Abstract
Abnormal neural activity, particularly in the rostrodorsal anterior cingulate cortex (rdACC), appears to be responsible for intense alcohol craving. Neuromodulation of the rdACC using cortical implants may be an option for individuals with treatment-resistant alcohol dependence. This study assessed the effectiveness and feasibility of suppressing alcohol craving using cortical implants of the rdACC using a controlled one-group pre- and post-test study design. Eight intractable alcohol-dependent participants (four males and four females) were implanted with two Lamitrode 44 electrodes over the rdACC bilaterally connected to an internal pulse generator (IPG). The primary endpoint, self-reported alcohol craving reduced by 60.7% (p = 0.004) post- compared to pre-stimulation. Adverse events occurred in four out of the eight participants. Electrophysiology findings showed that among responders, there was a post-stimulation decrease (p = 0.026) in current density at the rdACC for beta 1 band (13–18 Hz). Results suggest that rdACC stimulation using implanted electrodes may potentially be a feasible method for supressing alcohol craving in individuals with severe alcohol use disorder. However, to further establish safety and efficacy, larger controlled clinical trials are needed.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00851-4) contains supplementary material, which is available to authorized users.
Keywords: Alcohol dependence, Alcohol craving, Cortical stimulation, Anterior cingulate cortex
Introduction
Alcohol dependence is a worldwide debilitating disorder [1]. Apart from the health and social detriments for the affected individual, it impacts society. Its estimated societal cost is 223.5 billion dollars a year in the USA, with 125 billion dollars related to alcohol-involved vehicle accidents [1]. Compared to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [2], the most recent version, DSM-V [3], has removed the distinction between alcohol abuse (non-dependent hazardous use of alcohol) and alcohol dependence, and both conditions are now included in the single category of alcohol use disorder (AUD).
It has been proposed that people drink alcohol for mainly two reasons: for pleasure (reward drinking) or to avoid negative emotions (relief drinking) [4]. Particularly, relief drinkers are at higher risk of developing AUD [4], as alcohol intake is used as a way to self-medicate. The co-occurrence of relief drinking with AUD is in agreement with the fact that there is an unequivocally significant association between AUD and major depression, with 30% of major depression individuals reporting lifetime AUD [5]. Furthermore, patients with both AUD and major depression display a higher risk of relapse to alcohol dependence when compared to those with isolated disorders [6, 7]. Although the mechanism underlying the causal pathways between depression and AUD is unclear, there exists a bidirectional relationship with one study estimating that a diagnosis of one disorder doubles the risk of developing the other [8].
The optimal treatment goal for AUD is the achievement of long-term abstinence [9]. However, at least 60% of individuals resume hazardous consumption levels within 6 months of treatment (i.e. medication, inpatient or outpatient) [9]. This is related to three main factors: craving, stress and alcohol-related cues [10]. From a treatment point of view, of the three factors, cues cannot be altered. However, targeting craving could be a practical approach to treat alcohol addiction. Craving is multifaceted and encompasses the urge for reward, the necessity to reduce subsequent physiological distress and an intense compulsion identified by strong intent with or without loss of control [11]. Studies have shown that craving intensity predicts future alcohol relapse [12, 13]. In this study, we aim to reduce craving using an invasive neuromodulation technique.
For several decades, addiction has come to be viewed as a disorder of the dopamine neurotransmitter system [14]. From a neurobiological perspective, genetic factors account for an overall heritability of 40% for alcohol dependence [15], involving predominantly dopamine-related genes (dopamine receptors 1, 2, 3 and 4; dopamine transporter; dopamine hydroxylase), serotonin-related genes (receptor 2ac, transporter), monoamine-related genes (catechol-O-methyltransferase (COMT), monoamine oxidase A (MAO)) as well as gamma-aminobutyric acid (GABA) and opioid receptor genes [16]. The mesocorticolimbic dopaminergic reward systems is implicated in the pathophysiology of alcohol addiction [17], and many studies have zoomed in on the A1 allele of the TAQ1A dopamine D2 receptor gene [18]. This polymorphism is also implicated in the development of depression and anxiety [19, 20]. A reduction in density of the dopamine D2 receptors is linked not just to AUD but also to multiple addictive and compulsive behaviours [21]. Dopamine D2 receptor reduction decreases the sensitivity to negative action consequences, which may explain an increased risk of developing addictive behaviours in A1 allele carriers [22]. Applied to alcohol addiction, it could thus be suggested that when individuals drink to find relief, they may not be able to learn from the negative consequences of overconsumption. However, the generalisation of the dopamine theory of addiction has been questioned [14]. Even though a large body of evidence demonstrates that stimulants increase striatal dopamine levels and likely so that alcohol may have such an effect, there is little evidence to support that cannabis and opiates also increase dopamine levels [14]. Whereas striatal dopamine receptor availability and dopamine release are clearly diminished in stimulant or alcohol dependence, no such changes are evident in opiate, nicotine or cannabis dependence [14].
The brain’s reward system involves the ventral tegmental area (VTA) which has reciprocal connections with the nucleus accumbens and habenula, involved in reward and dysreward [23, 24]. The dorsal anterior cingulate cortex (dACC) receives projections from these reward processing regions, especially the habenula [24–26], and forms associations between rewards and action [27]. Moreover, activity in the dACC increases when obtained rewards are below the desired level, initiating modifications to selection of action [28]. It has been theorised that craving, i.e. a strong desire or wanting of a certain substance results from sensitisation and dissociation from liking [29, 30]. This leads to an increased desired level and, through the process of allostasis (i.e. stability through change [31] via reference resetting [30]), the vicious cycle of excessive consumption and withdrawal [30].
Craving in alcohol abuse has been linked to abnormal cue-evoked activity not only in the dACC, ventral striatum/nucleus accumbens and ventromedial prefrontal cortex [32] but also in the amygdala, posterior cingulate and parahippocampal cortex [32]. Craving in alcohol and stimulant abuse, i.e. in dopaminergic addiction, likely has a partially overlapping common neurobiological substrate, irrespective of the substance. This is shown in a meta-analysis where nicotine, alcohol and cocaine cravings elicited by cue reactivity overlapped in the dACC and ventral striatum/nucleus accumbens [33]. Self-reported alcohol craving, in contrast to cue-evoked alcohol craving, seems to be related to anteriorly located cingulate activity in the pregenual area [33, 34]. Craving has also been associated with the dopamine DRD3 receptor and alpha-synuclein polymorphism [35]. The craving-related activity in the ventral striatum/nucleus accumbens and anterior cingulate cortex is due to an upregulation of glutaminergic excitatory neurotransmission in these areas [36], suggesting that suppressive neuromodulation of these areas may subdue craving.
Transcranial magnetic stimulation (TMS) is a non-invasive technique used to modulate activity and connectivity in the brain [37]. Repetitive TMS (rTMS) has been shown to suppress alcohol [38] and cocaine craving [39] when using a figure-of-eight coil targeting the dorsolateral prefrontal cortex (DLPFC). rTMS of the DLPFC is known to increase the release of dopamine in the nucleus accumbens [40] and caudate nucleus [41] as well as modulate dopamine release in the subgenual anterior cingulate cortex (sgACC) and the orbitofrontal cortex [42]. The figure-of-eight coil has been shown to be able to reach targets with depths of 2 to 2.5 cm from the surface of the head (e.g. DLPFC) [43, 44]. However, the magnetic field generated by this coil is not sufficient to reach deeper cortical regions such as the dACC given the rapid decrease in electric field as a function of the tissue depth [43, 44]. The double cone coil, however, has been shown to stimulate regions between the depths of 3 to 4 cm [43, 44]. Indeed, it has been reported that the double cone coil can modulate the dACC and suppress alcohol craving transiently [45]. But apart from its direct effect, TMS modulates the network associated with the cortical target [46, 47], at least in awake people [48].
In view of the genetic vulnerability related to alcohol addiction and craving, it is to be expected that rTMS will only resort to a temporary improvement in craving, and that the dopaminergic reward deficiency will resume when the effect of rTMS wears off. This was already noted when an initial 2-week rTMS study targeting the dACC was highly beneficial but only outlasted the stimulation period for 3 weeks [45]. However, this can be resolved by surgically implanting an electrode on the dACC to provide permanent neuromodulation [49]. To our knowledge, there are two promising studies reporting that permanent neuromodulation can facilitate long-term abstinence in alcohol-dependent individuals [50, 51]. In the earlier study [51], five patients were treated for an average of 38 months with bilateral deep brain stimulation (DBS) of the nucleus accumbens with all patients reporting significant improvements in alcohol craving. In addition, two patients remained completely abstinent for more than 4 years. The more recent study by De Ridder and colleagues [50] reported a case where a patient remained abstinent for 18 months with reduced alcohol craving following stimulation of the rostrodorsal anterior cingulate cortex (rdACC). The current report is based on De Ridder et al.’s [50] methodology and details the effects and feasibility of suppressing alcohol craving using surgical electrode implantation in the rdACC in eight individuals with severe AUD.
Methods
Participants
This study was approved by the Southern Health and Disability Ethics Committee (ref: 14/STH/119), and the protocol is registered at the Australian New Zealand Clinical Trials Registry (ANZCTR) with the identifier ACTRN12614000859684. All protocol-related procedures were performed at the BRAI3N neuromodulation clinic of the University Hospital of Otago, Dunedin, New Zealand.
Participants were referred from the hospital’s outpatient department and from information resulting from press coverage. Five male and four female participants between the ages of 20 to 65, meeting the Mini-International Neuropsychiatric Interview (MINI) DSM-IV [52] (Table 1) criteria for alcohol dependence who have failed multiple prior treatments (i.e. at least one anti-craving medication and one residential or outpatient treatment) for alcohol dependence, were enrolled in the study. The MINI DSM-IV includes seven questions evaluating alcohol dependence criteria in the past 12 months [52]. To meet the alcohol dependency criteria, participants had to report at least three symptoms [52].
Table 1.
Mini-International Neuropsychiatric Interview (MINI) (DSM IV) criteria for alcohol dependence for each participant
| Participant number | MINI (DSM IV) criteria | Total number of criteria met | ||||||
|---|---|---|---|---|---|---|---|---|
| Did you need to drink a lot more in order to get the same effect you got when you started first drinking, or did you get much less effect with continued use of the same amount? | When you cut down on drinking, did your hands shake? Did you sweat or feel agitated? Did you drink to avoid these symptoms (for example, ‘the shakes’, sweating or agitation) or to avoid being hungover? | During the times when you drank alcohol, did you end up drinking more than you planned when started? | Have you tried to reduce or stop drinking alcohol but failed? | On the days that you drank, did you spend substantial time in obtaining alcohol or drinking or in recovering from the effects of alcohol? | Did you spend less time working, enjoying hobbies or being with others because of your drinking? | If your drinking caused you health or mental problems, did you still keep on drinking? | ||
| 1 | x | x | x | x | x | x | x | 7 |
| 2 | x | x | x | x | x | x | 6 | |
| 3 | x | x | x | x | x | x | x | 7 |
| 4 | x | x | x | x | x | x | x | 7 |
| 5 | x | x | x | x | x | x | x | 7 |
| 6 | x | x | x | x | x | x | x | 7 |
| 7 | x | x | x | x | x | x | x | 7 |
| 8 | x | x | x | x | x | 5 | ||
Of the nine individuals, one male participant declined having the implant. The study’s exclusion criteria included a history of epileptic seizures, psychiatric disorders with psychotic symptoms or maniac symptoms; have a pacemaker; or show contraindications for magnetic resonance imaging (MRI). All participants had a supportive social network (minimum one person) who provided contact details and were involved in pre-/post-surgery appointments. Participant’s demographic and clinical characteristics pre-implant are described in Table 2.
Table 2.
Participants’ demographic and clinical characteristics pre-stimulation
| Variable/participant number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Age at implant (years) | 53 | 47 | 48 | 58 | 33 | 32 | 63 | 41 | 46.9 (11.2) |
| Sex | Female | Female | Male | Female | Male | Male | Male | Female |
4 females 4 males |
| Age at onset (years) | 33 | 35 | 18 | 17 | 20 | 13 | 27 | 22 | 23.1 (7.9) |
| Number of inpatient/outpatient treatments | 4 | 1 | 11 | 3 | 7 | 3 | 3 | 3 | 4.4 (3.2) |
| Number of medicated assisted treatments | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1.1 (0.8) |
| Average self-reported daily intakea | 24 | 16 | 32 | 19 | 30 | 30 | 16 | 38 | 25.6 (8.1) |
| Self-reported alcohol cravingb | 8 | 8 | 5 | 7 | 5 | 10 | 9 | 9 | 7.6 (2.2) |
| ACQ-NOWc | 6.3 | 5.6 | 5.2 | 7 | 4.8 | 5.9 | 6.6 | 5.3 | 5.8 (0.8) |
| OCDS (total)d | 36 | 25 | 31 | 29 | 30 | 32 | 30 | 27 | 30.0 (3.3) |
| Obsessive subscale | 17 | 11 | 15 | 13 | 13 | 15 | 12 | 12 | 13.5 (2.0) |
| Compulsive subscale | 19 | 14 | 16 | 16 | 17 | 17 | 18 | 15 | 16.5 (1.6) |
| Other psychiatric disorders | Major depression, post-traumatic stress disorder, panic disorder | Obsessive compulsive disorder | Major depression, anxiety disorder, post-traumatic stress disorder | Major depression, anxiety disorder | Major depression, panic disorder, agoraphobia, antisocial personality disorder | Major depression | Major depression | ||
| MADRSe | 22 | 4 | 13 | 12 | 12 | 17 | 16 | 12 | 13.5 (5.2) |
| STAI-If | 57 | 59 | 66 | 47 | 47 | 42 | 67 | 51 | 54.5 (9.2) |
| STAI-IIf | 67 | 66 | 68 | 51 | 49 | 54 | 67 | 64 | 60.8 (8.0) |
aAlcohol Timeline Follow-Back over the past month. Average daily self-reported standard drinks
bSelf-reported alcohol craving ‘right now’; measured on a scale of 0 to 10 where 0 = not craving at all and 10 = the most ever
cAlcohol craving questionnaire-short form; ranges from 1 to 7
dObsessive compulsive drinking scale; total score ranges from 0 to 41. Obsessive subscale ranges from 0 to 20. Compulsive scale ranges from 0 to 21
eMontgomery-Asberg Depression Rating Scale; total score ranges from 0 to 60. Higher scores indicate increasing depressive symptoms
fSTAI-I measures state anxiety while STAI-II measures trait anxiety. Each subscale ranges from 20 to 80
Study Design and Procedures
The study was divided into four phases. During pre-stimulation evaluations (phase 1), a structured diagnostic interview [52] was carried out by a psychiatrist to establish alcohol dependence and to evaluate for other disorders. Physical examination (i.e. attention to physical effects of alcohol dependence, medication usage and other medical disorders), and routine blood tests were performed by a specialist in internal medicine. Participants’ resting-state electroencephalography (EEG) was compared to a group of healthy controls, matched for age and sex.
In phase 2, based on a previous study [45], non-invasive rTMS (Magstim Inc., Wales, UK) was performed, using double-cone coil, as a prognostic test. rTMS consisted of active stimulation at 1 Hz with 50% machine output in tonic mode for 5 consecutive days and placebo for 5 consecutive days in random order. For placebo rTMS, the coil was placed perpendicular to the scalp and was applied at the same frequency and intensity as active rTMS to ensure that the participants were exposed to the same clicking noise. Daily craving before rTMS session was assessed on a scale of 1 (not at all) to 10 (the most ever) to the question: “Please rate how strong your alcohol craving is right now by circling a number on the 10-point scale”. All participants (n = 9) enrolled in the study demonstrated ≥ 50% reduction in alcohol craving self-ratings to active rTMS stimulation and were considered eligible to continue to the next phase [45]. One participant declined to have the implant. At the end of the tenth session, all participants were able to identify the placebo and active rTMS sessions. However, from a clinical perspective, the participants’ speculation of order of stimulation did not seem to affect the therapeutic effect of active stimulation. This is evident from Table 3 that, among those who were implanted with the electrode (n = 8), there was a decrease in craving during the first 5 consecutive sessions in group 1 (active followed by placebo) compared to group 2 (placebo followed by active) (Table 3).
Table 3.
Daily repetitive transcranial magnetic stimulation (rTMS) craving score for each participant
| Group | Participant number | Self-reported alcohol craving1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | ||||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | ||
| 1 | 1 | 8 | 3 | 0 | 2 | 6 | 4 | 1 | 7 | 8 | 0 |
| 1 | 2 | 4 | 2 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | |
| 1 | 3 | 7 | 7 | 6 | 5 | 5 | 5 | 7 | 7 | 6 | 5 |
| 1 | 5 | 5 | 4 | 2 | 0 | 1 | 1 | 2 | 3 | 3 | 2 |
| 1 | 6 | 10 | 8 | 7 | 3 | 1 | 1 | 1 | 2 | 1 | 1 |
| Placebo | Treatment | ||||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | ||
| 2 | 4 | 7 | 7 | 6 | 6 | 6 | 7 | 3 | 2 | 1 | 1 |
| 2 | 7 | 5 | 5 | 5 | 5 | 5 | 6 | 5 | 2 | 1 | 0 |
| 2 | 8 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 0 | 1 | 0 |
1Daily craving before rTMS was assessed on a scale of 1 (not at all) to 10 (the most ever) to the question: ‘Please rate how strong your alcohol craving is right now by circling a number on the 10-point scale’
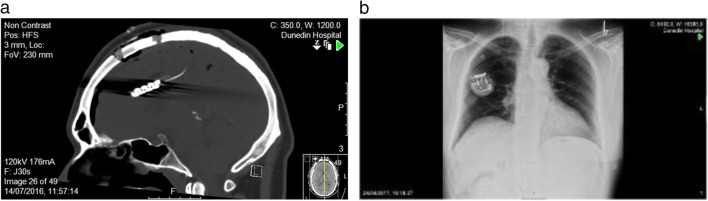
In the operative phase (phase 3), participants had routine pre-operative evaluations and MRI. Using MRI neuro-navigation, two Lamitrode 44 electrodes (Abbot, Neurodivision, Plano, Texas) were placed on the dACC under general anaesthesia (Fig. 1), followed by overnight observation in a neurosurgical high dependency unit.
Fig. 1.
Post-surgical computed tomography (CT) showing (a) the ‘back-to-back’ paddle sutured electrode on the rostrodorsal anterior cingulate cortex (dACC) and (b) the internal pulse generator (IPG) in the chest subcutaneously below the right clavicle
In phase 4, the electrodes were activated using an internal pulse generator (IPG) post-surgery. The original study design included a randomised immediate (3 days post-surgery) or delayed (17 days post-surgery) start protocol for activation of electrodes. However, due to adverse events, the original randomised study design was deemed unfeasible by the research team in consultation with the Data Safety Monitoring Board after the fourth implant. Time points of IPG activation and deactivation post-surgery as well as adverse events for each participant are presented in Table 4. By 2 months post-implant, all electrodes were activated.
Table 4.
Time points of IPG activation and deactivation post-surgery as well as adverse events for each participant
| Variable | Participant number | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| IPG activation | Day 3 | Day 14 | Day 3 | Week 8 | Week 4 | Week 4 | Week 8 | Day 14 |
| Reason | Early start | Delayed start | Early start | Psychosis | Infection | Impulsive behaviour | Right frontal venous infarct | Delayed start |
| IPG deactivation | Week 25 | Week 13 | Week 26 | |||||
| Reason | Infection (IPG removed) | Infection (IPG removed) | Seizures | |||||
| Data point used in analyses post-stimulation | Week 48 | Week 48 | Week 48 | Week 24 | Week 12 | Week 48 | Week 24 | Week 24 |
Participants 1 and 2 were on burst frequency of 10 Hz, participant 3 was on tonic frequency of 6 Hz, while participants 4 to 8 were on burst frequency of 6 Hz. Stimulation amplitude and length of electrical charge being delivered (cycle mode) were individually optimised for 48 weeks post-surgery. It has been previously shown that burst stimulation may be superior to tonic stimulation in activating cortical brain regions [53]. Unpublished study results from a clinical trial using implanted stimulators to suppress pain suggested that 6 Hz burst may be the optimal stimulation frequency. However, the Prodigy IPGs™ implanted in the first three participants allowed programming of the lowest frequency of 10 Hz burst or 6 Hz tonic. The third participant reported feelings of uneasiness at activation of device and was immediately switched to the lower frequency of 6 Hz tonic. Participants 4 to 8 were implanted with the Proclaim IPG™ which allowed the programming of 6 Hz burst.
Primary and Secondary Outcomes
The primary endpoint of the study was alcohol craving assessed on a numerical scale of 0 (not craving at all) to 10 (the most ever). Participants were asked to respond to the question: “Please rate how strong your alcohol craving is right now by circling a number on the 10-point scale”. Secondary endpoints of the study included different efficacy assessments: alcohol intake using the Timeline Follow-Back [54], the Obsessive Compulsive Drinking Scale (OCDS) [55] and the Alcohol Craving Questionnaire-NOW short form (ACQ-NOW) [56], and mood ratings using the State and Trait Anxiety Scale (STAI) [57] and the Montgomery-Asberg Depression Rating Scale (MADRS) [34]; adverse events were used to assess safety and tolerability throughout the study, and EEG was used to assess changes in brain activity.
The OCDS [55] is a 14-item scale measuring two different cognitive aspects of alcohol craving: obsessive and compulsive drinking, and the 10-item ACQ-NOW [56] provides an overall craving score reflecting domains related to alcohol craving. The STAI [57] has 40 items, measuring state (STAI-I) and trait (STAI-II) anxiety while MADRS [34], a 10-item diagnostic questionnaire, assesses the severity of depressive episodes.
Resting-state EEG was obtained using the Mitsar EEG 202 amplifier and was sampled with 19 electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, O2) in the standard 10 to 20 International placement, referenced to linked ears, and impedances were checked to remain below 5 kΩ. Standardised low-resolution brain electromagnetic tomography (sLORETA) was used to estimate the intracerebral electrical sources that generated the activity in each of the eight frequency bands: delta (2–3.5 Hz), theta (4–7.5 Hz), alpha 1 (8–10 Hz), alpha 2 (10.5–12.5 Hz), beta 1 (13–18 Hz), beta 2 (18.5–21 Hz), beta 3 (21.5–30 Hz) and gamma (30.5–45 Hz). These frequencies were according to oscillatory classes reported by Kubicki et al. [58]. Technical details of the EEG technique [29] and sLORETA [59] and its validity have been previously published.
The primary outcome measure (cravings scores) and mean standard drinks per day were assessed at baseline and weeks 4, 8, 12, 24 and 48 post-surgery. Secondary outcome measures were collected at baseline and weeks 12, 24 and 48 post-surgery.
Statistical Analyses
Primary and Secondary Outcomes
Descriptive statistics were used to summarise demographic characteristics of participants. Due to missing data, differences in primary and secondary outcomes using paired t test were assessed using the last available stimulation data point (post-stimulation) (Table 4) compared to baseline (pre-stimulation).
All statistical analyses were conducted using Stata 14 (StataCorp 2017).
EEG Analyses
EEG data of the control group was previously collected in clinic by the research group for a different study on tinnitus. Controls were healthy individuals, without a history of psychiatric or neurological disorders, drug or alcohol abuse, head injury (with loss of consciousness) or seizure, headache, physical disability or tinnitus.
At the sensor level, the power spectral density was calculated for each midline electrode (Fz, Cz, Pz) for controls and responders (i.e. participants who did not relapse at 12-month follow-up) pre- and post-stimulation using a Fourier transformation. The average band power for each frequency band mentioned above was calculated by integrating the power spectral density in the appropriate frequency ranges. Two-sample t tests were used for between-groups analyses (controls versus pre-stimulation and controls versus post-stimulation), and paired t tests were utilised to examine the average band power pre- compared to post-stimulation for each band and electrode, respectively. The different analyses were controlled for multiple comparisons using the Benjamini-Hochberg test [60]. The critical value for comparison was calculated with a false discovery rate of 25% [60]. Values above the largest p value that is smaller than the critical value were considered significant [60].
At a whole-brain level, sLORETA was used to 1) compare responders’ pre- and post- stimulation brain activity to a control group matched for age and sex and 2) comparison between pre- and post-stimulation. Similar to power spectral density analysis, multiple comparisons for whole-brain analyses were controlled using the Benjamini-Hochberg test.
As for region of interest (ROI) analysis, based on results from whole-brain analysis, beta 1 current density was extracted for the rdACC using sLORETA. Power in all voxels was normalised to a power of 1 and log transformed at pre- and post-stimulation. The ROI value therefore reflects the log-transformed fraction of total power across all voxels of the beta 1 band at the rdACC. Significant changes pre- and post-stimulation were determined using paired t tests. In addition, to ROI analysis, we conducted Pearson’s correlation between ROI current density and craving scores at pre-stimulation and post-stimulation and changes from pre- to post-stimulation.
Results
Demographic and Descriptive Statistics
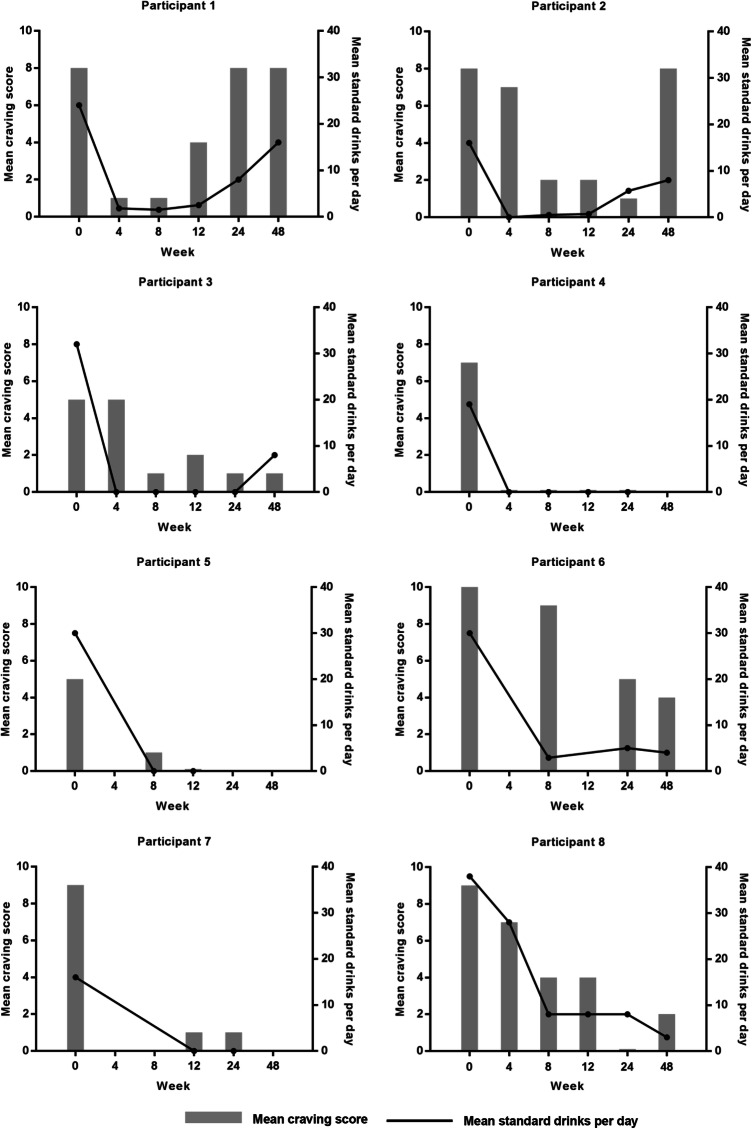
Six of the eight participants met all seven alcohol dependence criteria assessed on the MINI (DSM IV). One participant met 6 criteria while another met 5 out of the seven criteria (Table 1). Participants’ baseline demographic and clinical characteristics are reported in Table 2. Mean craving scores and mean standard drinks per day for each participant are demonstrated in Fig. 2. Two participants (participants 1 and 2) relapsed at 12-month follow-up.
Fig. 2.
Mean craving score and mean standard drinks per day for each participant
Primary and Secondary Outcomes
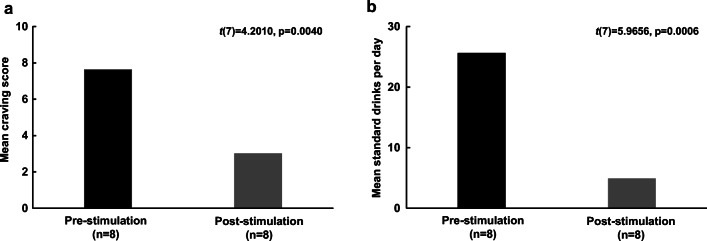
Self-reported alcohol craving was reduced by 60.7% (mean change ± SD = 4.6 ± 3.1) post-stimulation (mean ± SD = 3.0 ± 3.3) compared to pre-stimulation (mean ± SD = 7.6 ± 1.9) (t(7) = 4.2, p = 0.004) (Fig. 3a). There was a mean decrease of alcohol consumption by 21 standard drinks per day (80.0%) (mean change ± SD = 20.8 ± 9.8) post-stimulation (mean ± SD = 4.9 ± 5.6) compared to pre-stimulation (mean ± SD = 25.6 ± 8.2) (t(7) = 6.0, p = 0.0006) (Fig. 3b).
Fig. 3.
(a) Mean (± SE) craving score pre- and post- stimulation. (b) Mean (± SE) standard drinks per day pre- and post-stimulation
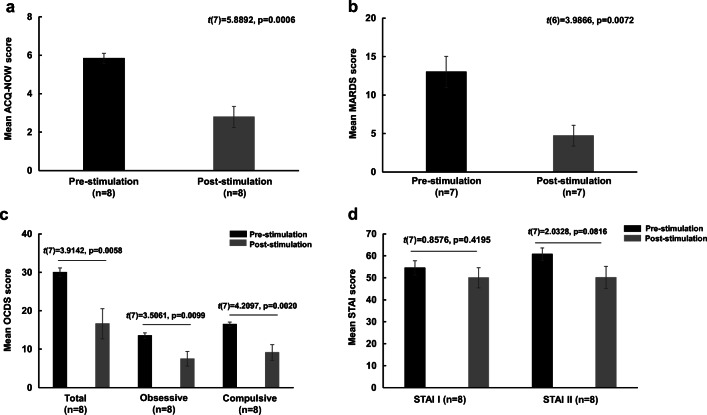
There was a mean decrease of 52.3% (mean change ± SD = 3.1 ± 1.5) on the ACQ-NOW post-stimulation (mean ± SD = 2.8 ± 1.5) compared to pre-stimulation (mean ± SD = 5.8 ± 0.8) (t(7) = 5.9, p = 0.0006) (Fig. 4a). Also, there was a mean decrease of 63.5% (mean change ± SD = 8.3 ± 5.5) on the MADRS post-stimulation (mean ± SD = 4.7 ± 3.6) versus pre-stimulation (mean ± SD = 13.0 ± 5.4) (t(6) = 4.0, p = 0.007) (Fig. 4b). There were reductions by 44.6% (mean change ± SD = 13.4 ± 9.7) post-stimulation (mean ± SD = 16.6 ± 11.1) versus pre-stimulation (mean ± SD = 30.0 ± 3.3) (t(7) = 3.9, p = 0.0006) on the total OCDS scale, 44.4% (mean change ± SD = 6.0 ± 4.8) post-stimulation (mean ± SD = 7.5 ± 5.4) compared to pre-stimulation (mean ± SD = 13.5 ± 2.0) (t(7) = 3.5, p = 0.0009) on the obsessive subscale and 44.7% (mean change ± SD = 7.38 ± 4.96) post-stimulation (mean ± SD = 9.1 ± 5.8) versus pre-stimulation (mean ± SD = 16.5 ± 1.6) (t(7) = 4.2, p = 0.002) on the compulsive subscale (Fig. 4c). However, results showed that there were no significant changes in the STAI-I (t(7) = 0.9, p = 0.4194) (mean change ± SD = 4.5 ± 14.84) post-stimulation (mean ± SD = 50.0 ± 12.9) compared to pre-stimulation (mean ± SD = 54.5 ± 9.2) and STAI-II (t(7) = 2.0, p = 0.0816) post-stimulation (mean ± SD = 50.1 ± 14.3) versus pre-stimulation (mean ± SD = 60.8 ± 7.9) (Fig. 4d).
Fig. 4.
Mean (± SE) scores pre- and post-stimulation for (a) Alcohol Craving Questionnaire-NOW (ACQ-NOW), (b) Montgomery-Asberg Depression Scale (MADRS), (c) Obsessive Compulsive Drinking Scale (OCDS) and (d) State and Trait Anxiety Index (STAI)
EEG Analyses
At a sensor level, in responders, there was a significant (t(10) = − 2.3, p = 0.0416) difference in average beta 2 band power for Fz when comparing controls (mean ± SD = − 25.4 ± 8.3) to pre-stimulation (mean ± SD = − 10.7 ± 13.0). A significant difference (t(5) = 2.7, p = 0.0440) was also observed in the beta 3 band for Fz when comparing pre-stimulation (mean ± SD = − 66.8 ± 48.0) to post-stimulation (mean ± SD = − 112.2 ± 10.4). However, these results were not significant after adjusting for multiple comparisons.
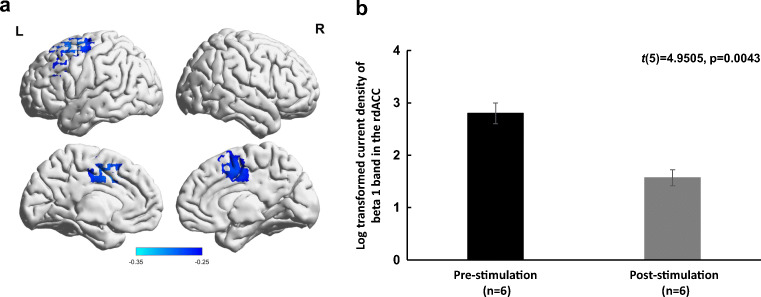
At a whole-brain level, after correcting for multiple comparisons, in responders, there was a significant decrease in current density at the rdACC for beta 1 band (t(5) = 1.6, p = 0.026) (Fig. 5a). No significant effects were observed for the delta, theta, alpha 1, alpha 2, beta 2, beta 3 and gamma bands in the rdACC. Also, there were no significant differences between responders at pre- and post-stimulation and healthy controls for all bands.
Fig. 5.
(a) Significant decrease in beta 1 activity (p = 0.026) in the rdACC in 6 participants (responders to stimulation) post- versus pre-stimulation. (b) Significant decrease (p = 0.0043) in log-transformed current density of the beta 1 band in the rdACC post- versus pre-stimulation of the responders
As for ROI analysis for beta 1 band in the rdACC, there was a significant decrease (mean change ± SD = 1.22 ± 0.61) (t(5) = 5.0, p = 0.004) in log-transformed current density pre-stimulation (mean ± SD = 2.8 ± 0.5) and post-stimulation (mean ± SD = 1.6 ± 0.4) (Fig. 5b).
There were no significant correlations between log-transformed current density for beta 1 in the rdACC and craving scores at pre-stimulation (r = 0.76, p = 0.08) and post-stimulation (r = 0.44, p = 0.39). However, there was a significant correlation between change in beta 1 band and change in craving (r = 0.9018, p = 0.0140).
Adverse Events
Adverse events are presented in Table 4. There were 2 cases of infection on the IPG insertion wound requiring IPG removal. One participant presented psychotic symptoms 3 days post-surgery. One participant suffered a right frontal venous infarct with patchy haemorrhagic change causing a transient left-sided weakness 1 day post-surgery. The hemiparesis completely resolved after 3 weeks. However, the participant had two seizure episodes requiring IPG deactivation 26 weeks post-surgery. One participant exhibited reckless impulsive behaviour for 3 weeks upon returning home post-surgery, requiring IPG activation to be delayed for a month.
Discussion
This is the first clinical trial reporting the effects of rdACC stimulation for alcohol craving using implanted electrodes in eight participants with severe, treatment-resistant alcohol dependence. Consistent with previous case reports [45, 50], there was a significant, 60.7% reduction in alcohol craving score following rdACC stimulation. Craving has been shown to be related to increased activity in the rdACC [29], which was confirmed in this study, and this relationship is very likely causal as the rdACC over-activity decreased with successful stimulation. It is worthy of note that results of the study were not influenced by limited access to alcohol as all participants were back to their daily lives with access to alcohol after an overnight observation in the high dependency unit post-surgery.
Even though there was a drastic reduction in alcohol craving at a group level, there were two non-responders, and participants did not completely discontinue drinking. Rather, alcohol consumption changed from uncontrolled use to a controlled alcohol intake. When questioned, participants attributed the reason for drinking to habit. This suggests that rdACC stimulation may be effective in controlling alcohol craving but not habitual overconsumption of the substance. It has been postulated that while appetitive conditioning is governed by the orbital frontal and anterior cingulate cortex, and temporal lobe including the amygdala [61], habit formation depends on interactions between the prefrontal cortex and dorsal lateral striatum [61].
It is of note that two participants relapsed at 1-year follow-up. Their relapse could potentially be a result of the use of higher-frequency burst stimulation (10 Hz) compared to other participants (6 Hz burst). Previous studies have shown that burst compared to tonic stimulation is a substantially more powerful cortex activator [53] and has beneficial effects when applied to the auditory cortex for tinnitus [62], somatosensory cortex for pain [63] and anterior cingulate for AUD [49], OCD [64] and tinnitus [65], as well as on the spinal cord [66, 67] and peripheral nerve [68] for pain. Based on case reports, it is suggested that theta frequencies between 4 and 7 Hz may be optimal for the anterior cingulate cortex [49, 64, 65]. In this study, as a result of technical alterations, the first three participants had Prodigy IPGs™ implanted (lowest possible frequency 10 Hz burst or 6 Hz tonic) and the remaining cohort the Proclaim IPG™ which allowed the programming of 6 Hz burst. Interestingly, the third participant on 6 Hz tonic responded positively.
It should be emphasised that the main aim of this study was to examine the effect of rdACC stimulation on craving. It has been reported that a score above 3 on the one-item VAS indicates moderate craving and can be utilised as a threshold to identify patients presenting harmful drinking [69]. Craving is more directly related to the severity of AUD while compulsion as measured by the OCDS is linked to the need to satisfy craving [69]. In the current study, these dimensions of AUD are not significantly correlated at baseline (r = 0.37, p = 0.3683).
Targeting the rdACC seems to also have a therapeutic effect on depression. Of the eight participants, six were diagnosed with current major depression at pre-stimulation with a mean MADRS score of 15.2 (SE, 1.64). Post-stimulation assessment demonstrated a significant (p = 0.0205) 8.1-point reduction in total score to a mean of 7.2 (SE, 1.92). Given that participants were on antidepressants before enrolment with similar dosage throughout the trial, results suggest that improvement is related to rdACC stimulation. One limitation of the current study is the inability to determine whether depression preceded the development of alcohol dependence or vice versa, and therefore, one can only assume these participants were relief drinkers (i.e. drinking to avoid negative emotions). Previous studies have suggested a bidirectional causal relationship, and that being diagnosed with one disorder doubles the risk of the onset of the other [8].
Collectively, the results suggest that rdACC stimulation improves depression and obsessive-compulsive drinking but not anxiety. Anxiety could be independent of craving resolution, as rdACC stimulation by rTMS seems to be beneficial for depression [45, 70, 71] and possibly obsessive-compulsive disorder [72]. Indeed, cingulotomies are performed for OCD [73, 74] and depression, both of which are related to increased activity in the rdACC, as evidenced by functional imaging. In contrast, anxiety may be related more to subgenual anterior cingulate activity changes [75] and might therefore require a different surgical target, even though depression and anxiety often are associated. It has been previously postulated that rdACC stimulation may have a primary effect on anxiety and secondarily on alcohol craving [50]. Results from this study, however, seem to indicate that rdACC stimulation may have a positive effect on alcohol craving in individuals whose alcohol dependence did not originate from anxiety.
This feasibility study had some important adverse effects. While results may point towards a trend that adverse events increased in responders and individuals implanted with the Proclaim stimulator, we maintain that it was not due to stimulation of the rdACC. Infections (participants 4 and 5) could have been prevented by using vancomycin instead of routine preventive antibiotics. The manic psychotic event of participant 4 and the development of impulsive behaviour in participant 6 were likely induced by perioperative stress given that they occurred before IPG activation. Moreover, participant 4 has a history of bipolar disorder, which the participant had failed to mention to the psychiatrist at enrolment. The haemorrhagic infarct with subsequent seizures (participant 7) was due to the occlusion of a draining vein into the superior sagittal sinus, which is a rare (0% in children [76] to 5.9% in adults [77]) but a known risk factor of this open surgical corridor.
It must be stated that the MINI (DSM-IV) [52] was used to assess alcohol dependence in this study. This questionnaire has been shown to have acceptably high validation and reliability scores and can be administered in a much shorter time when compared to the long version of DSM-IV [52]. The MINI (DSM-IV) has been used to identify alcohol-dependent individuals in both clinical and research settings [78–80]. In comparison to DSM-IV, DSM-V has been shown to identify a larger number of milder alcohol–related symptoms patients who have greater confidence in their capacity to modify their drinking habits [80]. In addition, when interpreting study results, the potential diffusion effect resulting from volume conduction should be taken into consideration. Although there is a scarcity of research in this matter, stimulation studies have reported that the volume of brain tissue being activated from an implant highly depends on the electrode’s height and diameter, the stimulation region and the relative position of the electrode [81]. Also, individual anatomical factors including lesions and anisotropic conductivity of white matter can influence the flow of volume currents [81]. For example, in a case report of two patients with implanted electrodes on the same target, i.e. rdACC, for tinnitus, an increased functional connectivity from the target was identified in contrast to the non-responder [65], similar to results from a larger group of patients on a different target [82].
In conclusion, the magnitude in alcohol craving improvement from the study suggests that rdACC stimulation using implanted electrodes may be effective in suppressing alcohol craving in individuals with severe AUD, based on a demonstrable pathophysiological mechanism. However, it must be highlighted that because of the small sample size, no final safety and efficacy conclusions can be drawn, and thus, this underscores the need for a larger cohort study to further investigate the beneficial effects of this procedure.
Electronic supplementary material
(PDF 1078 kb)
Acknowledgments
We would like to thank the Neurological Foundation of New Zealand and the University of Otago for funding the study and Abbott for support.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sook Ling Leong, Email: sookling.leong@tcd.ie.
Dirk De Ridder, Email: dirk.deridder@otago.ac.nz.
References
- 1.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal DL. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). The Corsini Encyclopedia of Psychology 2010:1–3.
- 3.Association AP . Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. 2013. [DOI] [PubMed] [Google Scholar]
- 4.Heinz A, Löber S, Georgi A, et al. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol and Alcoholism. 2003;38:35–9. doi: 10.1093/alcalc/agg005. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. The American Journal of Medicine. 2005;118:330–41. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Brière FN, Rohde P, Seeley JR, Klein D, Lewinsohn PM. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Comprehensive Psychiatry. 2014;55:526–33. doi: 10.1016/j.comppsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Archives of General Psychiatry. 2002;59:375–80. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- 8.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106:906–14. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Quintero C, de los Cobos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115:120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo D, Sinha R. The neurobiology of alcohol craving and relapse. Handbook of clinical neurology: Elsevier; 2014:355–68. [DOI] [PubMed]
- 11.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol and Alcoholism (Oxford, Oxfordshire) 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 12.Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol and Alcoholism. 2004;39:357–61. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- 13.Flannery BA, Poole S, Gallop R, Volpicelli J. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. Journal of Studies on Alcohol. 2003;64:120–6. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- 14.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–12. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 15.Uhl GR, Grow RW. The burden of complex genetics in brain disorders. Archives of General Psychiatry. 2004;61:223–9. doi: 10.1001/archpsyc.61.3.223. [DOI] [PubMed] [Google Scholar]
- 16.Blum K, Oscar-Berman M, Demetrovics Z, Barh D, Gold MS. Genetic Addiction Risk Score (GARS): molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS) Molecular Neurobiology. 2014;50:765–96. doi: 10.1007/s12035-014-8726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 18.Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. American Journal of Epidemiology. 2007;167:125–38. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- 19.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. International Journal of Neuropsychopharmacology. 2017;20:1036–46. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience. 2016;17:524. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohjalainen T, Rinne J, Någren K, et al. The A1 allele of the human D 2 dopamine receptor gene predicts low D 2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3:256. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 22.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–5. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 23.Lammel S, Lim BK, Ran C, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker PM, Jhou T, Li B, et al. The Lateral Habenula Circuitry: Reward Processing and Cognitive Control. J Neurosci. 2016;36:11482–8. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ely BA, Stern ER, Kim JW, Gabbay V, Xu J. Detailed mapping of human habenula resting-state functional connectivity. Neuroimage. 2019;200:621–34. doi: 10.1016/j.neuroimage.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrisi S, Nord CL, Balderston NL, Roiser JP, Grillon C, Ernst M. Resting state connectivity of the human habenula at ultra-high field. Neuroimage. 2017;147:872–9. doi: 10.1016/j.neuroimage.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 28.Preedy VR. Neuropathology of Drug Addictions and Substance Misuse Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids: Academic Press; 2016.
- 29.De Ridder D, Manning P, Leong SL, et al. The brain, obesity and addiction: an EEG neuroimaging study. Scientific Reports. 2016;6:34122. doi: 10.1038/srep34122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ridder D, Manning P, Leong SL, Ross S, Vanneste S. Allostasis in health and food addiction. Scientific Reports. 2016;6:37126. doi: 10.1038/srep37126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Mohan A, De Ridder D, Sunaert S, Vanneste S. The neural correlates of the unified percept of alcohol-related craving: a fMRI and EEG study. Scientific Reports. 2018;8:923. doi: 10.1038/s41598-017-18471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs–a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33:1318–26. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 34.Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery–Åsberg depression rating scale (MADRS) Journal of Affective Disorders. 2003;77:255–60. doi: 10.1016/s0165-0327(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal A, Wetherill L, Bucholz KK, et al. Genetic influences on craving for alcohol. Addict Behav. 2013;38:1501–8. doi: 10.1016/j.addbeh.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer J, Pedersen A, Scherbaum N, et al. Craving in Alcohol-Dependent Patients After Detoxification Is Related to Glutamatergic Dysfunction in the Nucleus Accumbens and the Anterior Cingulate Cortex. Neuropsychopharmacology. 2013;38:1401–8. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature Reviews Neuroscience. 2007;8:559. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 38.Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- 39.Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. American Journal on Addictions. 2008;17:345–6. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- 40.Erhardt A, Sillaber I, Welt T, Müller M, Singewald N, Keck M. Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology. 2004;29:2074. doi: 10.1038/sj.npp.1300493. [DOI] [PubMed] [Google Scholar]
- 41.Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task–a TMS–[11C] raclopride PET study. European Journal of Neuroscience. 2008;28:2147–55. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimulation. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu M, Ueno S. Comparison of the induced fields using different coil configurations during deep transcranial magnetic stimulation. PLoS One. 2017;12:e0178422. doi: 10.1371/journal.pone.0178422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Ridder D, Vanneste S, Kovacs S, Sunaert S, Dom G. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study. Neuroscience Letters. 2011;496:5–10. doi: 10.1016/j.neulet.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–43. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 49.De Ridder D, Manning P, Glue P, Cape G, Langguth B, Vanneste S. Anterior Cingulate Implant for Alcohol Dependence. Neurosurgery 2016. [DOI] [PubMed]
- 50.De Ridder D, Manning P, Glue P, Cape G, Langguth B, Vanneste S. Anterior cingulate implant for alcohol dependence: case report. Neurosurgery. 2016;78:E883–E93. doi: 10.1227/NEU.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 51.Voges J, Mueller U, Bogerts B, Muente T, Heinze H-J. Deep brain stimulation surgery for alcohol addiction. World Neurosurgery. 2013;80:S28. e1–S. e31. doi: 10.1016/j.wneu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry 1998. [PubMed]
- 53.Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nature Neuroscience. 2001;4:402. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 54.Sobell L, Sobell M. Timeline follow-back measuring alcohol consumption. Center for Psychological Studies, Nova Southeastern University 1992:41–72.
- 55.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 56.Raabe A, Grüsser SM, Wessa M, Podschus J, Flor H. The assessment of craving: psychometric properties, factor structure and a revised version of the Alcohol Craving Questionnaire (ACQ) Addiction. 2005;100:227–34. doi: 10.1111/j.1360-0443.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 57.Spielberger CD. State-trait anxiety inventory. The Corsini encyclopedia of psychology. John Wiley & Sons, Inc: Hoboken, NJ; 2010. [Google Scholar]
- 58.Kubicki S, Herrmann W, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmacopsychiatry. 1979;12:237–45. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 59.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12. [PubMed] [Google Scholar]
- 60.Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics. 2002;27:77–83. [Google Scholar]
- 61.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 62.De Ridder D, Vanneste S, van der Loo E, Plazier M, Menovsky T, Van de Heyning P. Burst stimulation of the auditory cortex: a new form of neurostimulation for noise-like tinnitus suppression. Journal of Neurosurgery. 2010;112:1289–94. doi: 10.3171/2009.10.JNS09298. [DOI] [PubMed] [Google Scholar]
- 63.De Ridder D, Vanneste S, Van Laere K, Menovsky T. Chasing map plasticity in neuropathic pain. World Neurosurg. 2013;80:901 e1–5. doi: 10.1016/j.wneu.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 64.De Ridder D, Leong SL, Manning P, Vanneste S, Glue P. Case Report: Anterior Cingulate Implant for Obsessive Compulsive Disorder. World Neurosurg 2016. [DOI] [PubMed]
- 65.De Ridder D, Joos K, Vanneste S. Anterior cingulate implants for tinnitus: report of 2 cases. J Neurosurg. 2016;124:893–901. doi: 10.3171/2015.3.JNS142880. [DOI] [PubMed] [Google Scholar]
- 66.Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation: Technology at the Neural Interface. 2018;21:56–66. doi: 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]
- 67.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80:642–9 e1. doi: 10.1016/j.wneu.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 68.De Ridder D, Plazier M, Menovsky T, Kamerling N, Vanneste S. C2 Subcutaneous Stimulation for Failed Back Surgery Syndrome: A Case Report. Neuromodulation 2013. [DOI] [PubMed]
- 69.Flaudias V, Teisseidre F, De Chazeron I, et al. A multi-dimensional evaluation of craving and impulsivity among people admitted for alcohol-related problems in emergency department. Psychiatry Research. 2019;272:569–71. doi: 10.1016/j.psychres.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 70.Kreuzer PM, Downar J, de Ridder D, Schwarzbach J, Schecklmann M, Langguth B. A Comprehensive Review of Dorsomedial Prefrontal Cortex rTMS Utilizing a Double Cone Coil. Neuromodulation 2018. [DOI] [PubMed]
- 71.Kreuzer PM, Schecklmann M, Lehner A, et al. The ACDC pilot trial: targeting the anterior cingulate by double cone coil rTMS for the treatment of depression. Brain Stimul. 2015;8:240–6. doi: 10.1016/j.brs.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 72.De Ridder D, Leong SL, Manning P, Vanneste S, Glue P. Anterior cingulate implant for obsessive-compulsive disorder. World Neurosurgery. 2017;97:754. e7–e16. doi: 10.1016/j.wneu.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 73.Banks GP, Mikell CB, Youngerman BE, et al. Neuroanatomical characteristics associated with response to dorsal anterior cingulotomy for obsessive-compulsive disorder. JAMA Psychiatry. 2015;72:127–35. doi: 10.1001/jamapsychiatry.2014.2216. [DOI] [PubMed] [Google Scholar]
- 74.Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM, Sheth SA. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. Journal of Neurosurgery. 2016;124:77–89. doi: 10.3171/2015.1.JNS14681. [DOI] [PubMed] [Google Scholar]
- 75.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biological Psychiatry. 2015;77:385–93. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNatt SA, Sosa IJ, Krieger MD, McComb JG. Incidence of venous infarction after sacrificing middle-third superior sagittal sinus cortical bridging veins in a pediatric population. Journal of Neurosurgery: Pediatrics. 2011;7:224–8. doi: 10.3171/2010.11.PEDS09261. [DOI] [PubMed] [Google Scholar]
- 77.Tsutsumi K, Shiokawa Y, Sakai T, Aoki N, Kubota M, Saito I. Venous infarction following the interhemispheric approach in patients with acute subarachnoid hemorrhage. Journal of Neurosurgery. 1991;74:715–29. doi: 10.3171/jns.1991.74.5.0715. [DOI] [PubMed] [Google Scholar]
- 78.Lejoyeux M, Delaroque F, McLoughlin M, Adès J. Alcohol dependence among elderly French inpatients. The American Journal of Geriatric Psychiatry. 2003;11:360–4. [PubMed] [Google Scholar]
- 79.Muramatsu K, Kamijima K, Yoshida M, et al. The patient health questionnaire, Japanese version: validity according to the mini-international neuropsychiatric interview–plus. Psychological Reports. 2007;101:952–60. doi: 10.2466/pr0.101.3.952-960. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi T, Lapham G, Chavez LJ, et al. Comparison of DSM-IV and DSM-5 criteria for alcohol use disorders in VA primary care patients with frequent heavy drinking enrolled in a trial. Addiction Science & Clinical Practice. 2017;12:17. doi: 10.1186/s13722-017-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drakesmith M, El-Deredy W, Welbourne S. Reconstructing coherent networks from electroencephalography and magnetoencephalography with reduced contamination from volume conduction or magnetic field spread. PLoS One. 2013;8:e81553. doi: 10.1371/journal.pone.0081553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Ridder D, Vanneste S. Targeting the Parahippocampal Area by Auditory Cortex Stimulation in Tinnitus. Brain Stimul 2014. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1078 kb)