysiological, and morphological properties [5, 10–15], but it has yet to be determined whether these represent functionally distinct populations. Given that the loss of inhibition in spinal circuits is thought to lead to aberrant processing of somatosensory input, the loss of pain suppression, and the development of a neuropathic pain-like state [16–18], these interneurons represent an obvious target for the development of novel pain management therapies. To facilitate this, we must first define the functional significance of various inhibitory interneuron subpopulations under normal conditions and then determine how the circuits contribute to change under pathological states that lead to chronic pain.
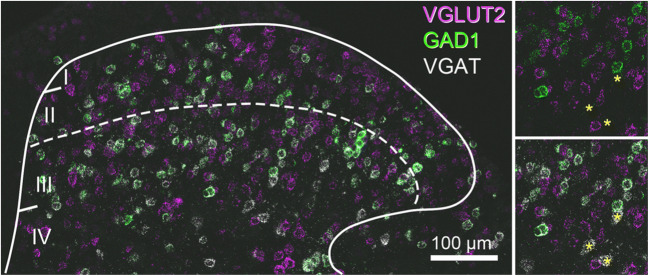
Fig. 1.
Neurotransmitter heterogeneity of dorsal horn neurons. Fluorescent in situ hybridization labelling for VGLUT2 (magenta), GAD1 (green), and VGAT (white) shows excitatory interneurons (magenta) outnumber inhibitory interneurons (green and white, or white only) in laminae I–III. VGLUT2-expressing interneurons are common in laminae I–III. Inhibitory interneurons can be split into three subpopulations based on their neurotransmitter content: those that express GABA, those that express glycine, and those that express both GABA and glycine. In this figure, inhibitory interneurons that only express GABA, and those likely to co-express both GABA and glycine, show co-expression for both GAD1 and VGAT (green and white, respectively) and are common in laminae I–III, whereas cells that express only glycine are common in lamina IV (insets, yellow asterisks). AM Bell, AJ Todd, and DI Hughes, unpublished observations
Neurochemical and Molecular-Genetic Heterogeneity of Inhibitory Interneuron Populations
GABA acts as the major inhibitory neurotransmitter throughout most regions of the central nervous system, although glycinergic neurotransmission predominates in parts of the spinal cord and brainstem, and in the retina. Immunohistochemical studies in the rat and mouse show that GABA-immunoreactive (GABA-IR) cells in the spinal dorsal horn are concentrated in laminae I–III, whereas glycine-IR cells are rarely seen in laminae I and II, but are common in laminae III and IV [7, 9, 19–22]. In the rat, inhibitory interneurons account for between 25 and 30% of all cells in laminae I and II, and approximately 40% of those in lamina III [7, 8], with similar patterns being reported in the mouse [9]. Virtually all interneurons in laminae I–III that are enriched with glycine are also GABA-IR [7–9], and immunolabelling studies have shown that most axon terminals in this region that are derived from inhibitory interneurons contain both GABA and glycine [23–27]. This supports the view that axon terminals of most inhibitory interneurons in laminae I–III of the spinal dorsal horn co-release both neurotransmitters [28–30], but whether the resultant inhibition has both GABAergic and glycinergic components (distinguished pharmacologically) depends on the presence of corresponding neurotransmitter receptors at postsynaptic sites.
All dorsal horn inhibitory interneurons are believed to express the developmental transcription factor Pax2 [31–34]. Inhibitory interneurons in the superficial dorsal horn (laminae I–II) can be assigned to 5 largely non-overlapping populations (Fig. 2) on the basis of their expression certain neurochemical markers: the neuropeptides galanin and dynorphin (which are co-expressed), neuropeptide Y, neuronal nitric oxide synthase (nNOS), and the calcium-binding proteins parvalbumin (PV) and calretinin (CR) [10]. It is important to note that these markers are not exclusive to inhibitory interneurons, as several are also expressed by excitatory neurons (dynorphin, nNOS, calretinin, parvalbumin) or by primary afferents (galanin, parvalbumin). Recent studies of dorsal horn populations using open-ended genetic screening or transcriptomic approaches provide an unprecedented means of assessing the neurochemical and molecular-genetic profile of spinal interneurons [5, 11, 12]. The findings of one such study in the mouse identified 15 molecularly distinct subtypes of inhibitory neurons when single-cell RNA sequencing was used to classify dorsal horn neurons [12], and these largely match the neurochemically distinct populations identified in the superficial laminae using immunohistochemical approaches (Fig. 2), with the Gal/Dyn, NPY, CR, and PV populations corresponding to the Gaba1–3, Gaba5–7, Gaba8–9, and Gaba 14 clusters. Given that glycinergic populations cannot be identified with any great precision [12], this scheme is not definitive, but is nonetheless an important resource that provides a means of identifying unique molecular signatures in neurochemically defined neuronal populations. With the ever-increasing development of new transgenic mouse lines that express site-specific recombinases (SSRs), we can use such schemes to develop intersectional strategies in which co-expression of two recombinases (e.g., Cre and Flp), driven from different genes, is used to target specific neuronal populations [35, 36]. This will provide a means of targeting and manipulating neuronal populations with far greater precision than was previously possible.
Fig. 2.
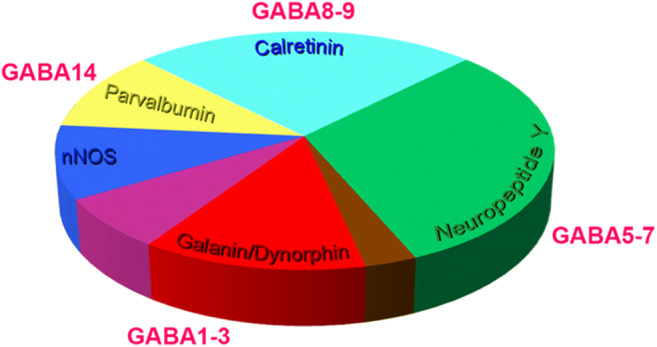
Neurochemical features of spinal inhibitory interneurons in laminae I and II. The estimated proportions of all inhibitory interneuron populations, as defined by their neurochemical profiles, are presented in the pie chart (modified from reference 10]. Four segments of this chart (parvalbumin, calretinin, neuropeptide Y, and galanin/dynorphin populations) correspond well with molecular clusters of inhibitory interneurons identified in single-cell RNA sequencing studies [12]. Taken together, these datasets provide a means of devising intersectional strategies to target subpopulations of interneuron with greater precision than possible previously
Morphological and Electrophysiological Features of Inhibitory Interneurons
Morphological heterogeneity among dorsal horn neuron populations has been a consistent finding, from early studies using Golgi labelling [37–39], to more recent studies where the morphology of individual cells was revealed following “blind” whole-cell recording in wild-type [13–15, 40, 41] animals and targeted recordings from transgenic mice [42–47]. The morphology of lamina II interneurons has been studied extensively. The most widely accepted scheme for classifying these neurons was developed from studies in hamsters [13], and defined four principal populations: islet, central, vertical, and radial cells, although ~ 20% of the neurons in their sample could not be assigned to any of these classes and were described as “unclassified.” Central cells were further subdivided into transient and tonic types, based on their action potential firing pattern in response to injected depolarizing current.
Similar morphological populations of lamina II neurons have been described in various species, including rat [14, 15, 40, 41, 48, 49] and mouse [42, 45, 46, 50]. It is still to be determined whether we can justifiably use morphological features alone as an indicator of whether cells are excitatory or inhibitory interneurons. A particular limitation is that most studies have used a purely subjective approach to assign cells to different morphological classes. Nonetheless, it is generally accepted that 3 of the classes identified by Grudt and Perl (radial, transient central, and vertical cells) in most cases correspond to subsets of excitatory interneurons, while islet cells are invariably inhibitory interneurons. However, it is also clear that many inhibitory interneurons in lamina II are not islet cells [46, 51].
Given that a variety of markers commonly used to define inhibitory interneurons in the dorsal horn can also be expressed in glutamatergic interneuron populations [5, 12, 45], using the expression of only a single neurochemical marker to identify neuronal populations can be misleading. The most widely used scheme for defining spinal interneurons is based on a system that combines the morphological and physiological properties of individual cells [13]. In this study, five morphologically distinct populations (islet, central, radial, vertical, and unclassified) were proposed, and three principal patterns of action potential firing were identified, namely tonic-, transient-, and delayed-firing discharge. Similar firing patterns have been described in the rat and mouse spinal cord [43, 50, 52, 53], with five distinct patterns being reported, namely tonic-, delayed-, and initial burst-firing, along with single spiking and phasic bursting. The incidence of cells displaying particular discharge properties appears to be correlated to the lamina in which the recordings were performed [52] and also on the holding potential used during these experiments [43, 53], but certain morphologically defined populations also appear to associate more commonly with certain firing patterns. For example, islet cells typically display tonic- or initial burst-firing action potential discharge patterns in response to depolarizing current injection steps, whereas radial cells, central cells, medial-lateral cells, vertical cells, and those cells of unclassified morphology displayed a range of other firing patterns including transient-, delayed-, and single spike-firing [13, 43, 45]. Several other studies have also reported similar correlations between firing patterns and morphology [15, 33, 40, 42], and these have helped propagate a general consensus that tonic- or initial burst-firing discharge patterns in representing recordings from inhibitory interneurons, whereas transient-, delayed-, and single spike-firing patterns are indicators of excitatory interneurons. Attempts to further define what appear to be homogenous populations by incorporating additional descriptive criteria can, however, be problematic. For example, calretinin-expressing cells (CRINs) in the spinal dorsal horn are largely confined to lamina II and have been considered to represent a population of excitatory interneurons [54]. More recently, interrogation of this neurochemically defined population of cells using immunohistochemistry, targeted whole-cell patch-clamp recordings, and transcriptomics have revealed that CRINs are morphologically, neurochemically, and physiologically diverse [12, 45, 55, 56] and display features found in both excitatory and inhibitory populations (Fig. 3). It therefore remains to be determined precisely which combinations of features are reliable indicators of transmitter content for other cell populations, and whether these morphological and electrophysiological signatures also apply to spinal neurons in other laminae.
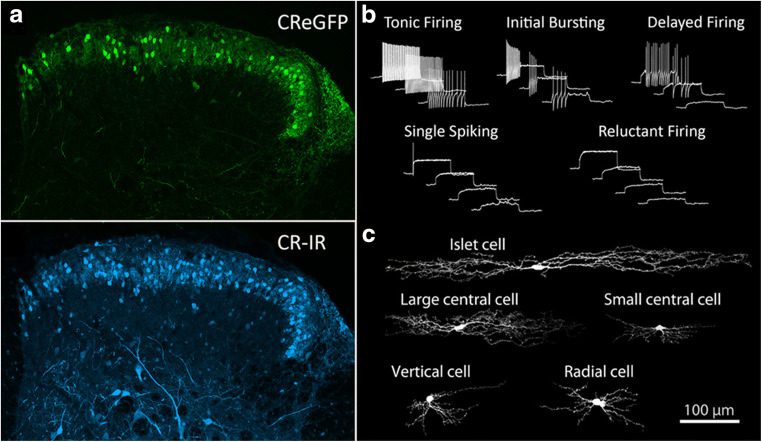
Fig. 3.
Morphological and electrophysiological diversity within a neurochemically-defined population lamina II neurons. Targeted whole-cell patch-clamp recordings were carried out in spinal cord slices maintained in vitro from a transgenic mouse line where enhanced green fluorescent protein (eGFP) was expressed in calretinin (CR) interneurons. (a) The distribution of eGFP-labelled cells mirrors that of calretinin cells labelled using immunohistochemical approaches (CR-IR). (b) Five distinct action potential firing patterns were recorded in eGFP cells from lamina II. (c) The morphological features of recorded neurons were also determined, with five morphologically distinct groups being recorded, as well as a group of unclassified cells. The only correlation between morphology and firing pattern that could be established was that cells with tonic-firing discharge patterns were always islet cells (and all islet cells were tonic firing). Modified from reference 45
Spinal Inhibition
Inhibitory interneurons in the spinal cord can generate two distinct forms of synaptic inhibition mediated through the release of GABA and/or glycine and activation of ligand-gated ion channels (GABAA and glycine receptors, respectively). Presynaptic inhibition is a GABA-mediated event resulting principally from the release of transmitter from axons (or presynaptic dendrites) that synapse with primary afferent terminals to act on GABAA receptors primarily [57–60], although GABAB receptors have also been implicated in this form of inhibition on both group Ia muscle afferents and Aβ cutaneous afferents [61, 62]. Postsynaptic inhibition results from the release (or co-release) of GABA and/or glycine at axodendritic and axosomatic synapses (primarily). While most inhibitory synaptic events typically have both GABA- and glycine-mediated components [60, 63–65] similar responses resulting from purely GABA- or glycinergic transmission have also been reported [66–68], and these support anatomical studies where axon terminals showing immunolabelling for only GABA or glycine have been described [24, 26, 69–71]. The presence of functional neurotransmitter receptors in the postsynaptic membrane will dictate the type of inhibition mediated at any given synapse, and the kinetics of these responses may also be highly dependent on the stoichiometry of receptors found at particular synapses [18, 72, 73]. Immunohistochemistry for the β3 subunit has been used to visualize GABAA receptors at synapses [25, 27], whereas the microtubule-associated protein gephyrin (which anchors to the β glycine receptor subunit to the underlying cytoskeleton) is commonly used to visualize glycine receptor expression [25, 74–77]. The β3 subunit of the GABAA receptor and gephyrin colocalize extensively at synapses formed by axon terminals containing both GABA and glycine [25, 27], but given that gephyrin-expressing synapses can also be found associated with axons enriched only in GABA [25, 27, 75], it is now widely considered to be a reliable marker of most inhibitory synapses in laminae I–III. The one notable exception to this generalized rule are axoaxonic synapses. These types of synapses are found on the central terminals of most types of primary afferents, with the exception of peptidergic C-fibers [78], and show immunolabelling for GABAA receptor subunits but not for gephyrin [79, 80]. A large-scale single-cell RNA sequencing of dorsal root ganglion neurons also shows very low expression levels for the genes that encode for gephyrin (GPHN), or for any of the splice variants of glycine receptor α subunits (GLRA1–4) that supports the view that primary afferents do not express functional glycine receptors [81], as implied in earlier studies where glycinergic membrane currents could not be shown in dorsal root ganglion neurons [82]. Furthermore, studies using optogenetic approaches to define the pharmacological basis of presynaptic inhibition in both myelinated LTMR afferents (A-LTMR) from the skin and of proprioceptive afferent groups have demonstrated that this form of inhibition is insensitive to strychnine but can be abolished by bicuculline [60, 83], whereas light-evoked postsynaptic inhibition mediated in unidentified neurons were sensitive to both antagonists. Taken together, these findings imply that the action of both transmitters at specific synapses serve important, but as yet largely undefined, roles in the resultant inhibition, although mechanisms where these transmitters tonically inhibit inhibitory interneurons in laminae I–III of the spinal dorsal horn have been proposed as being important in separating low-threshold mechanoreceptive information from pain circuits in lamina I [84].
At the ultrastructural level, the central terminals of both non-peptidergic C-fibers and Aδ down hair afferents display distinctive anatomical features where they form the central elements of Type I and Type II glomeruli, respectively [85–87]. These glomerular arrangements provide structural insights into how the synaptic circuitry of the spinal dorsal horn is arranged to provide stringent control over the passage of sensory information into the central nervous system. The central terminals relay afferent input to spinal neurons by the release of glutamate at axodendritic synapses, and like most classes of primary afferents, are subject to presynaptic inhibition via axoaxonic synapses. Ultrastructural studies using post-embedding immunogold labelling have shown diversity of transmitter content within individual axon terminals that synapse on to both spinal neurons [88] and the central terminals (and postsynaptic targets) of several classes of functionally-distinct cutaneous primary afferents [24, 26, 69–71]. These show that virtually all axon terminals that form axoaxonic synapses show immunolabelling for GABA, and most (but not all) also label for glycine, while inhibitory axodendritic synapses are typically formed by boutons that contain glycine, GABA, or both transmitters. These anatomical observations support earlier reports from electrophysiological studies that presynaptic inhibition is mediated through activation of GABAA (and possibly GABAB) receptors on the central terminals of primary afferents [57, 61, 63, 89], whereas postsynaptic inhibition can be mediated by either GABA or glycine, or the co-transmission of both [65, 84, 90]. Some inhibitory axon terminals form synaptic triads with the central terminals of primary afferents and dendrites that are themselves postsynaptic to the primary afferent [91–95], and these are likely to provide strict control over the passage of afferent input to postsynaptic dendrites by mediating both pre- and postsynaptic inhibition simultaneously. Most of the axon terminals involved in these triadic arrangements contain both GABA and glycine [24, 26, 69–71], and it is likely that while both transmitters are released at the axoaxonic and axodendritic synapses formed by these boutons, glycinergic inhibition only operates at the axodendritic synapses, given the absence of functional glycine receptors on primary afferents.
Cellular Basis and Behavioral Consequences of Spinal Disinhibition
The importance of spinal inhibition in somatosensory processing was demonstrated in studies where strychnine and bicuculline (glycine and GABAA receptor antagonists, respectively) were administered via intrathecal routes in rats [16]. This resulted in “a dose-dependent organized agitation response to light tactile stimulation,” which resembled tactile allodynia, a symptom reported by up to half of patients with neuropathic pain [96]. Tactile allodynia is often resistant to treatment, meaning that developing novel, more effective therapies presents a pressing clinical need [97]. Subsequent studies supported these initial findings [98–100], and the selective loss of spinal inhibition (spinal disinhibition) has been identified as an important contributor leading to the development of central sensitization and pathological pain [101–103]. Precisely how peripheral nerve injury induces spinal disinhibition, and the resultant effect this has on the activity of spinal circuits, remains a topic of considerable interest and debate (see reviews 104, 105, 106, 107, and 108). One of the most highly contested hypotheses proposes that peripheral nerve injury leads to selective loss of inhibitory interneurons in laminae I–III of the spinal dorsal horn through apoptosis [104–108]. However, a series of detailed anatomical studies have challenged these views, finding no loss of either GABA- or glycine-immunoreactive neurons in animals that had undergone partial peripheral nerve injuries that resulted in signs of neuropathic pain and showing that apoptosis in the dorsal horn after nerve injury was confined to microglia [8, 109, 110]. Although it has been shown that glutamic acid decarboxylase (the enzyme responsible for GABA synthesis) and mRNA encoding for the GAD65 isoform is down-regulated following nerve injury [107, 110, 111], other studies report no reduction of GABA levels in axon terminals of inhibitory interneurons from laminae I and II in the same partial nerve injury model [27].
Given that a loss of inhibitory interneurons and (or) a loss of GABA levels in the spinal dorsal horn remain topics of debate, other alternative mechanisms have been proposed to explain heightened excitability of dorsal horn circuits in conditions of neuropathic pain. One hypothesis implicates the downregulation of the chloride ion transporter KCC2 following peripheral nerve injury [112–115], brought about by the release of brain-derived neurotrophic factor (BDNF) from axotomized afferents [116, 117]. A reduction of KCC2 leads to disruption in chloride equilibrium, and this reduces the efficacy of inhibition mediated by the release of GABA and glycine in pain-transmitting neurons, whereas others have proposed that the reduced excitability of inhibitory interneurons and/or loss of their synaptic inputs are additional contributing factors [118–120].
Although the loss of spinal inhibition has been shown to allow A-LTMR afferent input to activate pain circuits in lamina I [121, 122], the route(s) through which this is achieved is/are poorly understood. Two distinct circuits through which this is achieved have been proposed, although both are gated by inhibitory PV-expressing interneurons (iPVINs) and involve the aberrant recruitment of vertical cells [60, 123]. Vertical cells have been proposed as likely candidates to facilitate the recruitment of pain circuits following A-LTMR activation given that their dendrites extend into lamina III and receive inputs from myelinated afferents, and their axon arborizes in lamina I to synapse on projection neurons that relay information to the spinoparabrachial nucleus [13, 124, 125]. PVINs are found primarily in the inner part of lamina II and in lamina III [126, 127], and most of these co-express both GABA and glycine [128]. These interneurons are known to play a crucial role in gating A-LTMR input into the spinal dorsal horn, given that selectively ablating them in naïve mice induces allodynia-like responses to mechanical stimuli, whereas chemogenetic activation of these cells in allodynic mice alleviates their mechanical hypersensitivity [123]. PVINs have been shown to be a source of axoaxonic inputs on to the central terminals of A-LTMRs [50, 60], and of axodendritic synapses on to several classes of interneuron populations known to receive direct input from myelinated afferents including vertical cells, interneurons that express the γ-isoform of protein kinase C (PKCγ), and other PV-expressing cells [124, 129]. The co-release of GABA and glycine at synapses formed by iPVINs initiates two types of inhibition (GABA-mediated presynaptic inhibition at axoaxonic synapses, and postsynaptic inhibition resulting from the action of both GABA and glycine), and supports the view that both transmitters play distinct roles in segregating A-LTMR afferent input from pain circuits and underlie their involvement in the development of different aspects of mechanical hypersensitivity [98, 130–133]. The most direct impact of losing inhibition mediated by PVINs would be the simultaneous reduction in presynaptic control of A-LTMRs and postsynaptic inhibition of vertical cells, allowing innocuous tactile inputs to activate lamina I pain circuits [60]. An additional consequence of losing PVIN-mediated inhibition is the loss of postsynaptic inhibitory drive to PKCγ interneurons [123]. These cells play an important role in neuropathic pain [134] by relaying A-LTMR information to pain circuits via transient central cells when glycinergic inhibition is compromised [41, 131, 132], and is brought about when PVIN-mediated inhibition is reduced after peripheral nerve injury [123].
Precisely how peripheral nerve injury leads to a loss of PV cell-mediated inhibition has yet to be established. There is no apparent loss of PV interneurons [60, 123], but whether the axons of these cells disconnect from their principal synaptic targets is yet to be resolved. What has become apparent is that some of the functional properties of PVINs change following peripheral nerve injury [60]. For example, the amplitude of current injection needed to maintain tonic firing for the entire stimulus in tonic-firing PV cells was significantly higher ipsilateral to the nerve injury than for the contralateral side, and the current-frequency relationship for action potential discharge was also significantly lower on the ipsilateral side. These changes are likely to result in a reduction of PV cell-mediated inhibition. An earlier targeted electrophysiological study of inhibitory interneurons in a GAD67::eGFP mouse line reported an impaired excitatory drive to GABAergic neurons after nerve-injured mice [135] but no change in either the excitability or discharge properties of these neurons [118, 119]. When similar experiments were conducted in a PVCre; Ai9 mouse line, no change in excitatory drive to PV cells was seen, but distinct differences in the excitability and action potential firing patterns of these interneurons were reported [60]. These findings suggest that subtle physiological differences may become apparent when discrete subpopulations of inhibitory interneurons are targeted specifically.
Future Directions—Novel Targets
One approach in helping to develop new therapies to tackle chronic pain states is to establish the functional significance of discrete neuronal populations in the spinal dorsal horn and then determine precisely how their associated anatomical features or electrophysiological properties change under pathological conditions. Experiments involving topical application of specific neurotransmitter receptor antagonists in freely moving animals were the first to establish the importance of spinal inhibition in somatosensory processing [16, 98–100]. These were followed by a series of in vitro electrophysiological studies where the properties of interneuron populations were recorded under normal or chronic pain conditions [42, 43, 107, 119], and more recently, where the activity of relatively large populations of cells was manipulated in vivo to determine [131–133, 136, 137]. Given recent technological advances and a better understanding of neurochemically distinct populations of spinal interneurons, we now have the unprecedented means of targeting and manipulating subpopulations of inhibitory interneurons with great precision [5, 11, 12, 36, 138].
For example, manipulating the activity of PV interneurons specifically in both naïve and nerve-injured mice in vivo has been instrumental in establishing their role in setting mechanical thresholds [123]. By determining that the inhibition mediated by these cells play important roles in gating both A-LTMR input directly, and the relay of information through vertical cells, we find that they are uniquely placed to exert significant influence on the segregation of innocuous tactile information from pain circuits [50, 60]. When PV cell-mediated inhibition is lost, the disinhibition on A-LTMRs will likely lead to increased recruitment of several excitatory interneuron populations [139–143], and the aberrant recruitment of the circuits they contribute to in turn may underlie the difficulties we have faced to-date in developing effective therapies to manage chronic pain states (Fig. 4). Simply targeting one spinal circuit may not be sufficient to alleviate chronic pain, but since disinhibition of afferent input is believed to contribute to the development of several chronic pain states [17] and lies upstream of these spinal circuits, re-establishing presynaptic control of A-LTMRs in chronic pain states may be a more effective strategy.
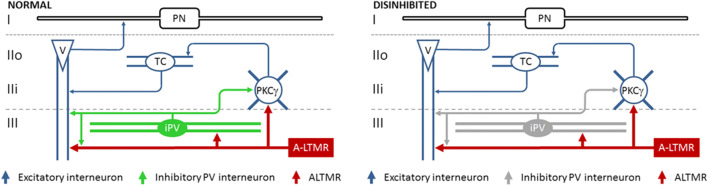
Fig. 4.
The role of inhibitory parvalbumin-expressing interneurons in gating low-threshold tactile input. Under normal conditions, inhibitory parvalbumin-expressing interneurons (iPV, green) mediate presynaptic inhibition of A-LTMR input (red) and postsynaptic inhibition of both vertical cells (blue, V) and PKCγ-expressing interneurons (blue, PKCγ). Peripheral nerve injury results in a reduction of iPV excitability (iPV, grey), leading to spinal disinhibition. The loss of iPV-mediated inhibition allows A-LTMR input to activate vertical cells directly, and through a polysynaptic route incorporating PKCγ-expressing interneurons and transient central cells (TC, blue). Under these conditions, vertical cells can relay A-LTMR input activate projection neurons (PN, black) in lamina I and recruit pain circuits. Based on references 48, 60, 123, 125
To achieve this selectively with pharmacological approaches may be challenging given the widespread distribution and heterogeneity of GABAA receptors in primary afferents and spinal dorsal horn neurons [79, 144], but by re-engaging specific neuronal populations and their outputs, rather than activating these receptors globally, it may be possible to restore normal function in experimental animal models with minimum additional consequences. Inhibition mediated by PV neurons is necessary to segregate A-LTMR input from pain circuits, and disinhibition of the circuits they serve is a significant contributor to the development of mechanical hypersensitivity. The loss of PV cell-mediated inhibition does not result from the death of these cells, but the reported changes in intrinsic electrophysiological properties of these cells imply that specific channelopathies within PV interneurons may be an important factor in the development of tactile allodynia. Although the precise mechanisms underpinning the changes in firing patterns and excitability seen in PV interneurons have yet to be determined, hyperpolarization-activated cyclic nucleotide–gated (HCN) channels are one of many possible targets given that they have been implicated in many pathological conditions including neuropathic pain [145–147]. HCN channels play critical roles in setting action potential firing patterns, and PV cells are known to show a high prevalence of Ih subthreshold currents and are enriched in both HCN1 and HCN4 subunits [50, 77]. It is tempting to speculate that changes in the properties of these channels in PV interneurons may contribute to the altered properties of these cells in chronic pain states. For example, a downregulation in HCN1 subunit expression (which confer faster kinetics on HCN channel complexes) in iPVINs, coupled with an increased expression of the more slowly conducting HCN4 subunits, could contribute to the reduced excitability seen in these cells after nerve injury [60]. Although changes in HCN subunit expression in distinct dorsal horn neuron populations have yet to be reported following peripheral nerve injury, it has been shown that mRNA for both HCN1 and HCN2 is markedly decreased in dorsal root ganglion neurons following axotomy [145, 148]. Should spinal interneurons undergo similar changes, restoring normal HCN subunit expression in these cells could re-establish spinal inhibition and alleviate the mechanical hypersensitivity seen in pathological conditions. The widespread expression of various HCN channel complexes in non-neuronal tissue poses significant problems when antagonists are administered systemically, but by developing intersectional strategies to target these channels in spinal interneurons specifically, it is now possible to study their contribution to the development of chronic pain states in a variety of animal models. The recent advances made in defining, targeting, and manipulating discrete neuronal populations now provide us with unprecedented means of studying distinct components of neuronal circuits in animal models, and this generates real hope that more effective treatments for treating chronic pain will soon be available.
Electronic Supplementary Material
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgments
Financial support from the Biotechnology and Biological Sciences Research Council (grants P007996/1 to DIH and N006119/1 to AJT), the Medical Research Council (grant MR/S002987/1 to AJT), and the Wellcome Trust (grant 102645 to AJT) is gratefully acknowledged.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Aligning New Approaches to Accelerate the Development of Analgesic Therapies
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Todd AJ. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Molecular Pain. 2017;13:1–19. doi: 10.1177/1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. Journal of Comparative Neurology. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- 3.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioural responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Science, USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168:295–310.e19. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. Journal of Comparative Neurology. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- 8.Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskár Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 9.Polgár E, Durrieux C, Hughes DI, Todd AJ. A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PLoS One. 2013;8:e78309. doi: 10.1371/journal.pone.0078309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle KA, Gutierrez-Mecinas M, Polgár E, Mooney N, O'Connor E, Furuta T, Watanabe M, Todd AJ. A quantitative study of neurochemically-defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience. 2017;363:120–133. doi: 10.1016/j.neuroscience.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behaviour. Cell Reports. 2018;22:2216–2225. doi: 10.1016/j.celrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lönnerberg P, Manno GL, Sharma N, Borgius L, Kiehn O, Lagerström MA, Linnarsson S, Ernfors P. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nature Neuroscience. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- 13.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. Journal of Physiology. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. Journal of Physiology. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaksh TL. Behavioral and anatomic correlates of the tactile-evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 17.Sandkühler J. Models and Mechanisms of Hyperalgesia and Allodynia. Physiological Reviews. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 18.Zeilhofer HU, Wildner H, Yévennes G. Fast synaptic inhibition in spinal sensory processing and pain control. Physiological Review. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin BJ, Barber R, Saito K, Roberts E, Wu J-Y. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. Journal of Comparative Neurology. 1975;164:305–322. doi: 10.1002/cne.901640304. [DOI] [PubMed] [Google Scholar]
- 20.Barber RP, Vaughn JE, Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Research. 1982;238:305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- 21.Ottersen OP, Storm-Mathisen J. Distribution of inhibitory amino acid neurons in the cerebellum with some observations on the spinal cord: An immunocytochemical study with antisera against fixed GABA, glycine, taurine, and β-alanine. Journal of Mind and Behaviour. 1987;8:503–518. [Google Scholar]
- 22.van den Pol A, Gorcs T. Glycine and glycine-receptor immunoreactivity in brain and spinal cord. Journal of Neuroscience. 1988;8:472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell K, Spike RC, Todd AJ. An immunocytochemical study of glycine receptor and GABA in laminae I-III of rat spinal dorsal horn. Journal of Neuroscience. 1993;13:2371–2381. doi: 10.1523/JNEUROSCI.13-06-02371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. European Journal of Neuroscience. 1996;8:2492–2498. doi: 10.1111/j.1460-9568.1996.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 25.Todd AJ, Watt C, Spike RC, Sieghart W. Co-localisation of GABA, glycine and their receptors at synapses in the rat spinal cord. Journal of Neuroscience. 1996;16:914–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson AH, Hughes DI, Bazzaz AA. Synaptic relationships between hair follicle afferents and neurones expressing GABA and glycine-like immunoreactivity in the spinal cord of the rat. Journal of Comparative Neurology. 2002;452:367–380. doi: 10.1002/cne.10410. [DOI] [PubMed] [Google Scholar]
- 27.Polgár E, Todd AJ. Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABA(A) receptors from synapses in laminae I-II of the ipsilateral spinal dorsal horn. Neuroscience. 2008;156:193–202. doi: 10.1016/j.neuroscience.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 29.Chéry N, de Koninck Y. Junctional versus extrajunctional glycine and GABAA receptor- mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. Journal of Neuroscience. 1999;19:7342–7355. doi: 10.1523/JNEUROSCI.19-17-07342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller AF, Coull JA, Chéry N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. Journal of Neuroscience. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen C-L, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nature Neuroscience. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 32.Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Punnakkal P, von Schoultz C, Haenraets K, Wildner H, Zeilhofer HU. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. Journal of Physiology. 2014;592:759–776. doi: 10.1113/jphysiol.2013.264937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson M. Pax2 is persistently expressed by GABAergic neurons throughout the adult rat dorsal horn. Neuroscience Letters. 2017;638:96–101. doi: 10.1016/j.neulet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenno LF, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nature Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gobel S. Golgi studies in the substantia gelatinosa neurons in the spinal trigeminal nucleus. Journal of Comparative Neurology. 1975;162:397–415. doi: 10.1002/cne.901620308. [DOI] [PubMed] [Google Scholar]
- 38.Beal JA, Cooper MH. The neurons in the gelatinosal complex (lamina II and ill) of the monkey (Macaca mulatta): a Golgi study. Journal of Comparative Neurology. 1978;179:89–122. doi: 10.1002/cne.901790107. [DOI] [PubMed] [Google Scholar]
- 39.Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis) Journal of Comparative Neurology. 1978;180:395–414. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. Journal of Physiology. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. Journal of Clinical Investigation. 2013;123:4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. Journal of Neuroscience. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkühler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. Journal of Physiology. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy J-M. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. Journal of Comparative Neurology. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 45.Smith KM, Boyle KA, Madden JF, Dickinson SA, Jobling P, Callister RJ, Hughes DI, Graham BA. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: implications for spinal pain processing. Journal of Physiology. 2015;593:4319–4339. doi: 10.1113/JP270855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwagaki N, Ganley RP, Dickie AC, Polgár E, Hughes DI, Del Rio P, Revina Y, Watanabe M, Todd AJ, Riddell JS. A combined electrophysiological and morphological study of neuropeptide Y–expressing inhibitory interneurons in the spinal dorsal horn of the mouse. Pain. 2016;157:598–612. doi: 10.1097/j.pain.0000000000000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boakye PA, Schmidt EKA, Rancic V, Kerr B, Ballanyi K, Smith PA. Characterization of Superficial Dorsal Horn Neurons from "Tamamaki" Mice and Stability of their GAD67-EGFP Phenotype in Defined-Medium Organotypic Culture. Neuroscience. 2018;372:126–140. doi: 10.1016/j.neuroscience.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. Journal of Neuroscience. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melnick I. Morphophysiologic properties of islet cells in substantia gelatinosa of the rat spinal cord. Neuroscience Letters. 2008;446:65–69. doi: 10.1016/j.neulet.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Graham BA. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. Journal of Physiology. 2012;590:3927–395. doi: 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganley R, Iwagaki N, del Rio P, Baseer N, Dickie AC, Boyle KA, Polgár E, Watanabe M, Abraira VE, Zimmerman A, Riddell JS, Todd AJ. Inhibitory interneurons that express GFP in the PrP-GFP mouse spinal cord are morphologically heterogeneous, innervated by several classes of primary afferent and include lamina I projection neurons among their postsynaptic targets. Journal of Neuroscience. 2015;35:7626–7642. doi: 10.1523/JNEUROSCI.0406-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. Journal of Physiology. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruscheweyh R, Ikeda H, Heinke B, Sandkühler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. Journal of Physiology. 2004;555:527–543. doi: 10.1113/jphysiol.2003.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang HY, Cheng J-K, Shih Y-H, Chen P-H, Wang C-L, Tsaur M-L. Expression of A-type K channel α subunits Kv 4.2 and Kv 4.3 in rat spinal lamina II excitatory interneurons and colocalization with pain-modulating molecules. European Journal of Neuroscience. 2005;22:1149–1157. doi: 10.1111/j.1460-9568.2005.04283.x. [DOI] [PubMed] [Google Scholar]
- 55.Smith KM, Boyle KA, Mustapa M, Jobling P, Callister RJ, Hughes DI, Graham BA. Distinct forms of synaptic inhibition and neuromodulation regulate calretinin positive neuron excitability in the spinal cord dorsal horn. Neuroscience. 2016;326:10–21. doi: 10.1016/j.neuroscience.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutierrez-Mecinas M, Davis O, Polgár E, Shahzad M, Navarro-Batista K, Furuta T, Watanabe M, Hughes DI, Todd AJ. Expression of calretinin among different neurochemical classes of interneuron in the superficial dorsal horn of the mouse spinal cord. Neuroscience. 2019;398:171–181. doi: 10.1016/j.neuroscience.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eccles JC, Schmidt RF, Willis WD. Pre-synaptic inhibition of the spinal monosynaptic reflex pathway. Journal of Physiology. 1962;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubbard JI, Willis WD. Reduction of transmitter output by depolarization. Nature. 1962;193:1294–1295. doi: 10.1038/1931294a0. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi A, Takeuchi N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. Journal of General Physiology. 1962;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyle KA, Gradwell MA, Yasaka T, Dickie AC, Polgar E, Ganley RP, Orr DPH, Watanabe M, Abraira VE, Kuehn ED, Zimmerman AL, Ginty DD, Callister RJ, Graham BA, Hughes DI. Defining a spinal microcircuit that gates myelinated afferent input: implications for tactile allodynia. Cell Reports. 2019;28:526–540. doi: 10.1016/j.celrep.2019.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in pre-synaptic inhibition of Ia EPSPs in cat spinal motoneurones. Journal of Physiology. 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salio C, Merighi A, Bardoni R. GABAB receptors-mediated tonic inhibition of glutamate release from Aβ fibers in rat laminae III/IV of the spinal cord dorsal horn. Molecular Pain. 2017;3:174480691771004. doi: 10.1177/1744806917710041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. Journal of Physiology. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. Journal of Physiology. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. Journal of Neuroscience. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inquimbert P, Rodeau J-L, Schlichter R. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and lamina III-IV of the young rat spinal cord. Journal of Neurophysiology. 2007;26:2940–2949. doi: 10.1111/j.1460-9568.2007.05919.x. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. Journal of Physiology. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bardoni R, Takazawa T, Tong C-K, Choudhury P, Scherrer G, MacDermott AB. Pre-and postsynaptic inhibitory control in the spinal cord dorsal horn. Annals of the New York Academy of Sciences. 2013;1279:90–96. doi: 10.1111/nyas.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutherland FI, Bannatyne BA, Kerr R, Riddell JS, Maxwell DJ. Inhibitory amino acid transmitters associated with axons in presynaptic apposition to cutaneous primary afferent axons in the cat spinal cord. Journal of Comparative Neurology. 2002;452:154–162. doi: 10.1002/cne.10374. [DOI] [PubMed] [Google Scholar]
- 70.Watson AHD. GABA- and glycine-like immunoreactivity in axons and dendrites contacting the central terminals of rapidly adapting glabrous skin afferents in rat spinal cord. Journal of Comparative Neurology. 2003;464:497–510. doi: 10.1002/cne.10812. [DOI] [PubMed] [Google Scholar]
- 71.Watson AHD. Synaptic interactions between the terminals of slow-adapting Type II mechanoreceptor afferents and neurones expressing γ-aminobutyric acid- and glycine-like immunoreactivity in the rat spinal cord. Journal of Comparative Neurology. 2004;471:168–179. doi: 10.1002/cne.20043. [DOI] [PubMed] [Google Scholar]
- 72.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proceedings of the National Academy of Science, USA. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taal W, Holstege JC. GABA and glycine frequently colocalize in terminals on cat spinal motorneurons. NeuroReport. 1994;5:2225–2228. doi: 10.1097/00001756-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Todd AJ, Spike RC, Chong D, Neilson M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. European Journal of Neuroscience. 1995;7:1–11. doi: 10.1111/j.1460-9568.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez FJ, Dewey DE, Harrington DA, Fyffe REW. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. Journal of Comparative Neurology. 1997;379:150–170. [PubMed] [Google Scholar]
- 77.Hughes DI, Boyle KA, Kinnon CM, Bilsland C, Quayle JA, Callister RJ, Graham BA. HCN4 subunit expression in fast-spiking interneurons of the rat spinal cord and hippocampus. Neuroscience. 2013;237:7–18. doi: 10.1016/j.neuroscience.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribeiro-da-Silva A, De Koninck Y. Morphological and neurochemical organization of the spinal dorsal horn. In: Bushnell MC, Basbaum AI, editors. Pain. New York: Academic Press; 2008. pp. 279–310. [Google Scholar]
- 79.Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABAA receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. Journal of Comparative Neurology. 2012;520:3895–3911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- 80.Lorenzo L-E, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABAA receptors. Journal of Neuroscience. 2014;34:8300–8317. doi: 10.1523/JNEUROSCI.0159-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature Neuroscience. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 82.Aguayo LG, Peoples RW, Yeh HH, Yévenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Current Topics in Medicinal Chemistry. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- 83.Fink AJP, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature. 2014;509:43–48. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. Journal of Physiology. 2010;588:2571–2587. doi: 10.1113/jphysiol.2010.188292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro-da-Silva A, Coimbra A. Two types of synaptic glomeruli and their distribution in laminae I-III of the rat spinal cord. Journal of Comparative Neurology. 1982;209:176–186. doi: 10.1002/cne.902090205. [DOI] [PubMed] [Google Scholar]
- 86.Rethelyi M, Light AR, Perl ER. Synaptic complexes formed by functionally defined primary afferent units with fine myelinated fibers. Journal of Comparative Neurology. 1982;207:381–393. doi: 10.1002/cne.902070409. [DOI] [PubMed] [Google Scholar]
- 87.Ribeiro-da-Silva A, Coimbra A. Capsaicin causes selective damage to type I synaptic glomeruli in rat substantia gelatinosa. Brain Research. 1984;290:380–383. doi: 10.1016/0006-8993(84)90961-2. [DOI] [PubMed] [Google Scholar]
- 88.Maxwell DJ, Todd AJ, Kerr R. Colocalization of glycine and GABA in synapses on spinomedullary neurons. Brain Research. 1995;690:127–132. doi: 10.1016/0006-8993(95)00613-u. [DOI] [PubMed] [Google Scholar]
- 89.Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. Journal of Physiology. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anderson WB, Graham BA, Beveridge NJ, Tooney PA, Brichta AM, Callister RJ. Different forms of glycine- and GABAA-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons. Molecular Pain. 2009;5:65. doi: 10.1186/1744-8069-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knyihár-Csillisk E, Csillik B, Rakic P. Periterminal synaptology of dorsal root glomerular terminals in the substantia gelatinosa of the spinal cord in the rhesus monkey. Journal of Comparative Neurology. 1982;210:376–99. doi: 10.1002/cne.902100405. [DOI] [PubMed] [Google Scholar]
- 92.Semba K, Masarachia P, Malamed S, Jacquin M, Harris S, Yang G, Egger MD. An electron microscopic study of primary afferent terminals from slowly adapting type I receptors in the cat. Journal of Comparative Neurology. 1983;221:466–481. doi: 10.1002/cne.902210409. [DOI] [PubMed] [Google Scholar]
- 93.Semba K, Masarachia P, Malamed S, Jacquin M, Harris S, Egger MD. Ultrastructure of pacinian corpuscle primary afferent terminals in the cat spinal cord. Brain Research. 1984;302:135–150. doi: 10.1016/0006-8993(84)91293-9. [DOI] [PubMed] [Google Scholar]
- 94.Semba K, Masarachia P, Malamed S, Jacquin M, Harris S, Yang G, Egger MD. An electron microscopic study of terminals of rapidly adapting mechanoreceptive afferent fibers in the cat spinal cord. Journal of Comparative Neurology. 1985;232:229–240. doi: 10.1002/cne.902320208. [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro-da-Silva A, Pignatelli D, Coimbra A. Synaptic architecture of glomeruli in superficial dorsal horn of rat spinal cord, as shown in serial reconstructions. Journal of Neurocytology. 1985;14:203–220. doi: 10.1007/BF01258448. [DOI] [PubMed] [Google Scholar]
- 96.Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Üçeyler N, Valet M, Wasner G, Treede R-D. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Woolf CJ. Overcoming obstacles to developing new analgesics. Nature Medicine. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 98.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia. Journal of Neurophysiology. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 99.Sorkin LS, Puig S. Neuronal model of tactile allodynia produced by spinal strychnine: effects of excitatory amino acid receptor antagonists and a mu-opiate receptor agonist. Pain. 1996;68:283–292. doi: 10.1016/s0304-3959(96)03130-2. [DOI] [PubMed] [Google Scholar]
- 100.Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- 101.Woolf CJ. Evidence for a central component of post- injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 102.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. The Journal of Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woolf CJ. Pain amplification—A perspective on the how, why, when, and where of central sensitization. Journal of Applied Biobehavioural Research 2018; e12124
- 104.Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Research. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- 105.Ibuki T, Hama AT, Wang X-T, Pappas GD, Sagen J. Loss of GABA immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 106.Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplants of immortalized serotoninergic precursors. Journal of Chemical Neuroanatomy. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 107.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. Journal of Neuroscience. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. Journal of Neuroscience. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Polgár E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 110.Polgár E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. Journal of Neuroscience. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lorenzo LE, Magnussen C, Bailey AL, St Louis M, De Koninck Y, Ribeiro-da-Silva A. Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury. Molecular Pain. 2014;10:57. doi: 10.1186/1744-8069-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei B, Kumada T, Furukawa T, Inoue K, Watanabe M, Sato K, Fukuda A. Pre- and post-synaptic switches of GABA actions associated with Cl- homeostatic changes are induced in the spinal nucleus of the trigeminal nerve in a rat model of trigeminal neuropathic pain. Neuroscience. 2013;228:334–348. doi: 10.1016/j.neuroscience.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Modol L, Cobianchi S, Navarro X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain. 2014;155:1577–1590. doi: 10.1016/j.pain.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Kahle KT, Schmouth JF, Lavastre V, Latremoliere A, Zhang J, Andrews N, Omura T, Laganiere J, Rochefort D, Hince P, Castonguay G, Gaudet R, Mapplebeck JC, Sotocinal SG, Duan J, Ward C, Khanna AR, Mogil JS, Dion PA, Woolf CJ, Inquimbert P, Rouleau GA. Inhibition of the kinase WNK1/HSN2 ameliorates neuropathic pain by restoring GABA inhibition. Science Signaling. 2016;9:ra32. doi: 10.1126/scisignal.aad0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. N-methyl-d-aspartate receptor- and calpain-mediated proteolytic cleavage of K+- Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. Journal of Biological Chemistry. 2012;287:33853–33864. doi: 10.1074/jbc.M112.395830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 117.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, de Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 118.Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. Journal of Neurophysiology. 2006;96:579–590. doi: 10.1152/jn.00087.2006. [DOI] [PubMed] [Google Scholar]
- 119.Schoffnegger D, Heinke B, Sommer C, Sandkühler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. Journal of Physiology. 2006;3:869–878. doi: 10.1113/jphysiol.2006.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H, Li Y, Yang Q, Liu X-G, Dougherty PM. Morphological and physiological plasticity of spinal lamina II GABA neurons is induced by sciatic nerve chronic constriction injury in mice. Frontiers in Cellular Neuroscience. 2018;12:143. doi: 10.3389/fncel.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 122.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. Journal of Neuroscience. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Reports. 2015;13:1246–1257. doi: 10.1016/j.celrep.2015.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yasaka T, Tiong SY, Polgár E, Watanabe M, Kumamoto E, Riddell JS, Todd AJ. A putative relay circuit providing low-threshold mechanoreceptive input to lamina I projection neurons via vertical cells in lamina II of the rat dorsal horn. Molecular Pain. 2014;10:3. doi: 10.1186/1744-8069-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cordero-Erausquin M, Allard S, Dolique T, Bachand K, Ribeiro-da-Silva A, De Koninck Y. Dorsal horn neurons presynaptic to lamina I spinoparabrachial neurons revealed by transynaptic labeling. Journal of Comparative Neurology. 2009;517:601–615. doi: 10.1002/cne.22179. [DOI] [PubMed] [Google Scholar]
- 126.Antal M, Freund TF, Polgár E. Calcium-binding proteins, parvalbumin- and calbindin-D 28k-immunoreactive neurons in the rat spinal cord and dorsal root ganglia: a light and electron microscopic study. Journal of Comparative Neurology. 1990;295:467–484. doi: 10.1002/cne.902950310. [DOI] [PubMed] [Google Scholar]
- 127.Antal M, Polgár E, Chalmers J, Minson JB, Llewellyn-Smith I, Heizmann CW, Somogyi P. Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-D-aspartate in the dorsal horn of the rat spinal cord. Journal of Comparative Neurology. 1991;314:114–124. doi: 10.1002/cne.903140111. [DOI] [PubMed] [Google Scholar]
- 128.Laing I, Todd AJ, Heizmann CW, Schmidt HH. Subpopulations of GABAergic neurons in laminae I-III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61:123–132. doi: 10.1016/0306-4522(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 129.Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for Sprouting of Aβ afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. Journal of Neuroscience. 2003;23:9491–9499. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loomis CW, Khandwala H, Osmond G, Heffean MP. Coadministration of intrathecal strychnine and bicuculline effects synergistic allodynia in the rat: an isobolographic analysis. Journal of Pharmacology and Experimental Therapeutics. 2001;296:756–761. [PubMed] [Google Scholar]
- 131.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKC gamma interneurons. PLOS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor-independent mechanical allodynia. Journal of Neuroscience. 2009;29:2519–2527. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hosli L, Haenraets K, Ghanem A, Conzelmann KK, Bosl M, Zeilhofer HU. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKC gamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 135.Leitner J, Westerholz S, Heinke B, Forsthuber L, Wunderbaldinger G, Jager T, Gruber-Schoffnegger D, Braun K, Sandkühler J. Impaired excitatory drive to spinal GABAergic neurons of neuropathic mice. PLoS One. 2013;8:e73370. doi: 10.1371/journal.pone.0073370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koga K, Kanehisa K, Kohro Y, Shiratori-Hayashi M, Tozaki-Saitoh H, Inoue K, Furue H, Tsuda M. Chemogenetic silencing of GABAergic dorsal horn interneurons induces morphine-resistant spontaneous nocifensive behaviours. Scientific Reports. 2017;7:4739. doi: 10.1038/s41598-017-04972-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Graham BA, Hughes DI. Rewards, perils and pitfalls of untangling spinal pain circuits. Current Opinion in Physiology. 2019;11:35–41. [Google Scholar]
- 139.Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science. 2016;354:578–584. doi: 10.1126/science.aaf8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moehring M, Halder P, Seal RP, Stucky CL. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron. 2018;100:349–360. doi: 10.1016/j.neuron.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alles SRA, Smith PA. The etiology and pharmacology of neuropathic pain. Pharmacological Reviews. 2018;70:315–347. doi: 10.1124/pr.117.014399. [DOI] [PubMed] [Google Scholar]
- 143.Peirs C, Dallel R, Todd AJ. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. Journal of Neural Transmission. 2020;127:505–525. doi: 10.1007/s00702-020-02159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- 145.Chaplan SR, Guo H-Q, Lee DH, Luo L, Liu C, Kuel C, Velumlan AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. Journal of Neuroscience. 2003;23:116–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan CS, Glajch KE, Gertler TS, Guzman JN, Mercer JN, Lewis AS, Goldberg AB, Tkatch T, Shigemoto R, Fleming SM, Chetkovich DM, Osten P, Kita H, Surmeier DJ. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nature Neuroscience. 2011;14:85–94. doi: 10.1038/nn.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reid CA, Phillips AM, Petrou S. HCN channelopathies: pathophysiology in genetic epilepsy and therapeutic implications. British Journal of Pharmacology. 2012;165:49–56. doi: 10.1111/j.1476-5381.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu H, Zhou J, Gu L, Zuo Y. The change of HCN1/HCN2 mRNA expression in peripheral nerve after chronic constriction injury induced neuropathy followed by pulsed electromagnetic field therapy. Oncotarget. 2017;8:1110–1116. doi: 10.18632/oncotarget.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)