Abstract
School-based vaccination (SBV) and checking students’ vaccination records at school have the potential to optimize vaccination coverage among school-aged children. The primary aim of this paper is to describe adoption of SBV by countries from 2008 to 2017, including target age groups and vaccines delivered in 2017, as reported annually through the World Health Organization (WHO)-United Nations Children’s fund (UNICEF) Joint Reporting Form (JRF). Expanding upon previous analyses, country-specific rates of primary school enrollment and home-based record (HBR) ownership were linked to the WHO-UNICEF JRF data, to identify countries with high potential to implement vaccination record checks at school. The proportion of countries reporting delivery of at least one routinely recommended vaccine dose in school settings increased from 95 (of 163 reporting; 58%) in 2008 to 108 (of 181 reporting; 60%) in 2017. The 13 additional countries that reported using SBV in 2017 were among 31 countries for which SBV data from the JRF were unavailable in 2017. The most common antigens delivered through SBV in 2017 were tetanus (94 countries), diphtheria (89 countries), and human papillomavirus (52 countries). Among 93 countries with data available for net primary school enrollment and HBR ownership, 52 (56%) countries had both ≥80% net primary school enrollment and ≥80% of children aged 12–23 months ever owning an HBR; 33 (63%) of these used SBV. If not already doing so, these 33 countries represent an opportunity to introduce routine checking of vaccination status at entry to, or during primary school. With the growing number of new vaccines and booster doses of childhood vaccines targeting school-age children, implementation of SBV and checking of student vaccination records at school may help improve vaccination coverage; however, additional data are needed to assess global prevalence of checking vaccination status at school and to identify factors facilitating optimal implementation of this strategy.
Keywords: School-based delivery, Routine immunization schedule, School-aged children, Adolescents, Vaccination coverage, Vaccines
1. Background
Health screening and delivery of preventive health services, including vaccination, [1] are often provided at primary and secondary schools. The World Health Organization (WHO) recommends delivery of five vaccine antigens to primary school-aged children and adolescents (tetanus, diphtheria, hepatitis B, rubella, and human papillomavirus [HPV]), though not all countries include these vaccines in national immunization schedules [2,3]. WHO also recommends providing hepatitis B vaccine to high-risk adolescents and varicella vaccine to adolescents in countries where the average age of acquisition of infections is high (≥15 years) [2]. School settings have gained prominence for administration of routinely recommended vaccines to children and adolescents, largely because the target population is easily identifiable in one location and reachable within a short period of time, with minimal logistical constraints [4-8].
School-based vaccination (SBV) has been defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery [9,10]. MacKroth et al. [11] and Vandelaer et al. [9] have previously described the extent to which SBV has been implemented globally. The former used an email survey of 143 countries, and the latter used the WHO-United Nations Children’s Fund (UNICEF) Joint Reporting Form (JRF) [10] data from 2012. In subsequent years, an increasing number of new vaccines (e.g., HPV vaccine) and booster doses of existing vaccines (e.g., tetanus and diphtheria-containing vaccines) have been recommended for primary school-aged children and adolescents [12]. In some countries, these recommendations have catalyzed establishment of a national or sub-national SBV platform and, in others, have catalyzed expanding existing platforms to new types of schools or new school grades [13-15].
Checking of vaccination status at the time of entry to school, or while school is in session (at school) is widely recommended at global, regional, and national levels [16-20], and in some countries is required by law [21-23]. Globally, however, understanding of the prevalence of policies and practices of checking vaccination status at school is limited. As of 2017, there has been no global census on the number of WHO member states or countries implementing this strategy, in part, because questions related to checking of vaccination status at school are not included in the WHO-UNICEF JRF. Evidence of the effectiveness of checking vaccination status at school is predominantly from high-income countries (HICs) and middle-income countries (MICs). In China, checking a child’s vaccination status at entry to primary school and kindergarten increased uptake of vaccines recommended for school-aged children and prompted catch-up vaccination for missed doses of infant vaccines, which increased population immunity in the cohorts of children whose vaccination status was checked [24,25]. Experiences from several HICs and MICs suggest that several factors influence the feasibility and effectiveness of checking vaccination status at school [20-23]. Three important factors include, collaboration between health and education sectors as is needed for any health initiative in schools, high levels of school enrollment to ensure the majority of the target population is available, and reliable sources of data on individual vaccination history, such as that recorded on home-based records (HBR). WHO defines HBRs as “a medical document (more often physical than electronic) — issued by a health authority (such as a national, provincial or district health department) on which an individual’s history of vaccinations received (including the name and number of doses of vaccine(s) along with dates given) from all healthcare providers is recorded” [26].
The primary aim of this paper is to update and expand on previous global summaries of 2017 WHO-UNICEF JRF data [9,11] about the prevalence of SBV in WHO member states and describe the target groups for vaccination, types of vaccine antigens and the number of doses delivered in these settings. By combining the WHO-UNICEF JRF data with country-specific rates of net primary school enrollment and HBR ownership, this paper also identifies countries with the potential to implement the practice of checking students’ vaccination status at school.
2. Methods
2.1. Data sources and variables
Self-reported country-level data on delivery of routinely recommended vaccines in schools (i.e., vaccines on the national immunization schedule and not delivered through campaign-style approaches) were extracted from the school-based immunization module in the WHO-UNICEF JRF (Fig. S1. questions 3000, 3050, and 3060–3170) [27]. This dataset includes data from calendar year 2008 (the first year the module was used) to calendar year 2017. The following country-level data were linked to this dataset: net enrollment rate of children in primary education from UNESCO Institute of Statistics (most recent year available, up to 2017; defined by UNESCO as the total number of students enrolled in the theoretical age group for primary education, as a percentage of the total population in that age group); ownership of HBRs among children 12–23 months of age from Multiple Indicator Cluster Surveys (MICS) or Demographic and Health Surveys (DHS) [28] (most recent year available, up to 2017); whether the country received support from Gavi, the Vaccine Alliance (Gavi) in 2017 (as indicated in the 2017 Annual Report) [29]; and countries’ income level in 2017, as defined by the World Bank (WB) [30]. Although neither currently owning or ever owning an HBR takes into account how long the HBR was retained, ever owning an HBR was chosen for the primary analysis because it includes a longer time period than currently owning, which only takes into account HBR retention at one point in time. For analysis purposes, WHO member states were classified into two categories based on net proportion of children enrolled in primary school and proportion of children aged 12–23 months ever owning an HBR; ≥80% and <80% for each.
2.2. Data cleaning
Country-level reports of SBV in the yearly WHO-UNICEF JRFs from 2008 to 2017 were prepared for analysis using SAS software (version 9.4). Vaccine names provided in a language other than English were translated into English, and each vaccine (e.g., measles-mumps-rubella vaccine) was coded into its component antigens (e.g., measles, mumps, and rubella). Data entries that seemed to be erroneous were excluded, unless confirmed by triangulation with other valid data sources, such as data on the WHO list of national immunization program schedules by country [3], review papers [31,32], or ministry of health web pages. Where a range of target school grades (e.g., grade 7–9) were reported for a single vaccine dose, the midpoint of the range (e.g., grade 8) was used as the grade in which the vaccine was delivered. For entries in which the grade was missing but the age of delivery was reported, the grade was estimated using the reference point of the country’s theoretical entrance age to the first grade of primary school, as defined by UNESCO’s International Standard Classification of Education (ISCED) [33]. For the small number of countries not mapped to ISCED, grade-related data were obtained from Classbase, an online education system database [34] or a country-specific ministry of education web page. Where multiple doses of the same vaccine given at different ages and grades were reported in the same JRF entry (e.g., tetanus toxoid vaccine [TT] given in 1st grade and 6th grade), unique entries were created by dose number and target group (e.g., Grade 1 – TT 1st dose). This method allowed for the calculation of the number of vaccine doses delivered in schools for each vaccine antigen and target grade or age.
2.3. Data analysis
Descriptive analyses were undertaken in Tableau (version 2018.1) to determine the number and proportion of WHO member states that reported implementing SBV, by year, from 2008 to 2017. For 2017, several characteristics were compared between WHO member states reporting SBV and those that did not, including, WB income level, WHO region, eligibility for Gavi funding, net proportion of children enrolled in primary school and HBR ownership. Opportunities to implement checking vaccination status in schools were identified at a country level by linking data from 2017 on presence of SBV, HBR ownership and net primary school enrollment. WHO member states were considered to have high potential for implementing checking of vaccination status at school, if they reported use of SBV, and both net primary school enrollment and HBR ownership was ≥80%.
3. Results
3.1. Prevalence of school-based vaccination
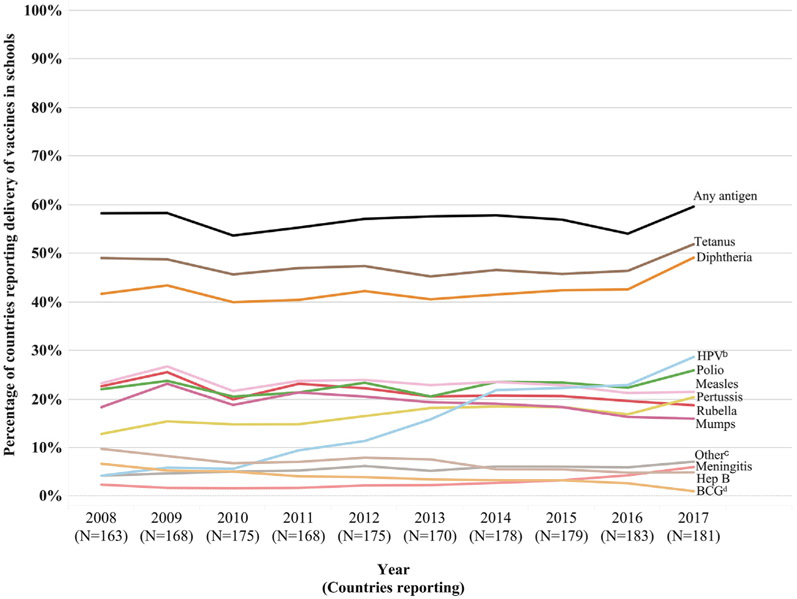
From 2008 to 2017, the number of WHO member states responding to the JRF SBV module ranged from 163 to 183 (Fig. 1, Table S1). From 2008 to 2017, the proportion of WHO member states reporting delivery of any vaccine antigen in schools increased from 58% (95 out of 163 reporting) in 2008 to 60% (108 out of 181 reporting) in 2017 (Fig. 1). Of the 68 WHO member states that reported not using SBV in 2008, 15 reported using SBV in 2017. Of the 95 WHO member states that reported using SBV in 2008, 11 had changed their answer and reported no longer using SBV in 2017, and four did not respond in 2017. Hence there was no change in the numerator among the original cohort of 163 countries that responded in 2008. However, among the 31 WHO member countries that did not respond to the JRF school-based vaccination module in 2008, 13 reported using SBV in 2017. The largest year-to-year increase occurred from 2016 to 2017, during which six countries reported SBV for the first time (Table S1).
Fig. 1.
Proportion of countries delivering a given antigen through school-based vaccinationa, among WHO member states responding to the school-based vaccination module in the World Health Organization–United Nations Children’s Fund Joint Reporting Form, 2008 to 2017. aSchool-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns). bHuman papillomavirus (HPV). cThe “Other” category of antigens includes varicella, influenza, yellow fever, typhoid, tick-borne encephalitis, hepatitis A, haemophilus influenzae B, pneumococcal, rotavirus, and dengue. dBacille Calmette-Guérin (BCG).
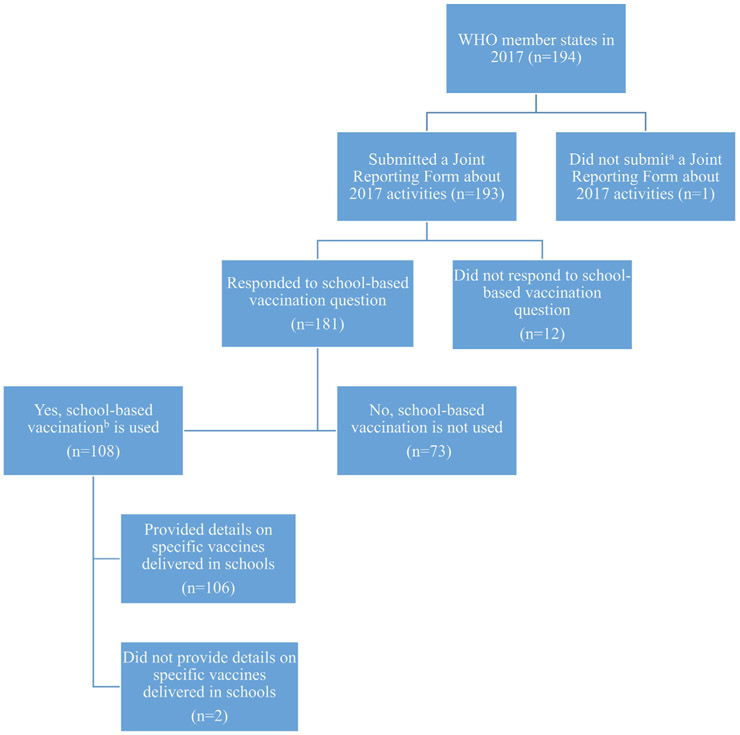
Of the 194 WHO member states (including countries and territories) in 2017, 193 (99%) submitted a JRF and, among these, 181 countries answered questions pertaining to the delivery of routine doses of vaccines in schools in 2017 (Fig. 2). Of the 181 responding WHO member states, 108 (60%) reported delivering routine doses of vaccines in schools in 2017 (Fig. 2, Table 1, and Fig. S2). Among WHO regions, SBV was most prevalent in the Western Pacific Region (81%), Americas Region (79%), and Eastern Mediterranean Region (68%). SBV was more common among upper-middle- income countries (UMIC) (77%), high-income countries (HIC) (67%), and lower-middle-income countries (LMIC) (58%) compared with low-income countries (LICs) (19%) and was more common in non-Gavi-supported countries (73%) compared with Gavi- supported countries (37%) (Table 1). Of the 108 countries reporting use of SBV in 2017, 89 (82%) indicated that vaccination was part of a comprehensive school health program that also delivered other health interventions (i.e. deworming, general health education) (data not shown).
Fig. 2.
Summary of World Health Organization (WHO) member states’ responses to the WHO–United Nations Children’s Fund (UNICEF) Joint Reporting Form module about use of school-based vaccination in 2017. aUruguay was the only country without any JRF data on activities that occurred in 2017 at the time data were accessed. bSchool-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns).
Table 1.
Characteristics of 181 World Health Organization (WHO) member states reporting on school-based vaccination activities occurring in 2017 in the WHO–United Nations Children’s Fund (UNICEF) Joint Reporting Form.
| School-based vaccinationan (%) |
No school- based vaccinationn (%) |
|
|---|---|---|
| Total | 108 (60%) | 73 (40%) |
| World Health Organization Region | ||
| African Region | 17 (38%) | 28 (62%) |
| Americas Region | 26 (79%) | 7 (21%) |
| Eastern Mediterranean Region | 13 (68%) | 6 (32%) |
| European Region | 25 (54%) | 21 (46%) |
| South-East Asia Region | 5 (45%) | 6 (55%) |
| Western Pacific Region | 22 (81%) | 5 (19%) |
| World Bank income category | ||
| High-income country (HIC) | 35 (67%) | 17 (33%) |
| Upper-middle–income country (UMIC) | 41 (77%) | 12 (23%) |
| Lower-middle–income country (LMIC) | 26 (58%) | 19 (42%) |
| Low-income country (LIC) | 6 (19%) | 25 (81%) |
| Gavi supportedb country in 2017 | ||
| Yes | 25 (37%) | 43 (63%) |
| No | 83 (73%) | 30 (27%) |
| Net Primary School Enrollment Ratec | ||
| Enrollment ≥ 80% | 97 (66%) | 49 (34%) |
| Enrollment < 80% | 8 (28%) | 21 (72%) |
| No data on enrollment | 3 (50%) | 3 (50%) |
| % of children aged 12–23 months whose caregiver had ever owned a home-based record of vaccination (HBR)d | ||
| Ever owned an HBR ≥ 80% | 34 (51%) | 33 (49%) |
| Ever owned an HBR < 80% | 3 (27%) | 8 (73%) |
| No data on ever-owned HBR | 71 (69%) | 32 (31%) |
School-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns); 13 countries did not respond to this question (5 high-income, 4 upper-middle-income, 3 low-income) in the JRF.
Received any amount of Gavi funds in calendar year 2017.
According to the United Nations Educational, Scientific, and Cultural Organization (UNESCO): Total number of students in theoretical age group for primary education enrolled in that level, divided by the total population in that age group.
According to countries’ most recent Demographic and Health Survey (DHS) or Multiple Indicator Cluster Survey (MICS).
3.2. Vaccine antigens delivered in schools
Prior to 2017, the proportion of WHO member states that reported delivery of specific vaccine antigens in schools varied little year-to-year for most antigens, with two exceptions: HPV, for which the proportion of countries delivering this vaccine increased steadily throughout this period, and bacille Calmette-Guérin (BCG) and hepatitis B vaccines, for which the proportions of countries delivering these vaccine antigen decreased steadily throughout this period. Between 2016 and 2017, HPV, tetanus-diphtheria or diphtheria-tetanus-pertussis (Td/DTP), and measles were the primary vaccine antigens delivered in schools.
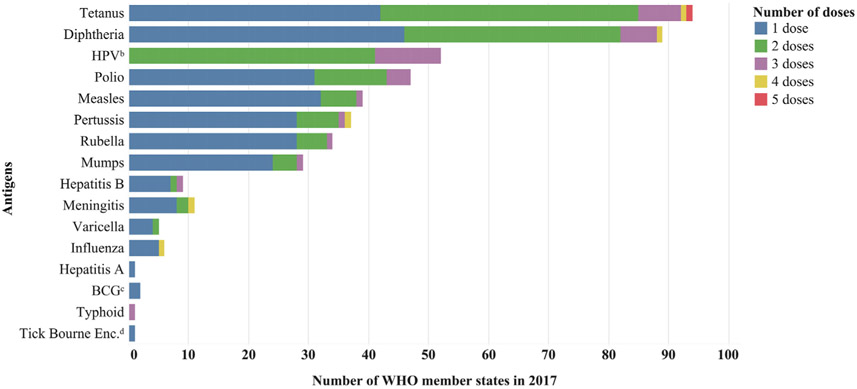
Among the 108 countries that reported delivering routine vaccine doses in schools in 2017, 106 (98%) reported additional information about the specific antigens delivered, including number of doses and school grade or age groups targeted (Figs. 1, 3, and S3). Among the 106 WHO member states reporting vaccine antigen data in 2017, the mean number of vaccine antigens reported to be delivered through SBV was 4 (range 1–10) (Fig. S3), which was the same number in 2008. On average, a larger variety of vaccine antigens were delivered in schools in UMICs and HICs (mean = 5, range 1–10), compared with LMICs and LICs (mean = 3, range 1–5) (Fig. S1). Of 18 different vaccine antigens delivered in schools globally in 2017, tetanus (94 countries) and diphtheria (89 countries) were the most common antigens delivered and were most frequently provided in combination vaccine presentations (e.g., Td) (Fig. 3). Of the 106 WHO member states that reported information on specific vaccine antigens given in schools in 2017, 60 (57%) had HPV vaccine in the routine immunization schedule. Of these 60 WHO member states, 52 (87%) reported delivering HPV vaccine in schools in 2017; 23 (44%) were HICs, 19 (37%) were UMICs, 8 (15%) were LMICs, and 2 (4%) were LICs. Of the 52 WHO member states reporting delivery of HPV vaccines in schools in 2017, 42 (81%) reported delivery of HPV vaccine in schools nationwide, 7 (13%) countries reported delivery in some sub-national areas, and 3 countries (6%) did not indicate the geographic reach of HPV vaccine delivery.
Fig. 3.
Number of WHO member states delivering a given vaccine antigen through school-based vaccinationa and the number of doses given in 108 World Health Organization (WHO) member states reporting specific vaccines delivered in schools in 2017 in the WHO–United Nations Children’s Fund (UNICEF) Joint Reporting Form. aSchool-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns). bHuman papillomavirus (HPV). cBacille Calmette-Guérin (BCG). dTick-borne encephalitis. Note: HPV vaccine was delivered to females only, with the exception of 10 countries that reported delivering HPV vaccine to both males and females. The vast majority of all other vaccine doses delivered in schools were given to both males and females, with just a few exceptions: four countries reported delivering at least one dose of tetanus-diphtheria containing vaccine to females only, and two countries reported delivering vaccine containing only tetanus toxoid to females only.
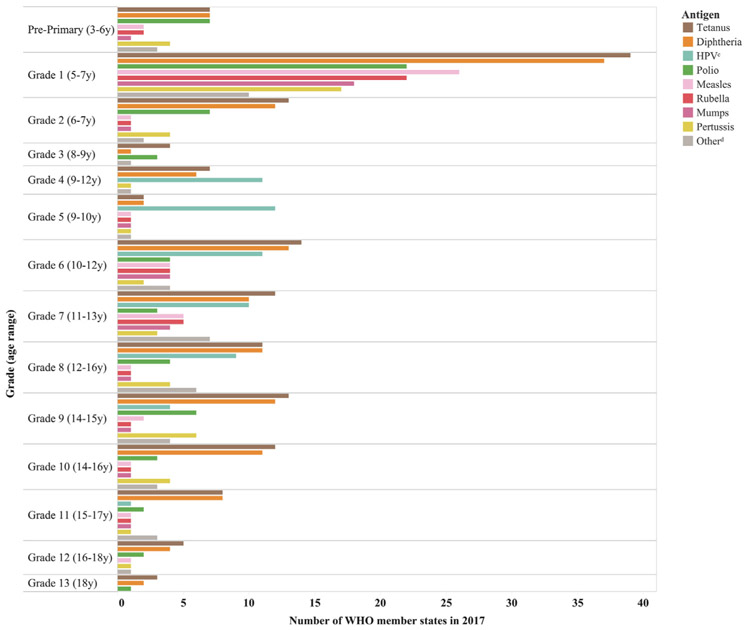
In 2017, delivery of routine vaccines in schools was most often conducted in the first grade of primary school among children 5–7 years of age (Fig. 4). The exception was HPV vaccine, which was most frequently delivered between 4th and 8th grade to females 9–13 years of age.
Fig. 4.
Distribution of antigens delivered through school-based vaccinationa by mean school gradeb and age rangeb targeted, among 106 World Health Organization (WHO) member states reporting specific vaccines delivered in schools in 2017 in the WHO–United Nations Children’s Fund (UNICEF) Joint Reporting Form. aSchool-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns). bFor countries that reported only the age(s) targeted but not the grade(s) targeted (and vice versa), the grade was determined by using, as a reference point, the country’s most recently reported theoretical age of entrance to primary school. cHuman papillomavirus (HPV). dThe “Other” category of antigens includes bacille Calmette-Guérin (BCG), varicella, influenza, yellow fever, typhoid, tick-borne encephalitis, hepatitis A, haemophilus influenzae B, pneumococcal, rotavirus, and dengue.
3.3. Net primary school enrollment, home-based record retention and school-based vaccination
Among the 181 WHO member states responding to the JRF question on delivery of vaccines in schools in 2017, 175 had data available on net primary school enrollment (146 [83%] had ≥80%, 29 [17%] had <80%) (Table 1). SBV was reportedly used in 97 (66%) of 146 WHO member states with net primary school enrollment ≥80%, and in 8 (28%) of 29 WHO member states with net primary school enrollment <80% (Table 1). Seventy-eight WHO member states had data available on ever owning an HBR among children aged 12–23 months (67 [86%] had ≥80%, 11 [14%] had <80%) (Table 1). SBV was used in 34 (51%) of 67 WHO member states reporting ≥80% of children aged 12–23 months ever-owning an HBR, and in 3 (27%) of 11 WHO member states with <80% of children ever owning an HBR (Table 1).
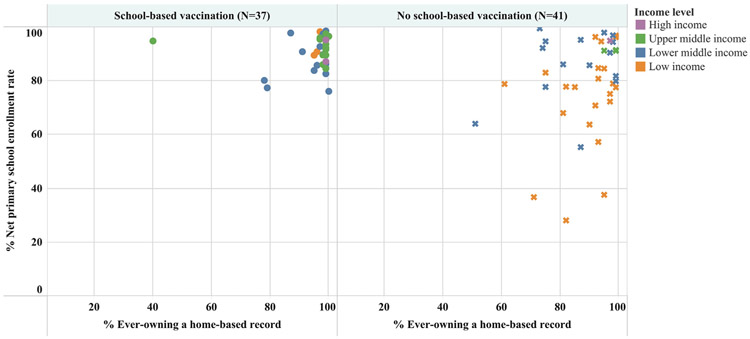
Among 78 WHO member states with data available for both variables (net primary school enrollment and children aged 12–23 months ever owning an HBR), 52 (67%) had ≥80% for both variables; 33 (63%) of these countries reported using SBV (Table 2) and 19 (37%) reported not using SBV (Table 3). The relationship between ever owning an HBR, net primary school enrollment, and SBV is illustrated in Fig. 5.
Table 2.
World Health Organization (WHO) member states delivering vaccines in schoolsa in 2017, according to the World Health Organization–United Nations Children’s Fund Joint Reporting Form, with high potentialb to implement checking of children’s vaccination status at school entry.
| Countries (N = 33) |
% Net Primary School Enrollment Rate d |
% Ever owned home-based record e |
|
|---|---|---|---|
| Gavi supported c | Rwanda | 96 | 99 |
| Sao Tome & Principe | 96 | 99 | |
| Sierra Leone | 98 | 97 | |
| Cuba | 95 | 99 | |
| Cambodia | 93 | 97 | |
| Bolivia | 90 | 98 | |
| Uganda | 91 | 96 | |
| Mongolia | 98 | 87 | |
| Mozambique | 90 | 95 | |
| Zambia | 86 | 99 | |
| Ghana | 85 | 99 | |
| Indonesia | 91 | 91 | |
| Honduras | 83 | 99 | |
| Côte d’Ivoire | 84 | 95 | |
| Non Gavi supported | Tunisia | 99 | 99 |
| Costa Rica | 97 | 100 | |
| Algeria | 98 | 99 | |
| Egypt | 97 | 98 | |
| Trinidad and Tobago | 95 | 99 | |
| Peru | 95 | 99 | |
| Belize | 96 | 97 | |
| Montenegro | 94 | 99 | |
| Philippines | 96 | 97 | |
| Mexico | 95 | 97 | |
| Jordan | 92 | 99 | |
| Macedonia | 92 | 99 | |
| Thailand | 90 | 99 | |
| Namibia | 90 | 98 | |
| Panama | 87 | 99 | |
| Dominican Republic | 86 | 98 | |
| El Salvador | 85 | 99 | |
| Guatemala | 85 | 99 | |
| Vanuatu | 86 | 96 |
School-based vaccination is defined as delivering a vaccine routinely in schools (excludes doses given during campaigns).
High potential is defined as having ≥ 80% primary school enrollment and ≥ 80% of children aged 12–23 months that ever owned an HBR.
Received any amount of Gavi funds in calendar year 2017.
Total number of students in theoretical age group for primary education enrolled in that level, divided by the total population in that age group.
Among children aged 12–23 months, according to countries’ most recent Multiple Indicator Cluster Survey (MICS) or Demographic Health Survey (DHS) data.
Table 3.
World Health Organization (WHO) member states not delivering vaccines in schoolsa in 2017, according to the World Health Organization– United Nations Children’s Fund Joint Reporting Form, with high potentialb to deliver vaccines in schools and implement checking of children’s vaccination status at school entry.
| Countries (N = 19) |
% Net Primary School Enrollment Rated |
% Ever owned home- based record e |
|
|---|---|---|---|
| Gavi supported c | Burundi | 97 | 99 |
| Malawi | 96 | 99 | |
| Nicaragua | 97 | 98 | |
| Viet Nam | 98 | 95 | |
| Guyana | 92 | 99 | |
| Nepal | 95 | 94 | |
| Bangladesh | 91 | 97 | |
| Benin | 96 | 92 | |
| Cameroon | 95 | 87 | |
| Kenya | 82 | 99 | |
| Togo | 85 | 95 | |
| Zimbabwe | 85 | 93 | |
| Congo | 86 | 90 | |
| Comoros | 81 | 93 | |
| Non-Gavi supported | Morocco | 94 | 98 |
| Poland | 95 | 97 | |
| Colombia | 91 | 99 | |
| Gabon | 91 | 95 | |
| Cabo Verde | 86 | 81 |
School-based vaccination is defined as delivering a vaccine routinely in schools (excludes doses given during campaigns).
High potential is defined as having ≥ 80% primary school enrollment and ≥ 80% of children aged 12–23 months that ever owned an HBR.
Received any amount of Gavi funds in calendar year 2017.
Total number of students in theoretical age group for primary education enrolled in that level, divided by the total population in that age group.
Among children aged 12–23 months, according to countries’ most recent Multiple Indicator Cluster Survey (MICS) or Demographic Health Survey (DHS) data.
Fig. 5.
Primary school enrollment and home-based record ownership among 78 World Health Organization (WHO) member states reporting delivery of routine doses of vaccines in schools in 2017 in the WHO–United Nations Children’s Fund (UNICEF) Joint Reporting Form that had data available on net primary school enrollment rateb and home-based record ownershipc, by World Bank income level. aSchool-based vaccination is defined as the delivery of routinely recommended doses of vaccines to school-aged children using the school as a venue for delivery (excludes doses given during campaigns). bTotal number of students in theoretical age group for primary education enrolled in that level, divided by the total population in that age group. cThe proportion of children aged 12–23 months who had ever owned a home-based record, according to a country’s most recent MICS or DHS survey data. Note: Countries having both net primary school enrollment rate and % ever owning an HBR ≥ 80% were identified as having the highest potential for delivering school-based vaccination and checking children’s vaccination status at school entry.
4. Discussion
New vaccines targeting school-aged children (e.g., HPV, meningococcal, and varicella vaccines), and recommendations for booster doses of childhood vaccines (e.g., tetanus-diphtheria–con taining vaccines), have led to an increased call to deliver vaccines to school-aged children [35]. Such calls are seen in strategic plans for the elimination of measles and rubella [36,37] and tetanus [38], both of which recommend SBV, and checking of vaccination status at school as strategies to contribute to elimination of these diseases. The response rate to the JRF school-based vaccination module has increased from 163 of 194 member states in 2008 to 181 of 194 member states in 2017. Despite this, there was little overall change from 2008 to 2017 in the proportion of WHO member states reporting delivery of at least one dose of a routine vaccine in a school setting (58%–60%); however, during the same period there was an increase in the total number of WHO member states reporting delivery of at least one routine vaccine through SBV (95–108). During the same period (2008–2017), there was no change in the average number of vaccine antigens delivered through SBV (mean = 4). Since 2008, the first grade of primary school has remained the most frequently targeted grade for SBV, but an increasing number of countries in 2017 reported delivering vaccines later in primary school and in secondary schools [2]. Gavi support for HPV vaccine introduction has helped catalyze the establishment of SBV in eligible LMICs, either alone or as part of existing school health programs [15,39]. However, SBV still remains more predominant in HIC and UMICs, than LMICs or LICs.
SBV may not be feasible or effective in all settings. Seventy-five percent of WHO member states responding to the school-based immunization section of the JRF in 2017 and with net primary school enrollment ≥80% reported implementing SBV in 2017, while less than 25% of reporting WHO member states with net primary school enrollment <80% reported implementing SBV. These findings support previous suggestions that SBV may not be as feasible or effective in settings with low school enrollment or attendance [9], such as LICs, secondary schools, or in rural areas [40-43]. Therefore, in these settings, SBV should be complemented by activities to reach out-of-school children.
Lessons learned from SBV in high and middle-income settings highlight a heterogeneous array of factors influencing feasibility of this strategy. Such factors include leadership and governance, organizational models and institutional relationships, workforce capacity and roles (particularly concerning the school nurse), available mechanisms to communicate about vaccination with parents and students (including methods for obtaining consent), and processes related to organization and delivery [8,44]. Similar influencing factors have been reported from LMICs implementing SBV, though the evidence from these settings was limited to delivery of HPV vaccine [13,45]. Operational cost of an SBV program, the cost of vaccine, and community preferences for vaccination delivery platforms (e.g. primary health care sector vs school-based) may also influence feasibility of implementation [46]. In addition to drawing on existing evidence about factors affecting delivery of vaccines in schools, countries planning to initiate SBV can determine their overall capacity to implement this strategy using the WHO school immunization readiness assessment tool [47]. This tool can also be used to monitor and inform improvements to delivery of vaccines in schools, as well as broader school health services.
Although many vaccines routinely recommended for school-aged children and adolescents were delivered in school between 2008 and 2017, the WHO-UNICEF estimates of national immunization coverage (WUENIC) did not include estimates of coverage for vaccines targeting school-aged children during this period [48,49]. Similarly, not all countries implementing SBV routinely monitor coverage of the vaccines delivered to school-aged children [50]. In countries where vaccination coverage among primary-school-aged children and adolescents has been assessed, coverage was generally higher in HICs with well-established SBV programs [9,43,51-54]. In LMICs, rapid uptake of HPV and Td vaccines in schools in recent years [39,43,51-53,55,56] illustrates that SBV can be a useful approach to reach school-aged children with primary or booster doses of routine vaccines. In 2018, WUENIC included estimates of national-level coverage of HPV vaccine, which was the first time that any vaccine targeting school-age children or adolescents was included in the report [57]. However, further efforts on a local to global level are still needed to accurately and consistently monitor vaccination coverage among school-age children and adolescents. Such information is critical to determine the success of the SBV approach.
Having comprehensive records of children’s vaccination history, as is recorded on an HBR, is critical for monitoring timely completion of individual vaccination status, assessing immunization program performance through vaccination coverage surveys, and evaluating individual and population vaccination status during disease outbreaks. Recording vaccines delivered in school on an HBR, which also includes all vaccines given during infancy and early childhood, has the potential to motivate parents to retain an HBR past their child’s infancy. Our findings concur with, and build upon, a previous analysis illustrating that countries implementing SBV had higher levels of current HBR ownership compared to those not implementing SBV [58]. In countries where electronic immunization registries or electronic medical record systems are not yet implemented, availability of an HBR is a critical component of checking children’s vaccination status at entry to, or during school. Checking of vaccination status in schools is a widely recommended strategy for increasing population-based coverage of routinely recommended vaccines in school-aged children [18,19] and could further motivate HBR retention, improve completeness of data in immunization registers or electronic medical records, and increase demand for complete and timely vaccination in childhood.
School enrollment, the start of each school year, and the point at which vaccines are delivered at the school all present opportunities to check children’s vaccination status. Thus, UMIC, LMICs, and LICs already implementing SBV have previously been identified as having the potential to implement checking of vaccination status in schools [56,58-60]. This is further supported by the reported results from our analysis in which 33 of the 78 WHO member states with source data available (42%), met three important criteria for successful implementation of checking vaccination status at school; presence of SBV program, ≥80% net primary school enrollment, and ≥80% ever owning an HBR. These countries are considered those with “high potential” to introduce checking of vaccination status at school and were spread across all WHO Regions. Additionally, 19 WHO member states had ≥80% net primary school enrollment and ≥80% ever owning an HBR but did not meet the third criteria of implementing SBV in 2017. The majority of these 19 countries were from the African Region. Checking a child’s vaccination status at school can occur with or without an official requirement to have certain vaccinations in order to enter school. The “nudge strategy” of checking vaccination status alone, without a financial or social penalty, focuses on building and strengthening systems and addressing barriers to accessing immunization services [61]. With this nudge strategy, accountability is placed on assessing vaccination status, facilitating access to missed doses of vaccines, and increasing demand for, and timeliness of, childhood vaccines in a country’s national immunization schedule. A more comprehensive understanding of the prevalence, potential benefits of, and challenges to checking children’s vaccination status, at entry to, or during school is needed, especially in LMICs and LICs.
As previously reported by Vandelaer et al. [9], the JRF data have inherent limitations because of their self-reported nature; however these data are currently the only available source from which the global prevalence of countries implementing SBV can be derived. Additionally, although the JRF instructions specify excluding reporting of doses delivered during vaccination campaigns, it is possible that some countries reported doses of vaccines delivered in campaigns targeting school-aged children or using schools as the vaccination site, thus creating the potential for over-reporting of SBV. Consistent with previous analyses of JRF data on SBV [9], JRF data from 2017 used in these analyses were not verified against other data sources unless entry errors or missing data points were evident, as described in the methods. Limitations of data on HBR ownership have been previously reported [62]. Missing data was a notable limitation for HBR ownership (available for 78 countries), as these data only existed for countries where a DHS or MICS survey had been conducted (i.e., primarily LIC, LMIC, and UMIC countries). Therefore, this analysis may not accurately reflect the status of HBR ownership globally [62-64]. Data on ever and current ownership of an HBR were most complete for children aged 12–23 months, though evidence suggests there is a reduction in HBR retention as age increases [58,65]. Therefore, the presented data on HBR ownership should be considered a maximum estimate of HBR retention [62,66,67]. Lastly, although the analysis was limited to the 194 WHO member states, regionally recognized countries and areas (e.g., Kosovo, Greenland, Taiwan) may also implement SBV, potentially increasing the prevalence of SBV beyond what is reported.
5. Conclusions
As national immunization programs evolve to introduce the growing number of new vaccines and booster doses of childhood vaccines targeting school-age children, more countries may establish an SBV platform. Despite the need to find ways to deliver routine vaccines to school-age children, there has been little increase in the proportion of WHO member states implementing SBV from 2008 to 2017. This may potentially be due to challenges that remain in settings where school enrollment or attendance is low, such as in LICs, rural areas, higher school grades, and in countries with funding and workforce constraints in the health and education sectors. There is need for further exploration of the association between SBV, primary school enrollment, and HBR ownership. Identification of other performance indicators of education and routine immunization systems that effect the potential for SBV is also needed, as is further understanding about the policies and practices that would optimize implementation of SBV in LICs, MICs and in Gavi-eligible countries. Countries already implementing SBV, especially those with high school enrollment and HBR ownership, could use the existing links with the education sector to discuss potential for routine checking of children’s vaccination status at school. Such a strategy would require collaboration between the health and education sectors to develop an acceptable policy, ensure availability of sufficient material and human resources, provide robust operational guidance, and conduct routine monitoring. Additional data are needed to assess global prevalence of checking vaccination status at school entry or during school, and to identify other factors facilitating optimal implementation of this strategy. To partly address this, additional questions about the policy and practice of checking vaccination at entry to, or during childcare or school are being added to the 2019 JRF.
Supplementary Material
Acknowledgements
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the U.S Centers for Disease Control and Prevention. The authors appreciate the contributions from the following colleagues, Margaret Watkins, Laure Dumolard, Stephanie Shendale, Aaron Wallace, and Susan Chu.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.10.054.
References
- [1].Baltag V, Pachyna A, Hall J. Global overview of school health services: data from 102 countries. Health Behavior Policy Rev 2015;2:268–83. [Google Scholar]
- [2].World health organization. WHO recommendations for routine immunization - summary tables (August 2018). Available from: http://www.who.int/immunization/policy/immunization_tables/en/ [accessed: 17 April 2018].
- [3].World health organization. Immunization schedules by antigens. Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules [accessed: 31 April 2018].
- [4].Pebody R. Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013/14 influenza season. Eurosurveillance 2014;19:22. [DOI] [PubMed] [Google Scholar]
- [5].Ward K, Quinn H, Bachelor M, Bryant V, Campbell-Lloyd S, Newbound A, et al. Adolescent school-based vaccination in Australia. Commun Dis Intell Q Rep 2013;37:E156–67. [DOI] [PubMed] [Google Scholar]
- [6].World health organization. School-based immunization. Available from: http://www.who.int/immunization/programmes_systems/policies_strategies/school_based_immunization/en/ [accessed: 3 April 2017].
- [7].Paul P, Fabio A. Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program. Vaccine 2014;32:320–6. [DOI] [PubMed] [Google Scholar]
- [8].Perman S, Turner S, Ramsay AI, Baim-Lance A, Utley M, Fulop NJ. School-based vaccination programmes: a systematic review of the evidence on organisation and delivery in high income countries. BMC Public Health 2017;17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vandelaer J, Olaniran M. Using a school-based approach to deliver immunization-global update. Vaccine 2015;33:719–25. [DOI] [PubMed] [Google Scholar]
- [10].World health organization. WHO/UNICEF joint reporting form 2018. Available from: http://www.who.int/immunization/monitoring_surveillance/routine/reporting/reporting/en/ [accessed: 30 April 2018].
- [11].Mackroth MS, Irwin K, Vandelaer J, Hombach J, Eckert LO. Immunizing school-age children and adolescents: experience from low- and middle-income countries. Vaccine 2010;28:1138–47. [DOI] [PubMed] [Google Scholar]
- [12].Loharikar A, Dumolard L, Chu S, Hyde T, Goodman T, Mantel C. Status of new vaccine introduction - worldwide, September 2016. MMWR Morb Mortal Wkly Rep 2016;65:1136–40. [DOI] [PubMed] [Google Scholar]
- [13].La Vincente SF, Mielnik D, Jenkins K, Bingwor F, Volavola L, Marshall H, et al. Implementation of a national school-based Human Papillomavirus (HPV) vaccine campaign in Fiji: knowledge, vaccine acceptability and information needs of parents. BMC Public Health 2015;15:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Binagwaho A, Wagner CM, Gatera M, Karema C, Nutt CT, Ngabo F. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull World Health Organ 2012;90:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Griffiths UK, Feletto M, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low-and middle-income countries. PLoS ONE 2017;12 e0177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].World health organization. South-east Asia regional immunization technical advisory group (SEAR-ITAG) report of the ninth meeting. Available from: http://www.who.int/immunization/sage/meetings/2018/october/5_SEAR_Report.pdf?ua=1 [accessed: 27 November 2018].
- [17].World health organization western Pacific region. 27th meeting of the technical advisory group on immunisation and vaccine preventable diseases in the Western Pacific Region, 2018 Available from: http://www.who.int/immunization/sage/meetings/2018/october/6_WPR_Report.pdf?ua=1 [accessed: 27 November 2018]. [Google Scholar]
- [18].Goodson JL, Alexander JP, Linkins RW, Orenstein WA. Measles and rubella elimination: learning from polio eradication and moving forward with a diagonal approach. Expert Rev Vaccines 2017;16:1203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].World Health Organization. Measles and rubella global strategic plan 2012-2020 midterm review. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- [20].Takashima Y, Schluter WW, Mariano KM, Diorditsa S, de Quiroz Castro M, Ou AC, et al. Progress toward measles elimination-Philippines, 1998–2014. MMWR Morb Mortal Wkly Rep 2015;64:357–62. [PMC free article] [PubMed] [Google Scholar]
- [21].Mengjuan D, Zheng J, Liwei L, Lei W, Lingsheng C, Lei C, et al. Evaluation of a school entry immunization record check strategy in 4 counties of Ningxia and Hubei provinces, China. Vaccine 2018;36:6231–6. [DOI] [PubMed] [Google Scholar]
- [22].Orenstein WA, Hinman AR. The immunization system in the United States - the role of school immunization laws. Vaccine 1999;17(Suppl 3):S19–24. [DOI] [PubMed] [Google Scholar]
- [23].Choe Y, Park K, Park E, Kong I, Lee J-K. School entry vaccination requirement program: Experience from the Republic of Korea. Vaccine 2018;36:5497–9. [DOI] [PubMed] [Google Scholar]
- [24].Yin X, Li X, Cao J. Effect of new procedures for immunization certificate inspections at kindergarten and school entry in Ningyang County of Shandong Province. Chin J Vaccines Immunization 2016;22:546–50. [Google Scholar]
- [25].Zuo S, Cairns L, Hutin Y, Liang X, Tong Y, Zhu Q, et al. Accelerating measles elimination and strengthening routine immunization services in Guizhou Province, China, 2003–2009. Vaccine 2015;33:2050–5. [DOI] [PubMed] [Google Scholar]
- [26].World health organization. Immunization in practice: a practical guide for health staff - 2015 update. Geneva, Switzerland; 2015. [Google Scholar]
- [27].World health organization. Data, statistics and graphics: 6.4 Immunization provided at school Available from: http://www.who.int/immunization/monitoring_surveillance/data/en/ [accessed: 9 April 2018].
- [28].Brown DW. Home-based record ownership prevalence. Available from: https://sites.google.com/site/vaccinationcardprevalence/ [accessed: 10 December 2016].
- [29].Gavi the vaccine alliance. Annual progress report 2016. Geneva, Switzerland: Gavi, The Vaccine Alliance; 2016. [Google Scholar]
- [30].World Bank. World Bank country and lending groups. Historical classification by income in XLS format. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [accessed: 1 August 2018].
- [31].D’Addario M, Redmond S, Scott P, Egli-Gany D, Riveros-Balta AX, Henao Restrepo AM, et al. Two-dose schedules for human papillomavirus vaccine: Systematic review and meta-analysis. Vaccine 2017;35:2892–901. [DOI] [PubMed] [Google Scholar]
- [32].PATH. Country HPV delivery strategy characteristics. Available from: http://www.rho.org/HPVlessons-map.htm [accessed: 18 June 2018].
- [33].United nations educational scientific and cultural organization. International standard of classification of education (ISCED) Mappings. Available from: http://uis.unesco.org/en/isced-mappings [accessed: 30 April 2018].
- [34].Classbase educational database. Country education systems. Available from: https://www.classbase.com/Countries [accessed: 30 April 2018].
- [35].World health organization. WHO recommendations for routine immunization - summary tables. Available from: http://www.who.int/immunization/policy/immunization_tables/en/ [accessed: 17 April 2018].
- [36].World health organization. Global measles and rubella strategic plan 2012–2020. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- [37].World Health Organization. Measles vaccines: WHO position paper – April 2017. Wkly Epidemiol Rec 2017;92:205–28.28459148 [Google Scholar]
- [38].World Health Organization. Protecting all against tetanus: Guide to sustaining maternal and neonatal tetanus elimination (MNTE) and broadening tetanus protection for all populations. Geneva: World Health Organization; 2019. [Google Scholar]
- [39].LaMontagne DS, Bloem PJN, Brotherton JML, Gallagher KE, Badiane O, Ndiaye C. Progress in HPV vaccination in low- and lower-middle-income countries. Int J Gynaecol Obstet 2017;138(Suppl 1):7–14. [DOI] [PubMed] [Google Scholar]
- [40].UNICEF.The state of the world’s children. Available from: https://www.unicef.org/publications/files/SOWC_2017_ENG_WEB.pdf[accessed: 18 September 2018].
- [41].World Health Organization. Explorations of inequality: Childhood immunization. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- [42].Arsenault C, Johri M, Nandi A, Mendoza Rodríguez JM, Hansen PM, Harper S. Country-level predictors of vaccination coverage and inequalities in Gavi-supported countries. Vaccine 2017;35:2479–88. [DOI] [PubMed] [Google Scholar]
- [43].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Burchett HED, LaMontagne DS, et al. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007–2016. Papillomavirus Res 2017;4:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cooper Robbins SC, Ward K, Skinner SR. School-based vaccination: a systematic review of process evaluations. Vaccine 2011;29:9588–99. [DOI] [PubMed] [Google Scholar]
- [45].Torres-Rueda S, Rulisa S, Burchett HE, Mivumbi NV, Mounier-Jack S. HPV vaccine introduction in Rwanda: Impacts on the broader health system. Sexual Reprod Healthcare: Official J Swedish Assoc Midwives 2016;7:46–51. [DOI] [PubMed] [Google Scholar]
- [46].LaMontagne DS, Cernusch T, Yakubu A, Bloem PJN, Watson-Jones D, Kim JJ. Chapter 15. School-based delivery of vaccines to 5- to 19-year olds In: Bundy DAP, de Silva N, Horton S, Jamison DT, Patton GC, editors. Disease control priorities 3rd edition child adolescent health and development. Washington D.C: International Bank for Reconstruction and Development / The World Bank; 2017. p. 199–209. [PubMed] [Google Scholar]
- [47].World health organization. School vaccination readiness assessment tool. Available from: http://apps.who.int/iris/handle/10665/90566 [accessed: 2 December 2018].
- [48].VanderEnde K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage - 2017. MMWR Morb Mortal Wkly Rep 2018;67:1261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].World health organization. Immunization, vaccines and biologicals—data, statistics and graphs. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726243/ [accessed: 29 June 2018].
- [50].SAGE working group on maternal and neonatal tetanus elimination and broader tetanus prevention. Report of the SAGE working group on maternal and neonatal tetanus elimination and broader tetanus prevention. Available from: http://www.who.int/immunization/sage/meetings/2016/october/1_Report_of_the_SAGE_Working_Group_on_Maternal_and_Neonatal_Tetanus_27Sep2016.pdf [accessed: 29 June 2018].
- [51].World health organization. School immunization programme in Indonesia. Available from: http://www.who.int/immunization/programmes_systems/policies_strategies/Indonesia-School-immunization.pdf?ua=1 [accessed: 18 September 2018].
- [52].World health organization. School immunization programme in Tunisia. Available from: http://www.who.int/immunization/programmes_systems/policies_strategies/Tunisia-School-Immunization.pdf?ua=1 [accessed: 18 September 2018].
- [53].World health organization. School immunization programme in Malaysia. Available from: http://www.who.int/immunization/programmes_systems/policies_strategies/Malaysia-school-immunization.pdf?ua=1 [accessed: 18 September 2018].
- [54].Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013;62:1–28. [PubMed] [Google Scholar]
- [55].Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016;4:e453–63. [DOI] [PubMed] [Google Scholar]
- [56].World health organization. School immunization programme in Sri Lanka. Available from: http://www.who.int/immunization/programmes_systems/policies_strategies/SriLanka-school-immunization.pdf?ua=1 [accessed: 18 September 2018].
- [57].World health organization. WHO-UNICEF estimates of national immunization coverage (WUENIC) 2018. Available from: https://www.who.int/immunization/monitoring_surveillance/data/en [accessed: 1 September 2019].
- [58].Brown DW, Gacic-Dobo M. Leveraging delivery of immunization services through schools to motivate improved current ownership of home-based records used to record vaccination services – an opportunity to further explore. New York City, New York UNICEF; 2015. [Google Scholar]
- [59].Zhang D, Mu Q, Dai L. Effect of evaluation on school entry immunization in Guizhou during 2003-2009 Modern Preventive Medicine. 2013;40:855–67. [Google Scholar]
- [60].Zhang M, Ran Z, Zheng J. Impact of immunization certificate examination on coverage rates of national immunization program vaccines among children entering kindergarten and school. Chin J Vaccines Immunization 2016;22:606–10. [Google Scholar]
- [61].MacDonald NE, Harmon S, Dube E, Steenbeek A, Crowcroft N, Opel DJ, et al. Mandatory infant & childhood immunization: Rationales, issues and knowledge gaps. Vaccine 2018;36:5811–8. [DOI] [PubMed] [Google Scholar]
- [62].Brown DW, Gacic-Dobo M. Home-based record prevalence among children aged 12–23 months from 180 demographic and health surveys. Vaccine 2015;33:2584–93. [DOI] [PubMed] [Google Scholar]
- [63].World health organization and UNICEF. Cameroon: WHO and UNICEF estimates of immunization coverage: 2017 revision. Available from: http://www.who.int/immunization/monitoring_surveillance/data/cmr.pdf [accessed: 2 September 2018].
- [64].United States agency for international development (USAID). The DHS program - where we work. Available from: https://dhsprogram.com/Where-We-Work [accessed: 18 September 2018].
- [65].Wei X Current status of school entry vaccination certificate inspection in China. Chin J School Health 2011;32:711–2. [Google Scholar]
- [66].Young SL, Gacic-Dobo M, Brown DW. Results from a survey of national immunization programmes on home-based vaccination record practices in 2013. Int Health 2015;7:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Brown DW, Gacic-Dobo M, Young SL. Home-based child vaccination records–a reflection on form. Vaccine 2014;32:1775–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.