Abstract
The splicing of tRNA introns is a critical step in pre-tRNA maturation. In archaea and eukaryotes, tRNA intron removal is catalyzed by the tRNA splicing endonuclease (TSEN) complex. Eukaryotic TSEN is comprised of four core subunits (TSEN54, TSEN2, TSEN34 and TSEN15). The human TSEN complex additionally co-purifies with the polynucleotide kinase CLP1; however, CLP1’s role in tRNA splicing remains unclear. Mutations in genes encoding all four TSEN subunits, as well as CLP1, are known to cause neurodegenerative disorders, yet the mechanisms underlying the pathogenesis of these disorders are unknown. Here, we developed a recombinant system that produces active TSEN complex. Co-expression of all four TSEN subunits is required for efficient formation and function of the complex. We show that human CLP1 associates with the active TSEN complex, but is not required for tRNA intron cleavage in vitro. Moreover, RNAi knockdown of the Drosophila CLP1 orthologue, cbc, promotes biogenesis of mature tRNAs and circularized tRNA introns (tricRNAs) in vivo. Collectively, these and other findings suggest that CLP1/cbc plays a regulatory role in tRNA splicing by serving as a negative modulator of the direct tRNA ligation pathway in animal cells.
INTRODUCTION
Transfer RNAs (tRNAs) play essential roles in the translation of mRNA into proteins. Eukaryotic tRNA genes are transcribed by RNA Pol III and undergo a series of post-transcriptional processing and modification steps prior to reaching their mature and functional state (1–6). A conserved subset of intron-containing tRNA genes is found across all three domains of life (7–9). Introns contained within pre-tRNA transcripts of these genes must be removed during tRNA maturation. In bacteria, tRNA introns are self-spliced (8), whereas archaea and eukaryotes utilize a tRNA splicing endonuclease (TSEN) complex to catalyze removal of tRNA introns (1,8,10–12). Archaeal tRNA splicing endonucleases have been sorted into four classes, based upon subunit organization, including: homotetramers (α4), homodimers (α2 or ϵ2), or dimers of dimers ((αβ)2) (1,13). Conversely, eukaryotic tRNA splicing endonuclease (TSEN) complexes are composed of four individual subunits (TSEN2, TSEN34, TSEN54 and TSEN15) (14,15) (Figure 1). TSEN2 and TSEN34 are metal ion independent nucleases that cleave the 5′ and 3′ splice sites, respectively. They generate the 5′ exon with a 2′3′-cyclic phosphate and the 3′ exon with a 5′-hydroxyl group (14,16) (Figure 1). TSEN15 and TSEN54 are non-catalytic structural proteins but their precise role in splicing is unknown (14,17). Following cleavage by the TSEN complex, the tRNA exons are joined together by ligation using two distinct pathways, a healing-and-sealing pathway used primarily in plants and fungi (18) and a direct ligation pathway used in metazoans that features the RNA ligase RTCB (10,19,20).
Figure 1.
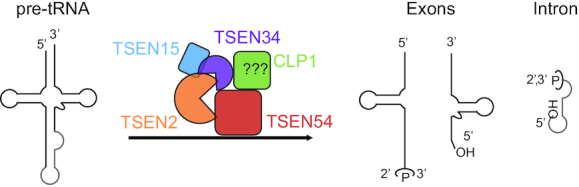
Cleavage of pre-tRNA by the TSEN complex, the current model of tRNA cleavage. The human TSEN complex is composed of four subunits including two endonucleases (TSEN2 and TSEN34) and two structural components (TSEN15 and TSEN54). Current models for tRNA splicing in metazoans suggest that the TSEN complex requires assistance from CLP1 to catalyze tRNA intron removal (light gray). Following cleavage by the TSEN complex, the 5′ exon and intron contain a 2′,3′ - cyclic phosphate and the 3′ exon and intron contain a 5′-OH.
Within the human genome there are 32 predicted intron-containing tRNAs (9). Recent advances in tRNA sequencing confirmed the expression of 26 of the 32 tRNA genes in HEK293 cells (21). The human introns are all located at the same position within the pre-tRNA, one base following the anticodon, between nucleotides 37 and 38 of the mature tRNAs (Supplementary Figure S1). Eukaryotic pre-tRNA 2° structures contain a distinctive base pair, known as the proximal base pair, that is absolutely critical for cleavage at the 3′ splice site (Supplementary Figure S1) (22,23). The eukaryotic TSEN complex has also been proposed to utilize a ‘molecular ruler’ mechanism whereby TSEN54 recognizes features in the mature tRNA domains that are used to recognize the 5′ splice site (10,17).
The TSEN complex is essential for eukaryotic life (20,24). Recent work in Saccharomyces cerevisiae revealed that each individual TSEN subunit is required, even when all intron-containing tRNA genes are replaced with intronless variants, suggesting that the complex has a fundamental cellular role beyond tRNA splicing (20). The eukaryotic TSEN complex was first discovered in budding yeast in the 1990s (14,25), however it was not until 2004 that the human TSEN complex was identified (15). Intriguingly, upon isolation of the TSEN complex from human cells, an additional TSEN binding partner was identified: the polyribonucleotide 5′-hydroxyl-kinase, CLP1 (15). CLP1 is also an established member of the mRNA 3′-polyadenylation machinery (26,27) and is conserved across eukaryotes. The S. cerevisiae homologue of CLP1 is an inactive kinase that cannot phosphorylate RNA substrates (28,29), whereas the CLP1 homologues from C. elegans and humans display robust RNA kinase activity in vitro (30,31). Mice that express catalytic deficient CLP1 develop a motor neuron disease that can be rescued by transgenic expression of wildtype CLP1 in neurons (32). This finding suggests that CLP1 kinase activity is essential within the nervous system but not in other cell types. The role of CLP1 in tRNA splicing is unclear, but several putative roles for CLP1 have been proposed, including: maintaining the integrity of the TSEN complex, the existence of a yeast-like ligation pathway, or promoting degradation/turnover of the intron (27).
The human TSEN complex and CLP1 are further linked via their association with pontocerebellar hypoplasia (PCH), a family of severe neurodegenerative autosomal recessive disorders (24,33–36). There is no curative treatment for PCH and patients rarely survive to adulthood (37). PCH is grouped into 11 subtypes based on phenotypic characteristics such as severe hypoplasia, microcephaly, trouble swallowing, and other motor and cognitive impairments (38). A mutation within CLP1 is linked to class PCH10 (35,39,40), while mutations in TSEN2, TSEN15, TSEN34 and TSEN54 are associated with PCH2, PCH4 and PCH5 (33,38). The other PCH subtypes are linked to mutations in other genes, many of which are associated with RNA processing and maturation (38,41–43). The underlying cause of PCH is unknown, but the association of PCH with several RNA processing enzymes suggests a link between disruption of RNA processing and neurological disorders. A homozygous mutation in CLP1 was recently shown to lead to a loss of TSEN complex association and reduced tRNA splicing (35,40). These results led to the hypothesis that, in humans, CLP1 protein is required for integrity of the TSEN complex and efficient splicing (27).
The goal of the present study was to reconstitute the core TSEN complex and characterize its activity in vitro. Here, we present the full reconstitution of the multi-subunit TSEN complex through use of an Escherichia coli co-expression system. The four subunits of the TSEN complex assemble into a stable heterotetramer that actively cleaves intron-containing pre-tRNAs as well as a mimic of the pre-tRNA anticodon stem loop. We further show that depletion of the Drosophila melanogaster orthologue of CLP1 leads to an increase in production of a mature tRNA reporter construct as well as endogenous and reporter tricRNAs (tRNA intronic circular RNAs). In parallel, overexpression of wildtype CLP1, but not kinase-dead CLP1, robustly decreased tricRNA formation in HEK cells. On the basis of these findings, we propose that CLP1 is not a critical component of the core TSEN tRNA cleavage machinery but instead functions as a regulatory factor for metazoan tRNA processing.
MATERIALS AND METHODS
Recombinant purification of the TSEN Complexes from E. coli
Each individual subunit of the TSEN complex was cloned into a polycistronic vector (pST39) (44). TSEN34, TSEN54, and TSEN2 remained untagged while a C-terminal 6x HIS-tag was cloned onto TSEN15. The TSEN34 (Y247A, H255A, K286A) and TSEN2 (Y369A, H377A, K416A) catalytic mutant co-expression plasmid was generated by Genscript. Please refer to Supplementary Table S1 for a list of all expression plasmids used in this study. Protein expression for all constructs was conducted using Rosetta II pLac(I) cells (Millipore) grown to high density at 37°C, cooled to 30°C, and induced with 0.2 mM ITPG. Induced cells were incubated for 5–6 h at 30°C. Upon the completion of protein expression, cells were harvested by centrifugation and pellets were stored at −80°C until use.
Cells were re-suspended in Lysis Buffer (50 mM Tris pH 8.0, 10 % glycerol, 500 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100) with the addition of 1 mM phenylmethylsulphonyl fluoride (PMSF). Cells were lysed using sonication and cell lysates were cleared at 15,000 × g for 50 min. Clarified lysates were incubated with HIS-60 (Takara) resin for 30–60 min, washed with Lysis Buffer, and then eluted in Lysis Buffer plus 250 mM Imidazole. The eluate was injected into a Superdex 200 Increase column (GE) equilibrated with 50 mM Tris pH 8.0, 200 mM NaCl, 5% glycerol and 5 mM MgCl2. Protein was concentrated in a 30 kDa molecular weight cut-off concentrator and then immediately assayed for tRNA cleavage. SDS-PAGE gels were visualized using SimplyBlue SafeStain (Invitrogen).
Characterization of the TSEN complex using SEC-MALS
Freshly prepared TSEN complex, as described above, was further purified on a Suprose 6 Increase column (GE) equilibrated with 20 mM Tris pH 8.0, 200 mM NaCl, 2% glycerol, and 5 mM MgCl2. Protein was then immediately concentrated and analyzed by SEC-MALS as previously reported (45), except using an S200 Increase column (GE) equilibrated with 20 mM Tris pH 8.0, 200 mM NaCl, 2% glycerol, and 5 mM MgCl2. An average value and the standard deviation from three independent experiments was calculated.
Preparation of synthetic RNA substrates
Fluorescently labeled anticodon stem loop RNA was obtained from IDT following HPLC purification (/5Cy5/UUGGACUUCUAGUGACGAAUAGAGCAAUUCAA). Full-length tRNA genes were synthesized and cloned into pUC19 vectors by Genescript, genes were flanked by a 5′ T7 promoter and a 3′ EcoRV restriction site (sequences are available in Supplementary Table S2). tRNA containing plasmids were linearized using restriction digestion and tRNAs were in vitro transcribed overnight using the HiScribe T7 transcription kit (NEB) per the manufacturer's protocol. RNA was then either cleaned up using the RNAeasy kit (Qiagen) following DNase digestion (Qiagen) or run on TBE-Urea gels (Novex), visualized by UV backlighting on a TLC plate, and purified from the gel. Briefly, the gel was shred through a syringe, RNA was extracted in buffer overnight at 4°C, gel pieces were removed by filtration through a 0.2 μM filter, and the samples were buffer exchanged into 10 mM Tris pH 8.0 with 1.0 mM EDTA and concentrated. Both methods were suitable for purification of RNA.
tRNA intron splicing assay
tRNA cleavage reactions were carried out in RNA Cleavage Buffer (50 mM KCl, 50 mM Tris pH 7.5, 5 mM MgCl2, 1 mM DTT and 1 unit/ml RNAsin (Promega)) at room temperature for 30 min (unless otherwise noted). Reactions were quenched with 6 M urea-loading dye. Substrates were then stored at −20°C or boiled at 95°C for 5 min and immediately run on a gel. Samples were separated on 10% or 15% TBE–Urea gels (Novex). Gels were then either stained with 1× SYBR Gold (Invitrogen) in 1× TBE, or washed three times in water and stained with 10 μM (5Z)-5-[(3,5-difluoro-4-hydroxyphenyl)methylene]-3,5-dihydro-2-methyl-3-(2,2,2-trifluoroethyl)-4H-imidazol-4-one (DFHBI-1T)(Tocris) in 50 mM KCl, 50 mM Tris pH 7.5 and 5 mM MgCl2. Gels stained by either method were imaged on a Typhoon set to 488 nm.
tRNA-R1 northern blot protocol
Samples were separated on 15% TBE–Urea gels (Novex) and then transferred to Amersham Hybond-N+ (GE Healthcare) membrane (equilibrated in 0.5× TBE) using the Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were UV crosslinked with 120 mJ/cm2 twice and then pre-hybridized for 2 h using DIG Easy Hyb (Roche). Fluorescent end-labeled oligos to the exons of tRNA-R1 were obtained from IDT (see Supplementary Table S3). The oligo was melted in hybridization buffer at 95°C and immediately added to the pre-hybridization solution for overnight annealing at 37°C (5′ exon) or 25°C (3′ exon) at a final concentration of 1.67 nM. The membrane was then washed 2 × 10 min with SSPE Wash 1 (746 mM NaCl, 77 mM NaPO4, 6 mM EDTA, 0.1% SDS, pH 7.4) and for 20 min with SSPE Wash 2 (77 mM NaCl, 5 mM NaPO4, 0.6 mM EDTA, 0.1% SDS, pH 7.4).
Molecular cloning, expression and isolation of the TSEN complex from HEK cells
Plasmids for pCDNA3.1-GFP-CLP1, pCDNA3.1-TSEN2-FLAG, pCDNA3.1-GFP-TSEN34 and pCDNA3.1-TSEN15-FLAG were obtained from the Genescript EZ orf collection (Ohu26557, Ohu21033, Ohu20820 and Ohu31733, respectively). The DNA for TSEN54 was codon optimized and provided by Genescript. Codon optimized genes for TSEN2, TSEN34 and TSEN15 were also produced by Genescript, including complete active site mutants for TSEN2 and TSEN34. Further subcloning was carried out as follows: TSEN54, and TSEN34 were cloned into pLexM with C-terminal and N-terminal FLAG-tags, respectively. TSEN2 WT and catalytic dead variant (Y369A, H377A, K416A) were also cloned into pLexM with a C-terminal FLAG-tag. TSEN34 and the catalytic dead variant (Y247A, H255A, K286A) were cloned into pCAG-OSF (N-terminal STREP-FLAG tag). The CLP1 mutant was generated using the NEB Q5 mutagenesis kit and verified by sequencing (Genewiz). A list of all mammalian expression plasmids is available in Supplementary Table S1.
HEK293 cells were grown in 40 ml suspension cultures, at a density of approximately 2 × 106 cells/ml. Cells were transfected with the aforementioned FLAG or GFP expression plasmids using polyethylenimine (PEI) (1 μg/ml) and grown for approximately 72 hrs, whereupon they were harvested, and frozen at −80°C until use. Pellets were lysed in HEK IP Buffer (50 mM Tris pH 8.0, 10% glycerol, 100 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 0.05% NP-40) with 1 mM PMSF at 4°C on a nutator for 1 h. Lysates were cleared by high speed centrifugation at 4°C. Cleared lysates were then incubated with appropriate resin (Anti-GFP, provided by the NIEHS protein expression core (46)), Anti-FLAG (Pierce™ Anti-DYKDDDDK Magnetic Agarose) and Anti-STREP (MagStrep ‘type3’ XT beads (IBA)), for ∼60 min at 4°C. Resin was washed 3 times with 1 ml of HEK IP Buffer. If used for RNA cleavage reactions, samples were equilibrated into RNA Reaction Buffer immediately prior to performing assays.
Western blots
Western blots were performed using standard procedures. Protein samples were separated on 4–20% Mini-PROTEAN® TGX™ precast gels (Bio-Rad) at 300 V for 18–20 min and transferred to 0.2 μM PDVF (Bio-Rad) using the Trans-Blot Turbo Transfer System (Bio-Rad). Odyssey blocking buffer (TBS) and fluorescent secondary antibodies (all used at 1:5000) were purchased from Licor. Blots were developed using a 2-minute exposure on the Licor FC, unless otherwise noted. The following conditions were used for primary antibodies: FLAG (SIGMA-F7425): 1:3000, GFP (ROCHE-11814460001): 1:3000, β-actin (Abcam-ab8224): 1:20 000.
Drosophila cell culture, RNAi, and in-gel staining
S2 cells were maintained in SF-900 serum-free medium (Gibco) supplemented with 1% penicillin-streptomycin and filter sterilized. S2 RNAi was performed as previously described (47) for 10 days, with dsRNA targeting Gaussia luciferase used as a negative control. The Broccoli dual reporter (23) was transfected on day 7, and cells were harvested on day 10. The reporter (2.5 μg plasmid DNA per well) was transiently transfected using Cellfectin II transfection reagent (Invitrogen) according to the manufacturer's protocol. Primers used to make PCR products for in vitro transcription (to make dsRNA) can be found in Supplementary Table S4. RNA was isolated using TRIzol Reagent (Invitrogen), with a second chloroform extraction and ethanol rather than isopropanol precipitation (48). To test knockdown efficiency, total RNA was treated with TURBO DNase (Invitrogen) and then converted to cDNA using the SuperScript III kit (Invitrogen) with random hexamer priming. Primers for cbc and 5S rRNA can be found in Supplementary Table S5.
To visualize Broccoli, RNA samples (5 μg) were electrophoresed through 10% TBE–Urea gels (Invitrogen). Gels were washed three times with dH2O to remove urea and then incubated in DFHBI-1T staining solution (40 mM HEPES pH 7.4, 100 mM KCl, 1 mM MgCl2, 10 μM DFHBI-1T (Lucerna)). Following staining, gels were imaged on an Amersham Typhoon 5. To visualize total RNA, gels were washed three times in dH2O, stained with ethidium bromide, and imaged on an Amersham Imager 600.
Northern blotting of Drosophila RNA
RNA samples (5 μg) were electrophoresed through 10% TBE-Urea gels (Invitrogen). Following electrophoresis, RNA was transferred to a nylon membrane (PerkinElmer). The membrane was dried overnight and UV-crosslinked. Pre-hybridization was carried out in Rapid-hyb Buffer (GE Healthcare) at 42°C. Probes were generated by end-labeling oligonucleotides (IDT) with γ-32P ATP (PerkinElmer) using T4 PNK (NEB), and then probes were purified using Illustra Microspin G50 columns (GE Healthcare). Upon purification, probes were boiled, cooled on ice, and added to the Rapid-hyb buffer for hybridization. After hybridization, the membrane was washed in saline-sodium citrate (SSC) buffer. For probe sequences, see Supplementary Table S3. Washing conditions are as follows. U1: hybridization at 65°C, washes (twice in 2× SSC, twice in 0.33× SSC) at 60°C. Dual reporter probe and tric31905 probes: hybridization at 42°C, two washes in 5× SSC at 25°C, two washes in 1× SSC at 42°C. For the dual reporter probe, two additional washes in 0.1× SSC at 45°C were performed. After washes, the membrane was exposed to a storage phosphor screen (GE Healthcare) and imaged on an Amersham Typhoon 5.
pTric co-expression experiments in HEK cells
Plasmids and methods for HEK cell co-expression experiments were performed as detailed above. The human pTric:Broccoli reporter has been previously reported (48). 1 ml of a large scale expression was used for RNA extractions, which were performed as described for S2 cells.
Statistical analyses of tRNAs
Quantifications of bands was performed using ImageJ software. For activity assays, the total cleaved tRNA was divided by the total tRNA (cleaved and uncleaved) in the lane. For the cell based assays, each band was normalized to the respective loading control band. Significance was calculated for three or more replicates using a student t-test of unpaired data with unequal variances.
RESULTS
Reconstitution of the human TSEN complex
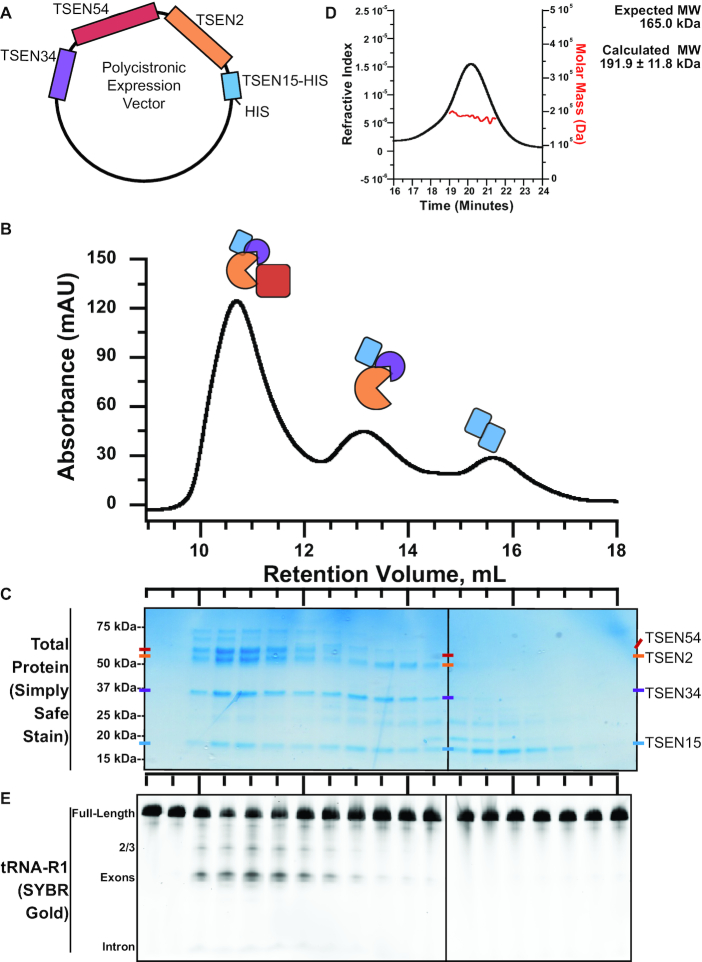
We developed a reconstitution protocol for purification of the human TSEN complex. The active eukaryotic TSEN complex has been successfully isolated from yeast, Xenopus, and mammalian cells (14,15,49). However, purification of the TSEN complex from native sources leads to heterogenous mixtures of the core components and variable amounts of known associating factors, such as CLP1 and components of the Pol II 3′ cleavage and polyadenylation machinery (15). In order to define the components necessary for tRNA intron cleavage, we developed a reconstitution system for the core TSEN machinery (TSEN2, TSEN15, TSEN34, and TSEN54). Our first approach was to purify the individual TSEN components by expressing them in Escherichia coli; however only TSEN15 was found to be soluble on its own. We hypothesized that the other TSEN components may depend upon one another for stability. Therefore, we engineered a polycistronic vector for the simultaneous expression of all four TSEN proteins (Figure 2A). We fused a hexa-histidine tag onto the C-terminus of TSEN15 so that the entire complex could be isolated with a nickel affinity column. The eluate from the affinity column was then run over a size exclusion column.
Figure 2.
The heterotetrameric human TSEN complex retains nuclease activity in the absence of CLP1. (A) Expression of the human TSEN complex in E. coli was possible through use of a polycistronic vector in which each TSEN protein is under independent ribosome binding sites. (B) Separation of the TSEN complex via gel filtration produces three distinct TSEN peaks. (C) A protein gel reveals the peaks contain the heterotetramer, a trimer (TSEN2, TSEN34 and TSEN15), and pool of soluble TSEN15. (D) Cleavage activity assays were performed on fractions from gel filtration separation of the TSEN complex. Cleavage reactions were separated on denaturing TBE-Urea gels, which were stained with SYBR Gold to visualize total RNA. (E) SEC-MAL analysis of the peak centered around 10.5 mL reveals the TSEN complex is a single heterotetramer with an average mass of 191.9 kDa.
We observed three prominent peaks from our size-exclusion profile located at retention volumes of 10.5, 13.5 and 15.5 ml (Figure 2B). We analyzed these peaks by SDS-PAGE to determine their protein composition (Figure 2C). The first peak at 10.5 ml contains all four subunits (TSEN2, TSEN15, TSEN34 and TSEN54). The identities of TSEN2, TSEN15, TSEN34 and TSEN54 were confirmed by mass spectrometry and the SDS-PAGE analysis is suggestive of an equimolar ratio of each subunit. To confirm the oligomeric state of the core complex, we analyzed the 10.5 ml peak fraction by size-exclusion chromatography, paired with in-line multi-angle light scattering (SEC-MALs) (Figure 2D). We measured an average molecular weight of 191.9 ± 11.8 kDa from three independent purifications, all with a polydispersity value of 1.0. These findings are consistent with the theoretical molecular weight of the TSEN heterotetramer. The second peak at 13.5 ml contains TSEN2, TSEN15 and TSEN34, suggesting that these components can form a stable sub-complex in the absence of TSEN54. The final peak at 15.5 ml contains TSEN15 alone. Previous work with the isolated TSEN15 subunit revealed that it forms a homodimer on its own (50), thus we infer that this peak represents a TSEN15 homodimer.
Next, we confirmed that the recombinant TSEN complex retained cleavage activity. To verify tRNA splicing endonuclease activity we transcribed the human tRNA-R1 (tRNAARG-TCT) in vitro, which includes a 15-nucleotide intron (Supplementary Figure S1). Expression of this gene was recently validated in human cells using a new tRNAseq methodology (21). The pre-tRNA was incubated with individual fractions from the SEC column and the cleavage reactions were analyzed on denaturing TBE-Urea gels. Gels were stained with SYBR Gold and visualized with a fluorescent imager. Prominent tRNA cleavage products were visible with the fractions corresponding to the full heterotetrametric TSEN complex but not for any of the other fractions (Figure 2E). This result confirms that our E. coli co-expression system produces the biochemically active TSEN complex and that, similar to studies of the S. cerevisiae TSEN complex (14), the human heterotetrametric TSEN core is sufficient for intron cleavage.
The human TSEN core is active on pre-tRNA substrates
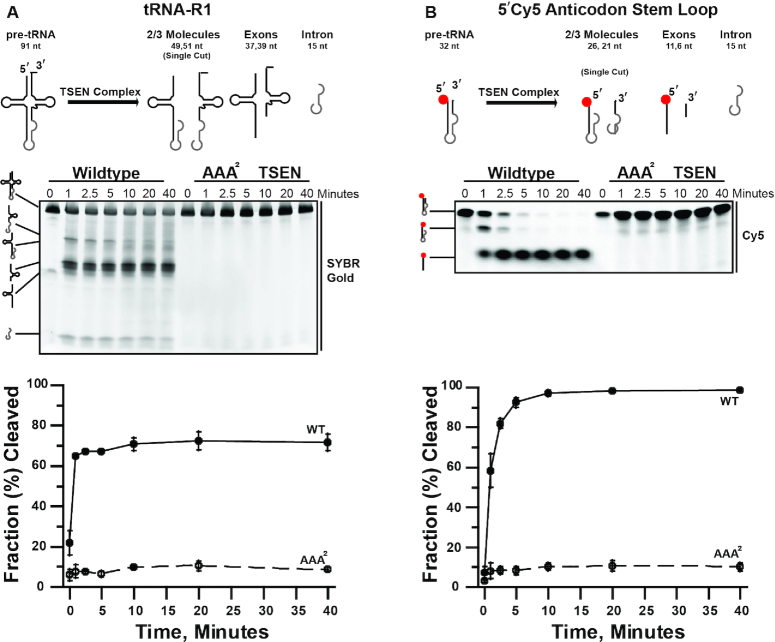
We characterized the endonuclease cleavage activity of the recombinant TSEN complex and found that it actively cleaves multiple intron-containing tRNA substrates. First, we carried out a time course with full-length in vitro transcribed pre-tRNA-R1 (Figure 3A) and the recombinant TSEN complex. Cleavage products were analyzed by denaturing gel and visualized with SYBR Gold (Figure 3A). We confirmed the identity of the cleavage products with northern blot probes to the 5′ and 3′ ends of tRNA-R1 (Supplementary Figure S2). To ensure that the cleavage products were generated by the TSEN complex and not a contaminant from our in vitro system, we created a catalytic deficient AAA2-TSEN variant. Sequence alignments across TSEN endonucleases suggests that the endoribonuclease active sites are well conserved (Supplementary Figure S3A) (16,51,52). Each endoribonuclease active site is composed of three invariant residues including a histidine, lysine, and tyrosine. A structure of the homodimeric tRNA splicing endonuclease machinery from Archaeoglobus fulgidus bound to RNA revealed that these invariant residues cluster around the cleavage site and suggests that they support catalysis through a general acid-base mechanism (53). To inactivate the human TSEN endonuclease subunits, TSEN34 (3′ site) and TSEN2 (5′ site), we made triple alanine substitutions of the catalytic triad from each active site; TSEN34 (Y247A, H255A, K286A) and TSEN2 (Y369A, H377A, K416A). The size exclusion profile for AAA2-TSEN complex overlays with that of the wildtype TSEN complex suggesting that the active site mutants do not interfere with assembly of the TSEN complex (Supplementary Figure S3B and C). We performed the tRNA-R1 time course with AAA2-TSEN and did not observe cleavage products by either SYBR Gold staining (Figure 3A) or northern blotting (Supplementary Figure S2B). Collectively, these results confirm that the specific cleavage products observed in our assays are from the active TSEN complex.
Figure 3.
Cleavage of tRNA substrates by the human TSEN complex. The heterotetrameric TSEN complex is efficient at cleaving (A) tRNA-R1 full-length (1.7 μM) and (B) anticodon stem loop (0.5 μM) tRNA substrates. Representative time courses (0, 1, 2.5, 5, 10, 20, 40 min) are shown for the cleavage of each tRNA substrates by 1.5 μM TSEN complex (both wildtype and endonuclease dead (AAA2) complexes). RNA cleavage products were separated on TBE–Urea denaturing gels and visualized using a typhoon. The average of three replicates is graphed, standard deviation is shown for each data point.
After confirming activity on a full length pre-tRNA, we found that the recombinant TSEN complex is also able to cleave a truncated pre-tRNA substrate. Due to a lack of structural information for the eukaryotic TSEN complex, our understanding of substrate recognition by the complex is limited. Previous work suggests that the eukaryotic TSEN complex has broad specificity. For example, the Xenopus TSEN complex can cleave a bulge-helix-bulge (BHB) substrate, the universal substrate for archaeal TSENs (49), whereas recent work with the S. cerevisiae TSEN complex identified the stem loop of CBP1 mRNA as a new TSEN substrate (54). To begin to address substrate recognition, we designed a synthetic mini intron construct with a 5′ fluorescent label (Figure 3B), that includes only the anticodon stem loop (ASL) of tRNA-R1 (Supplementary Figure S1). Using this substrate, we can detect the uncleaved product, the 5′ exon 2/3 tRNA molecules, and the 5′ exon (Figure 3B). We carried out a time course of the cleavage reaction with the ASL substrate and observed robust and efficient cleavage with the WT-TSEN complex, but not with the catalytic site mutant (Figure 3B). Therefore, the human TSEN complex does not require the full tRNA cloverleaf structure to efficiently process the anticodon stem in vitro.
Finally, we also characterized the activity of the recombinant TSEN complex using an alternative pre-tRNA substrate. Due to its small size, the excised wildtype tRNA intron is difficult to detect by conventional methods. Therefore, we designed a synthetic tRNA-I21 (tRNAILE-TAT) variant bearing a Broccoli-tagged intron (tRNA-I21-Broccoli) (55,56) (Supplementary Figure S4). The Broccoli aptamer binds to a fluorescent dye called DFHBI-1T, such that only intron(Broccoli)-containing RNAs are visualized (55). Thus, in DFHBI-1T stained gels, only pre-tRNA, intron-containing 2/3 tRNA molecules, and introns are detected (Supplementary Figure S4). We carried out a time course of the cleavage reaction with tRNA-I21-broccoli with the recombinant TSEN complex. Cleavage products were resolved by denaturing gel electrophoresis, refolded in the gel, stained with DFHBI-1T, and visualized with a fluorescent scanner. Using this approach, the intron cleavage product was clearly detectable with the wildtype TSEN complex (Supplementary Figure S4). Collectively, these results establish that our recombinant TSEN complex is active on a variety of intron containing pre-tRNA substrates in vitro.
CLP1 is not required for tRNA cleavage
To address questions regarding the role played by the polynucleotide kinase, CLP1, in the TSEN complex, we first attempted to form the CLP1•TSEN complex using a recombinant E. coli system. However, we could not generate enough of the CLP1•TSEN complex for biochemical experiments. This result suggests that there may be additional factors or post-translational modifications that mediate CLP1•TSEN association. To purify the CLP1•TSEN complex, we developed a mammalian expression system to isolate the TSEN complex bound to CLP1. To first establish the recombinant mammalian system, we individually and co-expressed each TSEN protein harboring a FLAG tag and then carried out immunoprecipitations using anti-FLAG resin (Figure 4A). Activity assays on our resin-bound isolates revealed detectable tRNA cleavage only following co-expression of all four TSEN subunits (Figure 4B). Correspondingly, we were unable to detect the presence of stoichiometric amounts of the individual endogenous TSEN proteins by SDS-PAGE analysis of isolates stained with SYPRO Orange (Figure 4C). The lack of nucleolytic activity as well as the absence of the full tetrameric TSEN complex within these immunoprecipitates suggests that, as in our recombinant E. coli system, co-expression of all four subunits is required to stably isolate the full TSEN complex cultured in human cells.
Figure 4.
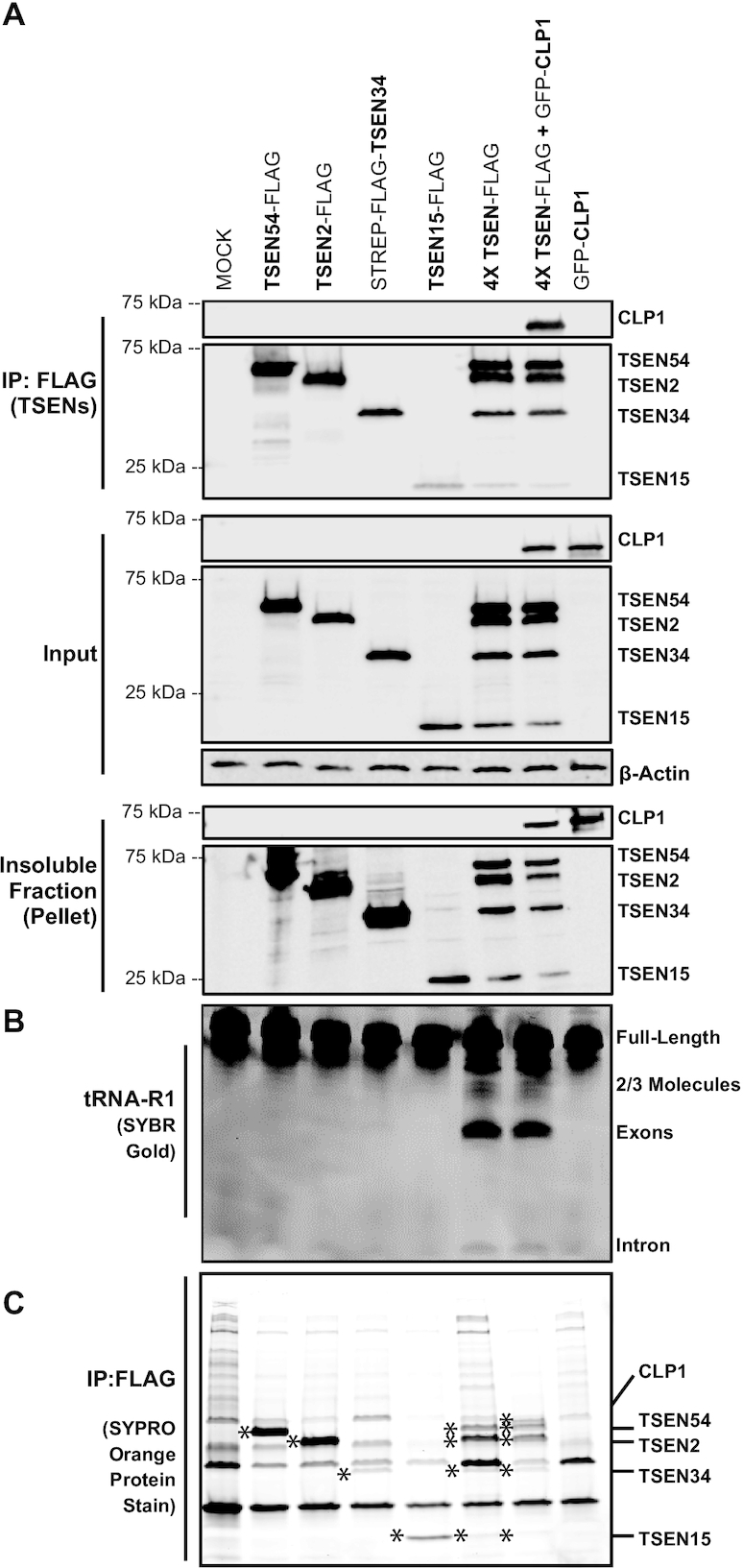
Co-expression and immunoprecipitation of the TSEN complex from HEK cells yields an active complex. (A) FLAG-tagged TSEN constructs were transiently co-expressed and immunoprecipitated, using Anti-FLAG resin, from HEK 293 cells. Isolated samples (IP), soluble cell lysates (input), and the insoluble fraction (pellet) were analyzed by Western blot. TSEN subunits were detected with an anti-FLAG antibody, CLP1 was detected with an anti-GFP antibody, and β-actin was used as a loading control. (B) Denaturing TBE-Urea gels were used to analyze immunoprecipitated samples assayed for endonuclease activity on pre-tRNA-R1, the gel was stained with SYBR Gold. (C) Finally, IP samples were separated on an SDS-PAGE gel and stained to visualize proteins using SYPRO Orange protein stain. TSEN proteins and CLP1 are denoted by ‘*’ in respective lanes.
To ensure that the observed cleavage was specifically from our overexpressed complexes and not endogenous TSEN subunits, we assayed tRNA cleavage using the recombinant TSEN complex with either wildtype or triple alanine endonuclease-dead variants of TSEN34 (Y247A, H255A, K286A) and TSEN2 (Y369A, H377A, K416A). Cells were transfected with plasmids harboring FLAG-tagged TSEN2, TSEN15, and TSEN54 along with Strep-FLAG tagged TSEN34. The active tetrameric TSEN complex was successfully isolated by co-immunoprecipitation with Strep-FLAG-TSEN34 and we observed no non-specific binding of the other subunits to the anti-Strep resin (Supplementary Figure S5A). Moreover, mutation of the active sites within TSEN34 and TSEN2 did not impair TSEN protein expression or TSEN complex formation (Supplementary Figure S5A, top two panels). We assayed the Strep-bound isolates with pre-tRNA-R1 and detected cleavage products by denaturing gel. We observed accumulation of the 3′ exon 2/3 tRNA molecule with the TSEN34-AAA mutant, whereas accumulation of the 5′ exon-2/3 tRNA molecule was observed with the TSEN2-AAA mutant (Supplementary Figure S5B). Co-expression of both active site mutants led to a loss of observable cleavage products (Supplementary Figure S5B).
After establishing the HEK293 expression system, we purified the TSEN•CLP1 complex by co-transfecting cells with plasmids harboring all four TSEN subunits and CLP1. Using this approach, we could isolate the TSEN•CLP1 complex by co-immunoprecipitation with FLAG-tagged TSEN subunits (Figure 4A) or Strep-tagged TSEN34 (Supplementary Figure S5). We saw no observable improvement in tRNA cleavage when GFP-CLP1 was co-expressed with the TSEN complex (Figure 4A, lane 6 vs 7; Supplementary Figure S5, lanes 1–6 versus lanes 7–12). We also confirmed the kinase activity of GFP-CLP1 isolates (Supplementary Figure S6). These results indicate that CLP1 does not play a central role in tRNA intron cleavage within the mammalian TSEN complex in vitro.
CLP1 and its Drosophila orthologue, cbc, negatively regulate tRNA exon ligation and intron circularization in vivo
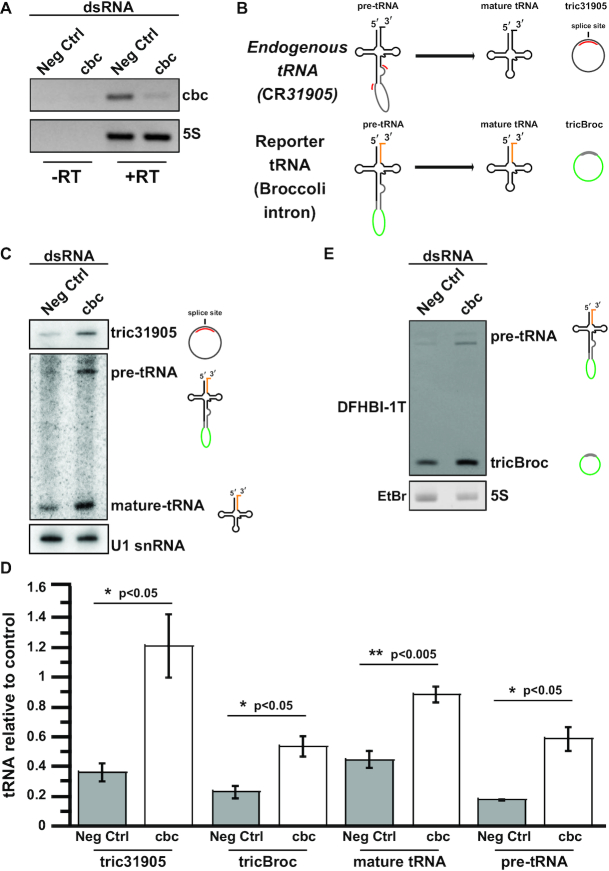
Given our finding that CLP1 is not required for TSEN-mediated tRNA intron removal in vitro, we sought to determine whether CLP1 might play a downstream role in tRNA processing in vivo. Previous work has shown that the CLP1•TSEN complex can cleave pre-tRNAs and phosphorylate both the 3′ tRNA exon and the intron (30). Phosphorylation of the exon prevents RTCB mediated ligation of the two exon halves, whereas phosphorylation of the intron prevents intron circularization (19). We therefore harnessed an in vivo expression system in Drosophila cells to determine if CLP1 activity is important for tRNA intron removal and maturation in metazoan cells.
Using a tRNA:Tyr ‘dual reporter’ construct that contains a Broccoli aptamer in its intron and four base changes in the 3′ exon (for specific recognition by northern blot) we assayed pre-tRNA transcripts for both intron circularization and tRNA maturation (23). We carried out RNA interference, targeting the Drosophila orthologue of CLP1 (called crowded-by-cid, or cbc) in S2 cells, and verified cbc knockdown by RT-PCR (Figure 5A). We then measured the efficiency of tRNA splicing by examining formation of circularized introns (tricRNAs), which are a known in vivo product of the metazoan tRNA splicing pathway (23,57) (Figure 5B). We observed a significant increase in levels of tricRNAs from both the endogenous tRNA CR31905 (Figure 5C, D, P < 0.05) as well in the tricBroc reporter (Figure 5D, E, P < 0.05). These data suggest that CLP1/cbc plays an inhibitory role in tricRNA biogenesis in vivo.
Figure 5.
Depletion of the Drosophila melanogaster CLP1 orthologue, cbc, in S2 cells does not prevent the generation of tricRNAs or tRNA maturation. S2 cells were treated with dsRNA to knock down cbc in vivo. Cells were then transfected with a D. melanogaster tRNA-Tyr reporter containing Broccoli in the intron (23). Total RNA was isolated from the cells. (A) RT-PCR was used to measure successful cbc knockdown by dsRNAs, with 5S rRNA and unamplified RNA as controls. (B) A cartoon depicting the final products formed from the in vivo cleavage of endogenous Drosophila tRNA CR31905 and the transfected reporter tRNA-Broccoli-intron tRNA. Only tric31905 is visualized by the tric31905 probe whereas introns, intron containing tRNAs, and the 3′ exon are detected for the dual reporter. (C) A northern blot probing tric31905 was performed to measure levels of endogenous circular intron production. We also carried out a northern blot to measure levels of pre-tRNA and mature-tRNA from the same sample. Levels of the snRNA U1 in bottom panel is the loading control for both northern blots. (D) Levels of tric31905 (n = 3), tricBroc (n = 4), mature tRNA (n = 4), and pre-tRNA (n = 4) levels were quantified and normalized to the U1 or 5S controls on their respective gels/blots. The average and standard error for biological replicates is shown. A student's t-test was used to calculate significance: *P < 0.05 and **P < 0.005. (E) RNA was run on a TBE-Urea gel and visualized using DFHBI-1T. The gel was then re-stained with ethidium bromide, and the 5S rRNA is shown as a loading control.
Previously, we showed that RtcB is the ligase responsible for tRNA intron circularization in Drosophila (23,57). Given the wealth of data demonstrating that RtcB-type ligases are inhibited by the presence of a 5′ phosphate (58–61), we hypothesized that CLP1/cbc would also inhibit formation of mature tRNAs. Consistent with this notion, northern blotting for the reporter tRNA revealed a similar highly significant increase in levels of the mature tRNA:Tyr construct following depletion of CLP1/cbc (Figure 5C,D, P < 0.005). We also observed a significant increase in pre-tRNA levels following cbc knockdown (Figure 5C, D, P < 0.05), perhaps indicating a role for CLP1/cbc in stages of tRNA gene expression that are upstream of TSEN (see Discussion).
Furthermore, we investigated the effect that CLP1 has on tricRNA formation in human cells. Using a pTric:Broccoli reporter construct described previously (48), we measured levels of the circularized intron (tricBroc) by DFHBI-1T fluorography. This reporter plasmid was transfected into HEK293 cells along with constructs harboring all four TSEN subunits in the presence or absence of an additional plasmid expressing CLP1. Overexpression of the TSEN complex together with the reporter leads to an accumulation of tricBroc over basal pTric levels (Figure 6A, lanes 4, 5), As expected, addition of GFP along with pTric and TSEN had little effect (Figure 6A, lane 5). Importantly, expression of CLP1 along with pTric and TSEN caused a significant decrease in tricBroc levels (Figure 6A, lane 6, Figure 6B) as compared to co-expression with a kinase-deficient version of CLP1 (Figure 6A, lane 7, Figure 6B, P < 0.05). Thus, CLP1 is a potent repressor of metazoan tRNA intron processing, as measured by tricRNA production. Collectively, our results provide strong evidence that the CLP1 kinase is neither required for tRNA intron cleavage, nor for tRNA exon ligation. We therefore propose that CLP1 and its orthologues are critical negative regulators of tRNA processing in animal cells (Figure 7).
Figure 6.
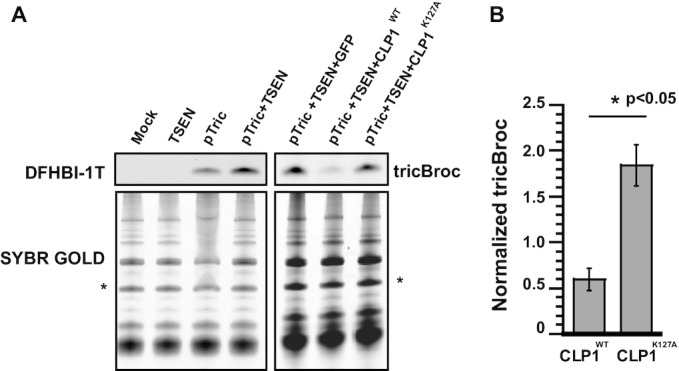
Co-expression of catalytically active CLP1 with a tricRNA reporter in HEK cells reduces tricRNA formation. HEK 293 cells were transfected with a human tRNA-Tyr reporter containing Broccoli in the intron. Co-expression was conducted using the TSEN proteins and wildtype or kinase dead CLP1. Total RNA was isolated from HEK cells. (A) RNA was run on TBE-Urea gels and visualized for Broccoli containing RNAs using DFHBI-1T. Gels were washed and re-staned with SYBR Gold to visualize total RNA, which is shown as a loading control. (B) For the co-expression experiments involving wildtype and kinase dead CLP1 with the tricRNA reporter and the TSEN proteins, the respective tricBroc band was normalized to a band from the SYBR Gold (see ‘*’ in A) and quantified. The average and standard error for two biological replicates and a technical replicate are shown, a student's t-test was performed. (*P < 0.05).
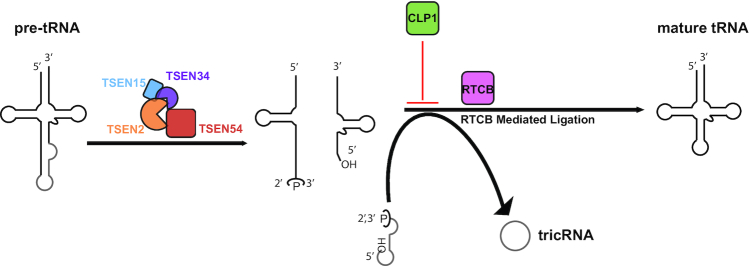
Figure 7.
CLP1 is a negative regulator of tRNA splicing. An updated model of tRNA splicing in which the TSEN complex can solely cleave the introns from pre-tRNAs and CLP1 plays a primary role in regulating the RTCB mediated ligation step of splicing.
DISCUSSION
The TSEN complex is required for catalysis of tRNA splicing in Archaea and Eukarya. In this manuscript, we describe the production of a recombinant human heterotetrameric TSEN complex. Our work shows that the human complex, as with other eukaryotic TSEN complexes, has broad substrate specificity. Extensive previous studies have shown that eukaryotic TSEN recognizes distinct structural motifs that orient the endonucleases for cleavage (17). Among these motifs, we previously reported the importance of the proximal base-pair for in vivo tRNA processing in Drosophila and human cells (23). In vitro cleavage of full-length tRNA substrates, as well as a fluorescently labeled ‘mini’ tRNA substrate, parallel those of Fabbri et al. (1998), who showed that the eukaryotic complex can recognize a BHB-like substrate that is missing the mature tRNA domain (49).
Beyond the subject of substrate specificity, our data provide a rationale for formation of the CLP1•TSEN complex in animal cells. Prior to this work, several putative roles for CLP1 in tRNA splicing have been proposed, including: supporting the integrity of the TSEN complex, the existence of an alternative yeast-like tRNA ligation pathway, turnover of the excised intron, or processing of non-tRNA substrates (27,28,30,35,40). Our reconstitution system demonstrates that the heterotetrametric TSEN core is functional in the absence of CLP1. Moreover, purification of the TSEN complex from transfected human cells in the presence or absence of CLP1 does not cause observable changes in tRNA splicing. These findings suggest that the core tRNA processing machinery is conserved from yeast to humans (14).
Our results are in contrast with previous studies that showed reduced tRNA cleavage in TSEN•CLP1 immunoprecipitated complexes of CLP1 wildtype compared with CLP1 variants, K127A and R140H. In those studies, complexes were immunoprecipitated via epitope-tagged CLP1. CLP1 variants display decreased association with the TSEN subunits (32,35,40), and hence in the previous studies there was overall less TSEN complex present in the immunoprecipitates. Direct immunoprecipitation of TSEN subunits (in the absence or presence of CLP1) more directly addresses the question of whether CLP1 plays an essential role in TSEN catalysis. Although our data show that CLP1 is not required for TSEN mediated cleavage in vitro, CLP1 clearly plays an important role in tRNA processing in vivo. We cannot exclude the possibility that CLP1 might be involved in certain aspects of tRNA transcription or TSEN substrate recognition. Such a role would be consistent with our finding that CLP1/cbc knockdown led to a buildup of the reporter pre-tRNA (Figure 5D). Beyond subtstrate recognition, there are likely additional factors or post-translational modifications that regulate TSEN. So, while CLP1 is clearly not required for the catalytic function of TSEN, future studies should elucidate the mechanism through which CLP1 and its binding partners regulate activity of the TSEN complex in vivo.
Unlike the unity we find regarding the eukaryotic tRNA endonuclease machinery, ligation of tRNA exons in yeast and humans is thought to be carried out via two distinct pathways. In the budding yeast S. cerevisiae, exons are ‘healed and sealed’ by the multicomponent enzyme, Trl1, which exhibits cyclic phosphodiesterase, polynucleotide kinase, and RNA ligase activities (11). Trl1 opens the 5′-exon's cyclic-phosphate termini, phosphorylates the 3′-exon's 5′-hydroxyl termini, and ligates the ends back together. Both CLP1 and the human phosphodiesterase CNPase were previously shown to be capable of rescuing Trl1 mutants in their respective domains (28,62), but no human tRNA ligase capable of joining the 3′-exon's 5′-phosphate to the 3′-hydroxyl has been identified. In contrast, the ‘direct ligation’ pathway in metazoan cells relies on RTCB to ligate the tRNA halves—joining a 5′ hydroxyl directly to the 2′,3′-cyclic-phosphate (31). CLP1-mediated phosphorylation of the 5′-hydroxyl of the 3′-exon is known to block RTCB-mediated ligation of the exon halves into mature tRNAs in vitro (19). Thus, CLP1 could either play a role in an alternative ‘yeast-like’ ligation pathway, or, as our data suggest, it could act as a negative regulator of RTCB-mediated ligation, which is believed to be the primary tRNA processing pathway in animal cells (63).
RTCB not only is responsible for tRNA ligation, but it is also responsible for biogenesis of tricRNAs in metazoa and archaea (23,57,64,65). Both metazoans and archaeons have been shown to produce tricRNAs, whereas yeast introns are primarily linear and rapidly degraded (66,67). Because circularized introns are very stable (57), CLP1 could also play a role in circular RNA homeostasis by inhibiting RTCB-mediated ligation and acting as negative regulator of tricRNA formation. Along these lines, 5′-phosphorylation of a tRNA intron has been shown to block circularization of this RNA in vitro (19). We found that knockdown of the Drosophila melanogaster CLP1 homologue, cbc, increased production of endogenous circularized tRNA introns (tricRNAs). Therefore, we propose that CLP1 acts as a critical negative regulator of tRNA processing. This model is further supported by RNA-sequencing and northern blotting analysis of PCH patient-derived fibroblasts, which showed a substantial accumulation of intronic sequences from patients harboring mutations in CLP1 (35,40). Beyond pre-tRNA splicing, CLP1 may also function as a negative regulator of other RNA processing pathways that depend on RtcB ligation, such as in the unfolded protein response (68–71).
In summary, we establish that the human TSEN complex assembles into a heterotetramer that is necessary and sufficient to support cleavage of tRNA introns in vitro. Moreover, we provide strong in vivo evidence supporting the model that CLP1 blocks RTCB-mediated ligation, thus serving as a negative regulator of both tricRNA biogenesis and tRNA maturation (Figure 7). Additional studies will be needed to firmly establish the precise mechanisms whereby CLP1 and its other binding partners participate in metazoan tRNA processing. Given that mutations in CLP1 and the genes encoding all four subunits of the TSEN complex are associated with the PCH family of severe neurological disorders, it stands to reason that CLP1 complexes could function in the spatiotemporal or developmental regulation of TSEN. We note that CLP1 and the TSEN complex are associated with distinct PCH subtypes (38). This finding suggests that the etiology of PCH might not be strictly limited to tRNA processing, but also linked to the biogenesis of other, yet to be characterized, RNAs. The TSEN reconstitution systems described here should prove useful in future studies aimed at characterizing additional RNA targets of the TSEN complex.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs Andrew Sikkema and Marcos Morgan as well as members of the Stanley Lab for their critical reading of this manuscript. We would like to acknowledge Robert Petrovich and the NIEHS Protein Expression Facility for assistance with protein expression in HEK293 suspension cultures and for supplying the anti-GFP resin. We thank the NIEHS Mass Spectrometry Research and Support Group for protein identification. We also thank Robert Dutcher for his assistance with running SEC-MALS.
Contributor Information
Cassandra K Hayne, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, 111 T. W. Alexander Drive, Research Triangle Park, NC 27709, USA.
Casey A Schmidt, Curriculum in Genetics & Molecular Biology and Integrative Program for Biological and Genome Sciences, University of North Carolina, Chapel Hill, NC 27599, USA.
Maira I Haque, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, 111 T. W. Alexander Drive, Research Triangle Park, NC 27709, USA; Department of Biology, North Carolina State University, Raleigh, NC 27695, USA.
A Gregory Matera, Curriculum in Genetics & Molecular Biology and Integrative Program for Biological and Genome Sciences, University of North Carolina, Chapel Hill, NC 27599, USA; Departments of Biology and Genetics, University of North Carolina, Chapel Hill, NC 27599, USA; Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599, USA.
Robin E Stanley, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, 111 T. W. Alexander Drive, Research Triangle Park, NC 27709, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
US National Institute of Health Intramural Research Program; US National Institute of Environmental Health Sciences (NIEHS) [ZIA ES103247 to R.E.S.]; US National Institute of Health Extramural Research Program; US National Institute of General Medical Sciences [R01-GM118636 and R35-136435, to A.G.M.]; Additional support was provided by the National Science Foundation Graduate Research Fellowship Program [DGE-1650116 to C.A.S.]; Dissertation Completion Fellowship from the University of North Carolina Graduate School (to C.A.S.); M.I.H. was supported by the 2019–2020 NIEHS Scholars Connect Program (NSCP). Funding for open access charge: NIEHS.
Conflict of interest statement. None declared.
REFERENCES
- 1. Hirata A. Recent insights into the structure, function, and evolution of the RNA-splicing endonucleases. Front. Genet. 2019; 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopper A.K., Nostramo R.T.. tRNA processing and subcellular trafficking proteins multitask in pathways for other RNAs. Front. Genet. 2019; 10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dixit S., Henderson J.C., Alfonzo J.D.. Multi-substrate specificity and the evolutionary basis for interdependence in tRNA editing and methylation enzymes. Front. Genet. 2019; 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatterjee K., Nostramo R.T., Wan Y., Hopper A.K.. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: location, location, location. Biochim. Biophys. Acta. 2017; 1867:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swinehart W.E., Jackman J.E.. Diversity in mechanism and function of tRNA methyltransferases. RNA Biol. 2015; 12:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackman J.E., Alfonzo J.D.. Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley Interdiscipl. Rev. RNA. 2013; 4:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujishima K., Kanai A.. tRNA gene diversity in the three domains of life. Front. Genet. 2014; 5:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abelson J., Trotta C.R., Li H.. tRNA splicing. J. Biol. Chem. 1998; 273:12685–12688. [DOI] [PubMed] [Google Scholar]
- 9. Chan P.P., Lowe T.M.. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009; 37:D37–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshihisa T. Handling tRNA introns, archaeal way and eukaryotic way. Front. Genet. 2014; 5:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopes R.R., Kessler A.C., Polycarpo C., Alfonzo J.D.. Cutting, dicing, healing and sealing: the molecular surgery of tRNA. Wiley Interdiscipl. Rev. RNA. 2015; 6:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calvin K., Li H.. RNA-splicing endonuclease structure and function. Cell. Mol. Life Sci.: CMLS. 2008; 65:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujishima K., Sugahara J., Miller C.S., Baker B.J., Di Giulio M., Takesue K., Sato A., Tomita M., Banfield J.F., Kanai A.. A novel three-unit tRNA splicing endonuclease found in ultrasmall Archaea possesses broad substrate specificity. Nucleic Acids Res. 2011; 39:9695–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trotta C.R., Miao F., Arn E.A., Stevens S.W., Ho C.K., Rauhut R., Abelson J.N.. The yeast tRNA splicing endonuclease: A tetrameric enzyme with two active site subunits homologous to the Archaeal tRNA endonucleases. Cell. 1997; 89:849–858. [DOI] [PubMed] [Google Scholar]
- 15. Paushkin S.V., Patel M., Furia B.S., Peltz S.W., Trotta C.R.. Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3′ end formation. Cell. 2004; 117:311–321. [DOI] [PubMed] [Google Scholar]
- 16. Trotta C.R., Paushkin S.V., Patel M., Li H., Peltz S.W.. Cleavage of pre-tRNAs by the splicing endonuclease requires a composite active site. Nature. 2006; 441:375–377. [DOI] [PubMed] [Google Scholar]
- 17. Reyes V.M., Abelson J.. Substrate recognition and splice site determination in yeast tRNA splicing. Cell. 1988; 55:719–730. [DOI] [PubMed] [Google Scholar]
- 18. Sawaya R., Schwer B., Shuman S.. Genetic and biochemical analysis of the functional domains of yeast tRNA ligase. J. Biol. Chem. 2003; 278:43928–43938. [DOI] [PubMed] [Google Scholar]
- 19. Popow J., Englert M., Weitzer S., Schleiffer A., Mierzwa B., Mechtler K., Trowitzsch S., Will C.L., Luhrmann R., Soll D. et al.. HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science (New York, N.Y.). 2011; 331:760–764. [DOI] [PubMed] [Google Scholar]
- 20. Cherry P.D., White L.K.. Genetic bypass of essential RNA repair enzymes in budding yeast. RNA. 2018; 24:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gogakos T., Brown M., Garzia A., Meyer C., Hafner M., Tuschl T.. Characterizing expression and processing of precursor and mature human tRNAs by hydro-tRNAseq and PAR-CLIP. Cell Rep. 2017; 20:1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baldi M.I., Mattoccia E., Bufardeci E., Fabbri S., Tocchini-Valentini G.P.. Participation of the intron in the reaction catalyzed by the Xenopus tRNA splicing endonuclease. Science (New York, N.Y.). 1992; 255:1404–1408. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt C.A., Giusto J.D., Bao A., Hopper A.K., Matera A.G.. Molecular determinants of metazoan tricRNA biogenesis. Nucleic Acids Res. 2019; 47:6452–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kasher P.R., Namavar Y., van Tijn P., Fluiter K., Sizarov A., Kamermans M., Grierson A.J., Zivkovic D., Baas F.. Impairment of the tRNA-splicing endonuclease subunit 54 (tsen54) gene causes neurological abnormalities and larval death in zebrafish models of pontocerebellar hypoplasia. Hum. Mol. Genet. 2011; 20:1574–1584. [DOI] [PubMed] [Google Scholar]
- 25. Rauhut R., Green P.R., Abelson J.. Yeast tRNA-splicing endonuclease is a heterotrimeric enzyme. J. Biol. Chem. 1990; 265:18180–18184. [PubMed] [Google Scholar]
- 26. Haddad R., Maurice F., Viphakone N., Voisinet-Hakil F., Fribourg S., Minvielle-Sébastia L.. An essential role for Clp1 in assembly of polyadenylation complex CF IA and Pol II transcription termination. Nucleic Acids Res. 2012; 40:1226–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weitzer S., Hanada T., Penninger J.M., Martinez J.. CLP1 as a novel player in linking tRNA splicing to neurodegenerative disorders. Wiley Interdiscipl. Rev. RNA. 2015; 6:47–63. [DOI] [PubMed] [Google Scholar]
- 28. Ramirez A., Shuman S., Schwer B.. Human RNA 5′-kinase (hClp1) can function as a tRNA splicing enzyme in vivo. RNA. 2008; 14:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noble C.G., Beuth B., Taylor I.A.. Structure of a nucleotide-bound Clp1-Pcf11 polyadenylation factor. Nucleic Acids Res. 2006; 35:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weitzer S., Martinez J.. The human RNA kinase hClp1 is active on 3′ transfer RNA exons and short interfering RNAs. Nature. 2007; 447:222–226. [DOI] [PubMed] [Google Scholar]
- 31. Dikfidan A., Loll B., Zeymer C., Magler I., Clausen T., Meinhart A.. RNA specificity and regulation of catalysis in the eukaryotic polynucleotide kinase Clp1. Mol. Cell. 2014; 54:975–986. [DOI] [PubMed] [Google Scholar]
- 32. Hanada T., Weitzer S., Mair B., Bernreuther C., Wainger B.J., Ichida J., Hanada R., Orthofer M., Cronin S.J., Komnenovic V. et al.. CLP1 links tRNA metabolism to progressive motor-neuron loss. Nature. 2013; 495:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Breuss M.W., Sultan T., James K.N., Rosti R.O., Scott E., Musaev D., Furia B., Reis A., Sticht H., Al-Owain M. et al.. Autosomal-recessive mutations in the tRNA splicing endonuclease subunit TSEN15 cause pontocerebellar hypoplasia and progressive microcephaly. Am. J. Hum. Genet. 2016; 99:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Budde B.S., Namavar Y., Barth P.G., Poll-The B.T., Nurnberg G., Becker C., van Ruissen F., Weterman M.A., Fluiter K., te Beek E.T. et al.. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat. Genet. 2008; 40:1113–1118. [DOI] [PubMed] [Google Scholar]
- 35. Karaca E., Weitzer S., Pehlivan D., Shiraishi H., Gogakos T., Hanada T., Jhangiani S.N., Wiszniewski W., Withers M., Campbell I.M. et al.. Human CLP1 mutations alter tRNA biogenesis, affecting both peripheral and central nervous system function. Cell. 2014; 157:636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bierhals T., Korenke G.C., Uyanik G., Kutsche K.. Pontocerebellar hypoplasia type 2 and TSEN2: review of the literature and two novel mutations. Eur. J. Med. Genet. 2013; 56:325–330. [DOI] [PubMed] [Google Scholar]
- 37. Namavar Y., Eggens V.R.C., Barth P.G., Baas F.. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A.. GeneReviews((R)). 1993; Seattle: University of Washington, Seattle University of Washington. [Google Scholar]
- 38. van Dijk T., Baas F., Barth P.G., Poll-The B.T.. What's new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet. J. Rare. Dis. 2018; 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wafik M., Taylor J., Lester T., Gibbons R.J., Shears D.J.. Two new cases of pontocerebellar hypoplasia type 10 identified by whole exome sequencing in a Turkish family. Eur. J. Med. Genet. 2018; 61:273–279. [DOI] [PubMed] [Google Scholar]
- 40. Schaffer Ashleigh E., Eggens Veerle R.C., Caglayan Ahmet O., Reuter Miriam S., Scott E., Coufal Nicole G., Silhavy Jennifer L., Xue Y., Kayserili H., Yasuno K. et al.. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell. 2014; 157:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eggens V.R.C., Barth P.G., Baas F.. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A.. GeneReviews((R)). 1993; Seattle: University of Washington, Seattle University of Washington. [Google Scholar]
- 42. Namavar Y., Barth P.G., Poll-The B.T., Baas F.. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet. J. Rare. Dis. 2011; 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lardelli R.M., Schaffer A.E., Eggens V.R., Zaki M.S., Grainger S., Sathe S., Van Nostrand E.L., Schlachetzki Z., Rosti B., Akizu N. et al.. Biallelic mutations in the 3′ exonuclease TOE1 cause pontocerebellar hypoplasia and uncover a role in snRNA processing. Nat. Genet. 2017; 49:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan S. A modular polycistronic expression system for overexpressing protein complexes in Escherichia coli. Protein Expr. Purif. 2001; 21:224–234. [DOI] [PubMed] [Google Scholar]
- 45. Pillon M.C., Sobhany M., Borgnia M.J., Williams J.G., Stanley R.E.. Grc3 programs the essential endoribonuclease Las1 for specific RNA cleavage. Proc. Natl Acad. Sci. U.S.A. 2017; 114:E5530–E5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schellenberg M.J., Petrovich R.M., Malone C.C., Williams R.S.. Selectable high-yield recombinant protein production in human cells using a GFP/YFP nanobody affinity support. Protein Sci. 2018; 27:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rogers S.L., Rogers G.C.. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat. Protoc. 2008; 3:606–611. [DOI] [PubMed] [Google Scholar]
- 48. Schmidt C.A., Noto J.J., Filonov G.S., Matera A.G.. A method for expressing and imaging abundant, stable, circular RNAs in vivo using tRNA splicing. Methods Enzymol. 2016; 572:215–236. [DOI] [PubMed] [Google Scholar]
- 49. Fabbri S., Fruscoloni P., Bufardeci E., Di Nicola Negri E., Baldi M.I., Attardi D.G., Mattoccia E., Tocchini-Valentini G.P.. Conservation of substrate recognition mechanisms by tRNA splicing endonucleases. Science (New York, N.Y.). 1998; 280:284–286. [DOI] [PubMed] [Google Scholar]
- 50. Song J., Markley J.L.. Three-dimensional structure determined for a subunit of human tRNA splicing endonuclease (Sen15) reveals a novel dimeric fold. J. Mol. Biol. 2007; 366:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim Y.K., Mizutani K., Rhee K.H., Nam K.H., Lee W.H., Lee E.H., Kim E.E., Park S.Y., Hwang K.Y.. Structural and mutational analysis of tRNA intron-splicing endonuclease from Thermoplasma acidophilum DSM 1728: catalytic mechanism of tRNA intron-splicing endonucleases. J. Bacteriol. 2007; 189:8339–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hirata A., Fujishima K., Yamagami R., Kawamura T., Banfield J.F., Kanai A., Hori H.. X-ray structure of the fourth type of archaeal tRNA splicing endonuclease: insights into the evolution of a novel three-unit composition and a unique loop involved in broad substrate specificity. Nucleic Acids Res. 2012; 40:10554–10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xue S., Calvin K., Li H.. RNA recognition and cleavage by a splicing endonuclease. Science (New York, N.Y.). 2006; 312:906–910. [DOI] [PubMed] [Google Scholar]
- 54. Tsuboi T., Yamazaki R., Nobuta R., Ikeuchi K., Makino S., Ohtaki A., Suzuki Y., Yoshihisa T., Trotta C., Inada T.. The tRNA splicing endonuclease complex cleaves the mitochondria-localized CBP1 mRNA. J. Biol. Chem. 2015; 290:16021–16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Filonov G.S., Moon J.D., Svensen N., Jaffrey S.R.. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J. Am. Chem. Soc. 2014; 136:16299–16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schmidt C.A., Noto J.J., Filonov G.S., Matera A.G.. Filonov G.S., Jaffrey S.R.. Methods in enzymology. 2016; 572:Academic Press; 215–236. [DOI] [PubMed] [Google Scholar]
- 57. Lu Z., Filonov G.S., Noto J.J., Schmidt C.A., Hatkevich T.L., Wen Y., Jaffrey S.R., Matera A.G.. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA. 2015; 21:1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desai K.K., Raines R.T.. tRNA ligase catalyzes the GTP-dependent ligation of RNA with 3′-phosphate and 5′-hydroxyl termini. Biochemistry. 2012; 51:1333–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tanaka N., Chakravarty A.K., Maughan B., Shuman S.. Novel mechanism of RNA repair by RtcB via sequential 2′,3′-cyclic phosphodiesterase and 3′-Phosphate/5′-hydroxyl ligation reactions. J. Biol. Chem. 2011; 286:43134–43143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanaka N., Meineke B., Shuman S.. RtcB, a novel RNA ligase, can catalyze tRNA splicing and HAC1 mRNA splicing in vivo. J. Biol. Chem. 2011; 286:30253–30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chakravarty A.K., Shuman S.. The sequential 2′,3′-cyclic phosphodiesterase and 3′-phosphate/5′-OH ligation steps of the RtcB RNA splicing pathway are GTP-dependent. Nucleic Acids Res. 2012; 40:8558–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schwer B., Aronova A., Ramirez A., Braun P., Shuman S.. Mammalian 2′,3′ cyclic nucleotide phosphodiesterase (CNP) can function as a tRNA splicing enzyme in vivo. RNA. 2008; 14:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmidt C.A., Matera A.G.. tRNA introns: presence, processing, and purpose. WIREs RNA. 2019; 11:e1583. [DOI] [PubMed] [Google Scholar]
- 64. Danan M., Schwartz S., Edelheit S., Sorek R.. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic. Acids. Res. 2012; 40:3131–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salgia S.R., Singh S.K., Gurha P., Gupta R.. Two reactions of Haloferax volcanii RNA splicing enzymes: joining of exons and circularization of introns. RNA. 2003; 9:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Knapp G., Ogden R.C., Peebles C.L., Abelson J.. Splicing of yeast tRNA precursors: structure of the reaction intermediates. Cell. 1979; 18:37–45. [DOI] [PubMed] [Google Scholar]
- 67. Wu J., Hopper A.K.. Healing for destruction: tRNA intron degradation in yeast is a two-step cytoplasmic process catalyzed by tRNA ligase Rlg1 and 5′-to-3′ exonuclease Xrn1. Genes Dev. 2014; 28:1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cherry P.D., Peach S.E., Hesselberth J.R.. Multiple decay events target HAC1 mRNA during splicing to regulate the unfolded protein response. eLife. 2019; 8:e42262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kosmaczewski S.G., Edwards T.J., Han S.M., Eckwahl M.J., Meyer B.I., Peach S., Hesselberth J.R., Wolin S.L., Hammarlund M.. The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep. 2014; 15:1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lu Y., Liang F.X., Wang X.. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol. Cell. 2014; 55:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jurkin J., Henkel T., Nielsen A.F., Minnich M., Popow J., Kaufmann T., Heindl K., Hoffmann T., Busslinger M., Martinez J.. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. 2014; 33:2922–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.