Abstract
C—H· · ·O hydrogen bonds constitute a unique class of cohesive interactions. Their properties are similar to those of canonical H-bonds, although their energy is significantly lower, typically in the 0.5–2.5 kcal/mol range. Polarised C—H groups, such as those adjacent to electronegative groups, or within aromatic moieties, are particularly strong donors. C—H· · ·O bonds are ubiquitous in nucleic acids and in proteins, notably stabilizing the p-sheet secondary structure. They have also been observed in numerous protein-ligand interactions. Here, we analysed crystal structures, deposited in the Protein Data Bank, of complexes of FDA-approved protein kinase inhibitors with cognate kinases, to assess the possible role of C—Hinhibitor· · ·Oprotein hydrogen bonds. The conserved hinge motif of protein kinases with two solvent-exposed carbonyl groups and one exposed backbone amide, is well known to be involved in canonical H-bonding with inhibitors. We now find that in virtually all complexes where the inhibitor interacts with the hinge backbone, at least one of the hinge carbonyl groups accepts an H-bond from a C—H inhibitor group, which is either aromatic or adjacent to an electronegative group. These observations are important for design of hinge-binding scaffolds of novel kinase inhibitors for therapeutic use.
Keywords: hydrogen bonds, protein kinase inhibitors, protein kinases
1 |. INTRODUCTION
Protein tyrosine and serine/threonine kinases are responsible for the regulation of much of the cellular signalling and constitute one of the most important families of drug targets.1–6 Inhibitors of protein kinases have the potential to be used in therapies of a large spectrum of diseases, ranging—for example—from neurodegeneration and inflammation, to cardiovascular pathophysiology and—most importantly—cancer. At the time when this manuscript was written, there were 48 Food and Drug Administration (FDA)-approved protein kinase inhibitors on the market, the vast majority for clinical use in cancer treatment and a few to treat inflammatory and autoimmune disorders.1,7,8
The design of kinase inhibitors is challenging, given the fact that the human kinome includes 517 protein kinases, each containing a homologous catalytic domain, with high significant amino acid and structural conservation, particularly within the adenosine triphosphate (ATP)-binding pocket, which is targeted by most inhibitors.9,10 The identification of a suitable chemical scaffold is a vital step in the design of kinase inhibitors. The majority of the FDA-approved drugs acting on protein kinases are Type I inhibitors, that is, competitive with respect to ATP. High-affinity binding requires that the inhibitors mimic the binding of ATP to its cognate pocket thus conferring on the inhibitor selectivity towards protein kinases in general. Once a scaffold is identified, selectivity towards a specific kinase is accomplished through derivatisation of the scaffold and introduction of various distal groups. The ATP-pocket of kinases is selective for adenine owing to a specific hydrogen bond (H-bond) network, which involves the solvent-exposed backbone of the “hinge”, a short oligopeptide that connects the N- and C-terminal lobes (or subdomains) of the catalytic kinase domain.11,12 The hinge includes three amino acids in an extended, β-sheet secondary conformation, and denote them—following other publications—in accordance to their position in the sequence downstream of the “gatekeeper” residue, as gk+1, gk+2 and gk+3. The adenine of ATP binds to the hinge in such a way that the N10 amino group of adenine donates an H-bond to the main chain carbonyl of gk+1, while the N1 nitrogen accepts an H-bond from the main chain amide of gk+3. The third interaction is a noncanonical H-bond, in which the C2-H of adenine donates a proton to the main chain carbonyl of gk+3 (Figure 1).
FIGURE 1.
Hydrogen bonding between the adenine moiety of ATP and the hinge oligopeptide of the kinase (based on the representative 1.8 Å resolution crystal structure of the N-terminal domain of the RSK2 in complex with AMPPNP, code 3G51)
Most inhibitor scaffolds mimic the interaction of adenine with the hinge, and contain H-bond donors and acceptors, which interact with the gk+3 and gk+1 carbonyl groups and the gk+1 amide. These canonical H-bond networks have been recently analysed in considerable detail, but only with respect to the canonical interactions involving electronegative donor and acceptor groups.13 However, it has been also reported in a number of instances that noncanonical, so-called “weak” H-bonds of the C—H· · ·O type are also found in kinase inhibitor complexes,14,15 although to our knowledge, there has been no systematic study of this subject. Hydrogen bonds in which C—H groups (typically polarised) donate protons to carbonyl groups are ubiquitous in nucleic acids, proteins, as well as protein-protein and protein-ligand interfaces.16,17 Here, we describe our analysis of crystal structures of complexes of 29 FDA-approved kinase inhibitors with their cognate kinases, available from the Protein Data Bank (PDB)18. Our results reveal the ubiquitous presence of C—H· · ·O interactions between inhibitors and the carbonyl groups of the hinge. Further, we note that the number of scaffolds found among the FDA-approved kinase inhibitors is very limited, but consistent with optimal exploitation of the carbonyl groups within the hinge region of kinases, as acceptors of both canonical H-bonds as well as weak H-bonds, in which the C—H groups are typically from aromatic rings, or adjacent to electronegative nitrogen atoms.
2 |. METHODS
We limited our study to the crystal structures of FDA-approved protein kinase inhibitors in complexes with their primary target kinases, when available, or with close homologues. The database was based in part by elegant recent reviews of protein kinase inhibitors by Roskoski.1,19 We initially identified 31 relevant crystal structures in the PDB. Of these, 21 structures included Type 1 inhibitors, seven included Type II inhibitors (i.e., those that bind to inactive, unique target kinase conformations) and three belonged to Type VI (covalent, irreversible inhibitors). In one case, that is, cobimetinib (Type VI) the inhibitor made no contact with the hinge, and the structures were excluded from further analysis, leaving a database of 30 structures (see Table 1 and Figure 2).
TABLE 1.
Full stereochemical details of all putative H-bonds detected between the inhibitors and the kinase hinge motif in the cohort of studied structures
| gk+1 (C=O) |
gk+3 (N–H) |
gk+3(C=O) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kinase | PDB | Donor | dH (Å) | 180-ξ (°) | ω (°) | χ (°) | Θ (°) | Acceptor | dH (Å) | (°) | Ψ (°) | Θ (°) | Donor | dH (Å) | 180-ξ (°) | ω (°) | χ (°) | Θ (°) | |
| Class I | |||||||||||||||||||
| Abemaciclib | CDK6 | 5L2S | C6 | 2.3 | 35.7 | 158.1 | −7.5 | −4.4 | N1 | 2.1 | 24.2 | 99.5 | 23.8 | N7 | 2.2 | 17.4 | 124.0 | 178.7 | 0.4 |
| Alectinib | ALK | 3AOX | C4 | 2.7 | 21.8 | 145.4 | −41.6 | −14.3 | O20 | 1.8 | 9.7 | 60.0 | 8.4 | C13 | 2.9 | 85.7 | 90.6 | 280.6 | −78.6 |
| Baricitinib | BMP2K | 4W9X | NAT | 1.7 | 11.9 | 171.3 | 6.1 | 1.3 | N1 | 2.2 | 15.3 | 87.2 | 15.3 | C2 | 2.9 | 47.0 | 132.7 | 225.9 | −31.7 |
| Bosutinib | SRC | 4MXO | CAH | 2.6 | 35.5 | 141.0 | 47.1 | 25.2 | NAT | 2.1 | 16.0 | 83.9 | 15.9 | CAK | 2.4 | 48.9 | 134.8 | 228.2 | −34.2 |
| Brigatinib | ALK | 6MX8 | C6 | 2.5 | 21.4 | 142.7 | −62.7 | −18.9 | N5 | 2.1 | 31.0 | 80.1 | 30.5 | N7 | 2.2 | 67.4 | 117.6 | 248.1 | −58.9 |
| Ceritinib | ALK | 4MKC | C21 | 2.3 | 24.2 | 154.2 | −39.6 | −15.1 | N20 | 2.1 | 36.0 | 83.6 | 35.7 | N18 | 2.3 | 51.0 | 143.2 | 226.1 | −34.1 |
| Crizotinib | ALK | 2XP2 | N22 | 2.1 | 22.8 | 168.4 | −86.7 | −22.8 | N23 | 2.0 | 11.3 | 53.1 | 9.0 | C5 | 2.4 | 69.4 | 132.8 | 237.8 | −52.4 |
| Dabrafenib | B-RAF | 4XV2 | C7 | 2.4 | 25.5 | 142.5 | 39.6 | 15.9 | N6 | 2.2 | 38.1 | 96.9 | 37.8 | N9 | 2.1 | 53.7 | 152.4 | 169.3 | 8.6 |
| Dasatinib | SRC | 3G5D | C1 | 2.9 | 19.5 | 127.9 | 28.8 | 9.3 | N1 | 2.1 | 27.1 | 89.3 | 27.1 | N | 2.0 | 49.9 | 147.7 | 212.5 | −24.3 |
| Erlotinib | EGFR | 1M17 | C19 | 2.4 | 38.5 | 164.1 | −36.2 | −21.6 | N2 | 2.1 | 14.1 | 78.5 | 13.8 | C17 | 2.7 | 57.0 | 118.0 | 261.5 | −56.0 |
| Fostamatinib | STK | 3FQS | C22 | 2.5 | 19.3 | 137.7 | −27.5 | −8.8 | N17 | 2.1 | 7.4 | 79.0 | 7.3 | N21 | 2.0 | 43.8 | 162.1 | 211.6 | −21.3 |
| Gefitinib | EGFR | 4122 | C2 | 2.2 | 30.3 | 166.9 | −39.5 | −18.7 | N3 | 2.0 | 21.9 | 98.8 | 21.6 | CAH | 2.2 | 55.6 | 139.5 | 243.3 | −47.5 |
| Lenvatinib | VGFR2 | 3WZD | C7 | 2.2 | 41.8 | 178.3 | −23.0 | −15.1 | N6 | 2.0 | 28.0 | 101.4 | 27.4 | C4 | 2.4 | 64.8 | 131.9 | 244.0 | −54.4 |
| Lepatinib | EGFR | 1XKK | C19 | 2.4 | 35.8 | 147.4 | −60.6 | −30.6 | N18 | 2.0 | 15.3 | 112.1 | 14.2 | C16 | 2.6 | 56.8 | 149.4 | 209.3 | −24.2 |
| Neratinib | EGFR | 3W2Q | CAQ | 2.4 | 47.0 | 160.8 | −30.8 | −22.0 | NBA | 2.1 | 26.8 | 80.1 | 26.4 | CAT | 2.4 | 54.6 | 114.1 | 265.9 | −54.4 |
| Palbociclip | CDK6 | 5L2I | C07 | 2.5 | 25.8 | 157.5 | −4.7 | −2.0 | N01 | 2.3 | 8.7 | 140.0 | 5.6 | N04 | 2.0 | 55.5 | 160.6 | 130.4 | 38.9 |
| Ribociclib | CDK6 | 5L2T | C4 | 2.4 | 42.9 | 150.9 | 51.2 | 32.0 | N1 | 2.3 | 16.9 | 141.4 | 10.4 | N15 | 1.9 | 30.4 | 160.2 | 162.2 | 8.9 |
| Ruxolitinib | SRC | 4U5J | CAE | 2.6 | 26.5 | 142.7 | −0.7 | −0.3 | NAP | 2.2 | 29.3 | 95.0 | 29.2 | NAN | 2.1 | 39.8 | 137.5 | 219.9 | −24.2 |
| Tofacitinib | JAK3 | 3LXK | N12 | 1.9 | 17.0 | 178.3 | −3.5 | −1.0 | N17 | 2.2 | 11.9 | 180.9 | −0.2 | C18 | 2.6 | 58.3 | 121.1 | 262.7 | −57.6 |
| Vandetanib | RET | 2IVU | C2 | 2.4 | 48.3 | 169.8 | −45.6 | −32.2 | N3 | 2.1 | 14.4 | 70.2 | 13.5 | CAI | 2.7 | 50.1 | 139.4 | 225.9 | −33.4 |
| Vemurafenib | B-Raf V600E | 5JRQ | N21 | 2.0 | 28.6 | 168.9 | −25.9 | −12.1 | N26 | 2.2 | 30.0 | 51.4 | 23.0 | C25 | 2.4 | 39.2 | 132.1 | 215.3 | −21.4 |
| Class II | |||||||||||||||||||
| Axitinib | VEGFR | 4AG8 | N15 | 1.9 | 26.7 | 168.2 | −2.1 | −0.9 | N14 | 2.1 | 37.6 | 106.9 | 35.7 | C16 | 2.7 | 60.9 | 125.4 | 238.4 | −48.1 |
| Imatinib | Abl | 3K5V | N3 | 2.0 | 11.2 | −106.4 | −10.7 | C2 | 2.4 | 40.4 | 132.9 | 228.3 | −28.9 | ||||||
| Nilotinib | p38 b | 3GP0 | N44 | 1.9 | 23.5 | −75.3 | −22.7 | C45 | 2.6 | 81.6 | 121.3 | 260.5 | −77.3 | ||||||
| Nintedanib | VEGFR2 | 3C7Q | N9 | 1.8 | 28.5 | 174.5 | −6.4 | −3.0 | O10 | 1.8 | 9.2 | 170.7 | 1.5 | C19 | 2.5 | 58.6 | 128.7 | 294.1 | −51.2 |
| Ponatinib | Bcr-Abl | 3OXZ | C1 | 2.4 | 51.2 | 163.7 | 12.1 | 9.4 | N1 | 1.9 | 29.6 | 76.1 | 28.7 | C81 | 2.7 | 23.3 | 132.0 | 207.4 | −10.5 |
| Sunitinib | KIT | 3G0E | N24 | 1.9 | 36.3 | 171.3 | −12.5 | −7.4 | O27 | 1.8 | 14.0 | 102.1 | 13.7 | ||||||
| Sorafenib | KDR | 3WZE | C25 | 2.4 | 42.3 | 136.6 | 25.6 | 16.9 | N26 | 2.3 | 25.4 | 86.8 | 25.4 | N30 | 1.9 | 48.1 | 135.9 | 236.5 | −38.4 |
| Class VI | |||||||||||||||||||
| Ibrutinib | BTK1 | 5P9J | NAB | 2.1 | 32.7 | 144.8 | −26.0 | −13.7 | N1 | 2.1 | 18.4 | 64.8 | 16.6 | C2 | 2.4 | 61.8 | 126.8 | 248.0 | −54.8 |
| Dacomitinib | EGFRK | 4I24 | C8 | 2.4 | 40.6 | 160.0 | −50.9 | −30.3 | N7 | 2.1 | 14.7 | 99.1 | 14.5 | C3 | 2.2 | 60.0 | 131.0 | 251.8 | −55.4 |
| Mean (C–H) | 2.4 | 34.4 | 153.7 | −15.5 | −7.1 | Mean | 2.1 | 20.6 | 82.9 | 16.6 | Mean (C–H) | 2.5 | 52.6 | 134.0 | 231.5 | −38.8 | |||
| SD | 0.1 | 9.9 | 11.9 | 36.0 | 19.2 | SD | 0.1 | 9.2 | 56.1 | 13.7 | SD | 0.2 | 11.7 | 6.4 | 14.9 | 15.3 | |||
| Mean (N–H) | 1.9 | 25.6 | 168.2 | −19.6 | −7.5 | Mean (N–H) | 2.1 | 45.7 | 144.1 | 199.5 | −14.4 | ||||||||
| SD | 0.1 | 8.0 | 10.1 | 29.4 | 8.2 | SD | 0.1 | 14.0 | 15.5 | 37.5 | 28.5 | ||||||||
Note: The color code: yellow—tyrosine kinases (all other are Ser/Thr kinases); blue—putative H-bonds involving a nitrogen donor or acceptor from the inhibitor; light red—H-bonds involving an oxygen acceptor from the inhibitor; grey—C–H· · ·O interactions that fall outside the expected stereochemistry of an H-bond; light green—averages and standard deviations for each class of interactions.
FIGURE 2.
Chemical structures of the kinase inhibitors studied in this work (hydrogen atoms are implicit and not shown). The blue boxes enclose the donor/acceptor atoms that form the H-bonding interaction with the hinge fragment of the cognate kinase. The red asterisk indicates a carbon atom, which serves as a potential donor in a C—H· · ·O bond. The arrow indicates the orientation of the H-bonding group with respect to the hinge motif, that is, the direction of the arrow is from gk+1 to gk+3. (a) Group I inhibitors; (b) Group II and (c) VI inhibitors
All coordinate sets available from the PDB lacked explicit hydrogen atoms. In order to improve the accuracy of the coordinates, to remove any bias imposed by different refinement strategies and to introduce explicit hydrogens, we re-refined all structures using deposited diffraction data (reflection data were available for all structures, except vandetanib, 2IVU, which was used directly from the PDB). In one case (ibrutinib, 5P9J) the reported resolution of 1.08 Å was not warranted by the quality of the deposited data—we cut the resolution to 1.5 Å). Hydrogens were added by ReadySet (PHENIX20), isotropic displacement parameters (B factors) were re-set to 15 Å2 and the models were refined to convergence with update of solvent structure using phenix.refine and REFMAC5.21,22 Hydrogens were treated as “riding” on their parent atoms. In several cases, electron density maps obtained in this way and inspected in COOT 23 indicated errors, such as omission of specific amino acids, clearly visible in electron density, wrong conformations etc. We corrected the obvious errors manually and re-refined the model again to convergence.
In order to identify putative H-bond donor and acceptor groups, we identified in each complex all hydrogen atoms of the inhibitors a 3 Å radius of the gk+3 and gk+1 carbonyl oxygens, and all potential acceptors (N,O) within 2.5 Å of the gk+1 amide nitrogen of the hinge of the kinase. Once potential H-bond partner groups were identified, we proceeded with detailed analysis of the stereochemical parameters.
In the case of the H-bonds involving the gk+3 amide group and the acceptor from the inhibitor, we measured the H· · ·A distances (dH), where A denotes the acceptor, the N—H· · ·A angles (ξ), and the dihedral angle Cα—N—H· · ·A(χ). This allowed us to calculate the elevation angles of the acceptor (θ), i.e., the angle between the H· · ·A bond and the plane of the peptide group. The angle θ was calculated from the relationship sin θ = sin ξ sin χ.24
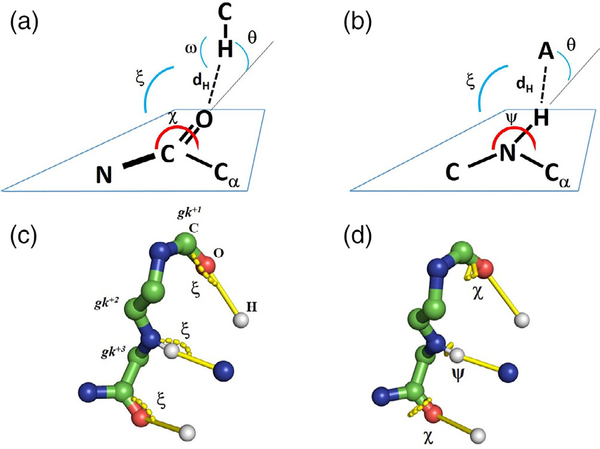
We were particularly interested in the interactions involving the backbone gk+1 and gk+3 carbonyl groups, as these involved both canonical electronegative donors as well as polarised C—H groups from inhibitors. To assess if these interactions displayed the stereochemistry expected of a genuine H-bond, we analysed the O· · ·H (dH) distances, the C=O· · ·H angles (ξ) and the N—C=O· · ·H dihedral angle (χ), and the elevation angles (θ) of the donor atom from the sp2 oxygen plane, calculated as explained above. Lastly, we also measured the C—H· · ·O angles (ω) to assess the linearity of the H-bond. All relevant stereochemical parameters are depicted in Figure 3.
FIGURE 3.
Definition of stereochemical parameters determined for the hydrogen bonds between the inhibitors and the hinge carbonyl and amide groups. (a) The five parameters determined for the H-bonds involving a hydrogen atom from the inhibitor and the kinase hinge carbonyl group; (b) the four parameters determined for the H-bonds involving the amide N—H group as a donor; (c) visualization of the ξ angles in the three H-bonds investigated; atoms are colored by type—the blue sphere representing the acceptor atom for the gk+3 carbonyl is blue to represent the majority of basic nitrogens as acceptors, although two oxygen atoms are also found in this position; (d) visualization of the dihedral angles χ and ψ using the same convention as in C
The stereochemistry of the interactions was analysed in PYMOL (version 2.3.3. Schrodinger LLC), which was also used to generate figures.
3 |. RESULTS
3.1 |. Re-refinement of atomic models
For approximately a third of the atomic models in our study, re-refinement significantly improved the overall R-factors, stereochemical parameters or both. Overall, the root-mean square deviation (RMSD) between the initial and final sets of coordinates was typically in the range of 0.2 Å, although in select models much larger corrections were also observed, even though they did not in general affect the atoms within the ATP-binding site. Details of the re-refinement are given in Table S1.
3.2 |. Identification of the H-bond donor and acceptor groups in inhibitors
All measured stereochemical parameters of H-bonds between inhibitors and kinases are shown in Table 1.
Among Type I inhibitors—with the exception of alectinib—all inhibitors appear to saturate the H-bonding potential of the hinge backbone by accepting an anchoring, canonical H-bond from the amide of the gk+3 residue and donating two H-bonds to the carbonyl oxygens of gk+1 and gk+3. In most cases, the canonical H-bond to the backbone amide of gk+3 is mediated by a basic nitrogen from a heterocycle as an acceptor; in alectinib, nintedanib and sunitinib, a carbonyl group acts as an acceptor. Importantly, in every structure at least one of the two putative H-bonds to the gk+1 and gk+3 carbonyls involves a C—H group from the inhibitor. In seven complexes, the second group is also C—H, while in the remaining ones it is N—H. In those complexes where one putative C—H· · ·O bond was identified, the carbon is located next to a non-basic nitrogen within a heterocycle. If a second C—H· · ·O bond is observed, it invariably originated from an aromatic C—H group. Among the seven analysed Type II inhibitor complexes, the interaction with the hinge region appears to be distinctly weaker, with some inhibitors not donating H-bonds to either the gk+1 or gk+3 carbonyls. These include imatinib, nilotinib and sunitinib. Finally, the two Type VI inhibitors show intimate H-bonding to the hinge, similar to the interactions involving the Type 1 inhibitors, with three H-bonds, one mediated by a C—H group in ibrutinib, and two in dacomitinib.
3.3 |. Stereochemistry of H-bonds to the amide group of gk+1
For H-bonds involving the gk+3 amide group as a donor, we expected to find only strong, canonical bonds to electronegative atoms in the inhibitors. The expectation for a strong canonical H-bond is that the dH distance is <2.5 Å, the deviation from linearity of the N—H· · ·A ensemble is not greater than 45°, and the elevation angle is also <45°, placing the acceptor close to the peptide plane.
In all of the studied structures, the H· · ·A distances fall within the 1.8–2.3 Å range, with an average of 2.1 ± 0.1 Å. All but three of these interactions involve heterocyclic, sp2-hybridised nitrogen atoms as acceptors. The three remaining cases—alectinib, nintedanib and sunitinib—involve carbonyl groups within the inhibitor acting as acceptors. The average deviation from linearity of the N—H· · ·A ensemble is only 20.5 ± 9.2°, with the largest deviation of 38.1° (dabrafenib). Finally, the average elevation angle (i.e., the angle between the H· · ·A bond and the plane of the peptide), is 16.6 ± 13.6° with the largest value of 37.8° in dabrafenib. All these parameters attest to the notion that the central, canonical H-bonds invariably conform to the stereochemistry expected for a strong interaction, probably accounting for at least ~5 kcal/mol in energy.
3.4 |. Stereochemistry of H-bonds to the carbonyl groups of the hinge
The H-bonds anchoring the inhibitors to the gk+1 and gk+3 carbonyls are quite diverse.
In the case of the gk+1 carbonyl, in all but two cases (Type II inhibitors imatinib and nilotinib), the oxygen accepts an H-bond from the inhibitor. Eight of these bonds involve N—H groups as donors (i.e., canonical H-bonds), originating from pyrrole, pyrazole, or amino groups of the inhibitor. The average dH distance is 1.9 ± 0.1 Å. The average deviation from the linearity of the C=O· · ·H ensemble in these interactions is 25.6 ± 8.0°, and the mean dihedral angle χ is—19.6 ± 29.4°, while the elevation angle (the angle between the H· · ·O bond and the sp2 plane of oxygen) is −7.5 ± 8.2°. Finally, the N—H· · ·O bonds are very linear, with the average ω angle of 168.2 ± 10.1°. These values show that the canonical C=O· · ·H—N bonds that involve the gk+1 carbonyl conform to the stereochemistry of strong H-bonds.
In the remaining 20 complexes, the gk+1 carbonyl oxygen is located close to a C—H group. All but one dH distances are ≤2.7 Å, as expected for a good C—H· · ·O bond (the structure of dasatinib where the long distance occurs was excluded from further calculation of stereochemical parameters). The average dH is 2.4 ± 0.1 Å, with a mean deviation from linearity of the C=O· · ·H ensemble of 34.9 ± 9.9°. The mean elevation angle θ is −7.1 ± 19.4°, indicating that the interactions for the hydrogen atoms to cluster close to the sp2 plane of the carbonyl oxygen. The mean dihedral angle χ is—15.5 ± 36.0°. Except for one case of an aromatic C—H group (alectinib) all others are polarised because of the adjacent one or two nitrogens within a heterocyclic moiety. In summary, all these values are consistent with a typical geometry of an H-bond, except that—as expected—the dH distance is longer than a canonical H-bond, but well within the range defined for C—H· · ·O bonds.
Of note is the mean dihedral angle χ (close to 0° for both N—H· · ·O and C—H· · ·O bonds). For H-bonds involving the carbonyl oxygen of the peptide group, the hydrogen atom tends to approach either one of the two lone sp2 electron pairs to maximise the covalent component of the bond 25 (the electrostatic component is maximised when the H-bond is linear). Thus, the C—O· · ·H angle should be ~120°, and the H-bond should lie within the plane of the peptide bond, as defined by the dihedral angle N—C—O· · ·H close to 0° (for the syn sp2 orbital) or 180° (for the anti sp2 orbital). (For a definition and visualization of the sp2 orbitals refer to Figure 4). The gk+1carbonyl group is at the N-terminus of the hinge, and has no H-bonding partner groups, but the anti sp2 free electron pair is not freely accessible due to steric occlusion. The observed mean deviation from linearity of ~30° for all H-bonds suggests that these interactions are often partly bifurcated and engage both orbitals. This seems particularly true of the N—H· · ·O bonds, where the deviation from linearity is smaller.
FIGURE 4.
Representative visualization of the location of three H-bonds between inhibitors and kinase hinge fragments. The two grey spheres denoted with H represent hydrogen atoms from donor groups of the inhibitor. The blue sphere represents the acceptor group, which is predominantly basic nitrogen. The syn and anti sp2 orbitals which are preferentially engaged by the donor groups are shown in yellow
Finally, it is important to note that the C—H· · ·O bonds are close to linear, with the average ω angle of 153.7 ± 11.9°.
In the case of the gk+3 carbonyl group, 10 H-bonds are accepted from N—H groups, again typically from pyrrole, pyrazole or amino moieties of the inhibitor. The average dH distance is 2.1 ± 0.1 Å, the average deviation from the linearity of the C=O· · ·H ensemble is 45.7 ± 14.0°, and the mean dihedral angle χ is 199.5 ± 37.5°, with the elevation angle of −14.4 ± 28.5°. The N—H· · ·O angle (ω) deviates from linearity and its average is 144.1 ± 15.5°. This reflects the fact that virtually all aromatic scaffolds of the inhibitors position the respective N—H donor groups above the plane of the gk+3 peptide (see Figure 5). The two inhibitors that notably show a distinctly more favourable interaction are palbociclip and ribociclip, both CDK4/6 inhibitors that take advantage of the different hinge stereochemistry in the target kinases.
FIGURE 5.
The structures of the 21 complexes of tyrosine kinase inhibitors with cognate kinases superposed on the hinge motif; only H-bond donor/acceptor groups from the inhibitors are shown, coloured by atom type, and only one representative hinge backbone is shown. The transparent blue line depicts the average plane of the inhibitor’s aromatic ensemble containing the donor/acceptor groups, the red line shows the plane of the gk+1 gk+3 acceptor and donor backbone groups, and the grey line shows the plane of the gk+2—gk+3 peptide group
In one complex (sunitinib), there is no apparent H-bond donor group close to the gk+3 carbonyl. In 19 complexes, the gk+1 carbonyl oxygen is located close to a C—H group. Eight of the C—H groups that are donors to the gk+3 carbonyl are aromatic while the remainder are polarised owing to the adjacent nitrogen(s). However, in seven cases either the dH distance exceeds the 2.7 Å cutoff or the position of the hydrogen (as defined by the χ and θ angles) is too far from the sp2 plane for a genuine H-bond to occur. In the remaining 12 complexes, the average dH is 2.5 ± 0.2 Å, with a mean deviation from linearity of the C=O· · ·H ensemble of 52.6 ± 11.7°, and a mean elevation angle θ is −38.8 ± 15.3°. The mean dihedral angle χ is 231.5 ± 14.9°. All these values are once more consistent with the expected stereochemistry of a relatively strong C—H· · ·O bond, although the stereochemistry appears to be less favourable than that seen with gk+1 carbonyl. Interestingly, the mean χ angle and the deviation from linearity of the C=O· · ·H ensemble indicate that unlike the gk+1 carbonyl, the gk+3 carbonyl oxygen engages its N—H and C—H donor groups exclusively via its anti sp2 orbital. The deviation from linearity of the C=O· · ·H ensemble, ~50°, is close to the expected 60°. This is easily reconciled when one recognises the fact that in the majority of protein kinases, the gk+3 carbonyl oxygen is engaged via its syn sp2 orbital in a H-bond with the gk+6 amide group, stabilizing a β-turn.
Finally, the C—H· · ·O bonds to the gk+3 carbonyl oxygens significantly deviate from linearity with the average ra angle of 134.0 ± 6.4°. Again, as shown in Figure 5, the typical relative orientation of the inhibitors is such that the plane of core aromatic scaffold is significantly above the plane of the gk+3 peptide, forcing the non-linearity of the C—H· · ·O interaction.
3.5 |. The core H-bonding templates in the inhibitors
The two H-bond donor groups and the acceptor within the inhibitor must be close together to be able to interact with the gk+1/gk+3 main chain. A representative arrangement of the three groups is shown in Figure 4. Perhaps not surprisingly, this creates constraints on the chemistry of the H-bonding moiety within the inhibitor. Figure 5 shows a subset of all the donor/acceptor groups from tyrosine kinase inhibitors superposed on the kinase average hinge structure. This figure illustrates clearly how the plane of the inhibitor is tilted with respect to the three main chain groups of the hinge, by about 25°. The central H-bond with the gk+3 amine as a donor is the key “anchoring” group, and the canonical strong H-bonds here are mostly linear. The steric hindrance of the C-terminal p-loop of the hinge lifts the inhibitor plane so that the H-bonds to the gk+3 carbonyl do not display favourable stereochemistry, with donors approaching the carbonyl from above the peptide plane; in contrast, as a result of the pivot, donor groups typically approach the gk+1 carbonyl from below of the peptide plane.
We analysed all inhibitors, which contain three H-bonding groups, and we find that there is remarkable conservation of the chemistry. The three donor-acceptor atoms are almost invariably coplanar, and arranged so that they accommodate only one other inserted atom, either between two nitrogen donors, or a nitrogen and a carbon. The three main templates are shown in Figure 6. Interestingly, the first two are very similar and differ only by the identity of the donor to the gk+3 carbonyl. Together, the three templates account for 18 of the inhibitors, even though the underlying chemistry varies considerably.
FIGURE 6.
The three conserved H-bonding templates identified in 18 of the inhibitors, with the representative examples for each and H-bonding geometry shown at the bottom
4 |. DISCUSSION
The concept of hydrogen bonds was first reported exactly 100 years ago. For many decades, these interactions were defined as occurring between two electronegative atoms. In the 1960s, Sutor26,27 was the first to recognise that polarised C—H groups can also act as donors in cohesive interactions akin to H-bonds, but the ubiquitous role of such interactions—which were also defined as weak hydrogen bonds—in nucleic acids, proteins, as well as protein-protein and protein-ligand interfaces became evident only much later.16,17 The first observation of C—H· · ·O bonds in kinase-inhibitor complexes was reported by Pierce et al.14 Their database included 184 complexes, available at the time from the PDB, and supplemented with an in-house database from Vertex Pharmaceuticals. The authors identified three types of C—H groups involved in these interactions: (1) those adjacent to at least one heteroatom, typically N; (2) groups within aromatic rings between two other carbons; and (3) those adjacent to a positively charged nitrogen (pyridinium C—H groups). Groups 1 and 3 are significantly polarised, enhancing the relative strength of the H-bond, while group 2 is only slightly polarised. These authors were also the first—to our knowledge—to explicitly recognise the unique C—H· · ·O bond between adenine (C2) of ATP and the carbonyl of gk+3. Nevertheless, this pioneering study did not identify specific groups within the protein kinase responsible for the interactions with the inhibitors’ C—H moieties. The issue was revisited by Panigrahi15, who analysed a larger dataset of 233 kinase-inhibitor structures and focused specifically on the types of amino acids within the protein most likely to serve as donors and acceptors in both canonical and weak H-bonds. Panigrahi noted that the majority of such interaction involve main-chain groups (i.e., backbone carbonyls and amides), but he did not characterise the stereochemistry of these interactions. More recently, Xing et al.13 analysed the canonical H- bonding network between the kinase hinges and inhibitors, but unfortunately, this otherwise extensive study did not discuss the C—H· · ·O bonds.
Our study provides a detailed analysis of the stereochemistry of H-bonds in the known crystal structures of protein kinases in complexes with FDA-approved inhibitors. The most important conclusion of our analysis is that the “weak” H-bonds of the C—H· · ·O type are ubiquitous in kinase-inhibitor complexes and account for the majority of the H-bonds with the gk+1 and gk+3 carbonyl oxygens of the hinge. We observe that most of these interactions display stereochemistry expected of relatively strong H-bonds. Specifically, the distances between the hydrogen and oxygen atoms are systematically shorter than those expected between non-bonded atoms (i.e., 2.7 Å), and the mean values determined by us (~2.4 Å) agree well with the study of distances in organic compounds determined at high resolution.28 It is also important to note that we observe clear directionality of the C—H· · ·O bonds. The hydrogen atoms are typically located close to the sp2 orbital plane of the accepting oxygen, and they approach the oxygen atoms close to either the syn, or the anti free sp2 orbital. There is a difference between the interactions involving the gk+1 and the gk+3 carbonyls: the former are decidedly stronger as judged by the stereochemistry.
Interestingly, the three donor-acceptor atoms within inhibitors occur with highly conserved motifs. In the vast majority of cases, the C—H donor to gk+1 and the N acceptor to the gk+3 amide are directly bonded and within an aromatic ring. This explains why the C—H· · ·O bonds to the gk+1 carbonyl appear stronger: the donor C—H group is highly polarised by the adjacent basic nitrogen. In contrast, most of the C—H donor groups interacting with the gk+3 carbonyls originate from aromatic rings but are not adjacent to electron withdrawing atoms.
Our observations will hopefully guide future development of new chemical scaffolds with the propensity to bind to the hinge fragment of protein kinases with high affinity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the National Institutes of Health for financial support (HL147555). Izabela Hawro was a visiting graduate student partly supported by the Office of the Dean of the School of Medicine.
Abbreviations:
- ATP
adenosine triphosphate
- FDA
Food and Drug Administration
- PDB
Protein Data Bank
- RMSD
root-mean square deviation
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. [DOI] [PubMed] [Google Scholar]
- 2.Carlson DA, Singer MR, Sutherland C, et al. Targeting Pim kinases and DAPK3 to control hypertension. Cell Chem Biol. 2018; 25: 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yli-Kauhaluoma J, Tuominen RK. Protein kinases as drug targets. Curr Top Med Chem. 2011;11:1304–1304. [DOI] [PubMed] [Google Scholar]
- 4.Arslan MA, Kutuk O, Basaga H. Protein kinases as drug targets in cancer. Curr Cancer Drug Tar. 2006;6:623–634. [DOI] [PubMed] [Google Scholar]
- 5.Akritopoulou-Zanze I The identification of new protein kinase inhibitors as targets in modern drug discovery. IDrugs. 2006;9: 481–487. [PubMed] [Google Scholar]
- 6.Roskoski R Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res. 2015; 100:1–23. [DOI] [PubMed] [Google Scholar]
- 7.Roskoski R Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016; 111:784–803. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Florez D, Valor L. Protein-kinase inhibitors: a new treatment pathway for autoimmune and inflammatory diseases? Reumatol Clin. 2016;12:91–99. [DOI] [PubMed] [Google Scholar]
- 9.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. [DOI] [PubMed] [Google Scholar]
- 10.Fabbro D, Cowan-Jacob SW, Mobitz H, Martiny-Baron G. Targeting cancer with small-molecular-weight kinase inhibitors. Methods Mol Biol. 2012;795:1–34. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011;36: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. [DOI] [PubMed] [Google Scholar]
- 13.Xing L, Klug-Mcleod J, Rai B, Lunney EA. Kinase hinge binding scaffolds and their hydrogen bond patterns. Bioorg Med Chem. 2015;23:6520–6527. [DOI] [PubMed] [Google Scholar]
- 14.Pierce AC, Sandretto KL, Bemis GW. Kinase inhibitors and the case for CH...O hydrogen bonds in protein-ligand binding. Proteins. 2002;49:567–576. [DOI] [PubMed] [Google Scholar]
- 15.Panigrahi SK. Strong and weak hydrogen bonds in protein-ligand complexes of kinases: a comparative study. Amino Acids. 2008;34:617–633. [DOI] [PubMed] [Google Scholar]
- 16.Derewenda ZS, Lee L, Derewenda U. The occurrence of C-H...O hydrogen bonds in proteins. J Mol Biol. 1995;252:248–262. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz S, Trievel RC. Carbon-oxygen hydrogen bonding in biological structure and function. J Biol Chem. 2012;287: 41576–41582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman H, Henrick K, Nakamura H, Markley JL. The world-wide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 2007;35:D301–D303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roskoski R Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol Res. 2016;103:26–48. [DOI] [PubMed] [Google Scholar]
- 20.Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst D. 2010;66:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murshudov GN, Skubak P, Lebedev AA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst D. 2011;67:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalevskiy O, Nicholls RA, Long F, Carlon A, Murshudov GN. Overview of refinement procedures within REFMAC5: utilizing data from different sources. Acta Crystallogr D Struct Biol. 2018;74:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Cryst D. 2010;66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor R, Kennard O. Crystallographic evidence for the existence of C-H. O, C-H. N, and C-H. C1 hydrogen-bonds. J Am Chem Soc. 1982;104:5063–5070. [Google Scholar]
- 25.Isaacs ED, Shukla A, Platzman PM, Hamann DR, Barbiellini B, Tulk CA. Covalency of the hydrogen bond in ice: a direct x-ray measurement. Phys Rev Lett. 1999;82:600–603. [Google Scholar]
- 26.Sutor DJ. C-H … O hydrogen bond in crystals. Nature. 1962; 195:68. [Google Scholar]
- 27.Sutor DJ. Evidence for existence of C-H … O hydrogen bonds in crystals. J Chem Soc. 1963;1105. [Google Scholar]
- 28.Rowland RS, Taylor R. Intermolecular nonbonded contact distances in organic crystal structures: comparison with distances expected from van der Waals radii. J Phys Chem. 1996;100:7384–7391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.