Abstract
Outer-membrane vesicles (OMVs) produced by Helicobacter pylori deliver bacterial components to host cells, provide a mechanism for stabilization of secreted components and may allow the bacteria to exert ‘long-range’ effects in the gastric niche, promoting persistence. In addition to their well-characterized host cell interactions, membrane vesicles improve stress survival in other bacterial species, and are constitutively produced by both pathogenic and non-pathogenic bacteria. We aimed to determine whether OMVs could improve H. pylori survival of a range of stressors. The effects of purified OMVs on the resistance of H. pylori to a range of environmental and antimicrobial stresses were determined using growth curves and survival assays. Addition of purified OMVs to H. pylori cultures provided dose-dependent protection against hydrogen peroxide-mediated killing. Supplementation with OMVs also partially protected H. pylori against the bactericidal effects of the antibiotics clarithromycin and levofloxacin, but not against amoxicillin nor metronidazole. Addition of purified OMVs allowed H. pylori to grow in the presence of inhibitory concentrations of the antimicrobial peptide LL-37. In the presence of 50 µg OMVs ml−1, significantly enhanced H. pylori growth was observed at higher LL-37 concentrations compared with lower LL-37 concentrations, suggesting that OMV–LL-37 interactions might facilitate release of growth-promoting nutrients. Taken together, these data indicate that production of membrane vesicles could help H. pylori to survive exposure to antibiotics and host antimicrobial defences during infection.
Keywords: antimicrobial, antibiotic resistance, Helicobacter pylori, membrane vesicles, survival, stress
Introduction
Helicobacter pylori is a Gram-negative, microaerophilic bacterium that infects the human stomach during early childhood. If untreated, infection persists lifelong despite a robust immune response [1] and causes asymptomatic gastritis, which may progress to ulceration and the development of gastric cancer [2, 3]. Treatment of H. pylori infection typically comprises a combination of amoxicillin with either clarithromycin or metronidazole administered with a proton pump inhibitor, but the failure rates of first-line triple therapies, particularly those containing clarithromycin [4], have been climbing. A range of alternative triple and quadruple therapies are now recommended, depending on the local antibiotic-resistance rates [5]. Levels of antibiotic resistance in H. pylori are escalating [4–7] and clarithromycin-resistant H. pylori was recently listed as one of the world’s highest priority antibiotic-resistant pathogens of concern by the World Health Organization [8]. There is a need for alternative therapies and better understanding of how H. pylori is able to persist lifelong in the harsh gastric environment.
Gram-negative bacteria, including H. pylori , constitutively release outer-membrane vesicles (OMVs) during normal growth [9, 10]. OMVs are small (20–300 nm), spherical vesicles predominantly containing outer membrane and periplasmic components from the bacterial cell [11]. H. pylori OMVs contain virulence factors including the toxin VacA [10, 12, 13] and are readily taken up by host cells [14, 15].
Roles for bacterial OMVs in host–pathogen interactions have been widely reported (reviewed by Schwechheimer and Kuehn, and MacDonald and Kuehn [11, 16]), but non-pathogenic bacteria also produce OMVs. Production of OMVs is upregulated in response to, and associated with survival of, stress in Pseudomonas aeruginosa [17] and Escherichia coli [18, 19], and OMVs contribute to bacterial survival of antibiotic treatment in E. coli [20, 21] and Pseudomonas syringae [22].
Protective effects of OMVs against oxidative stress in H. pylori were recently reported using strains P12 and 18943 [23]. If the production of OMV can help H. pylori to survive stressors such as the host immune response and antibiotic treatment, then it might be possible to increase the susceptibility of H. pylori to immunity and therapy, and/or reduce the virulence of the infection, by designing new therapies that interfere with vesiculation.
In this study, we aimed to determine whether OMVs could protect H. pylori against a range of stressors. Hydrogen peroxide and the cathelicidin derivative LL-37, a cationic antimicrobial peptide involved in the human gastric mucosal defence against H. pylori [24], were used to simulate immune-mediated stressors, and the protective effects of OMVs against antibiotics commonly used to treat H. pylori infections were also assessed.
Methods
Culture of H. pylori
H. pylori strain 60190 was provided by Professor John Atherton and his team at the University of Nottingham, UK. The bacteria were routinely cultured on blood agar base no. 2 (Oxoid) supplemented with 7.5 % defibrinated horse blood (TCS Biosciences) under microaerobic conditions (85 % N2, 10 % CO2, 5 % O2) at 37 °C.
Purification of OMVs
For most of the reported assays, OMVs were isolated from late exponential or early stationary phase broth cultures of H. pylori in serum-free media [brain heart infusion (BHI) broth supplemented with 0.2 % β-cyclodextrin]. For some of the antimicrobial survival assays, to improve the yield of OMVs (since H. pylori growth in serum-free liquid media is very slow), OMVs were isolated directly from bacteria that were grown on agar plates then re-suspended in media. Bacterial cells were removed by centrifugation at 4000 g for 10 min and sequential filtration of the culture supernatant through 0.45 and 0.20 µm syringe filters. OMVs were purified from the culture supernatants by centrifugation at 100 000 g for 2 h at 4 °C, with a preceding 40 % ammonium sulfate precipitation step to concentrate the secreted proteins and OMVs from larger culture volumes, as previously described [25]. The OMV pellets were washed using particle-free Dulbecco’s PBS (Sigma Aldrich) and finally re-suspended in 200–500 µl PBS. OMVs were quantified using a Pierce BCA protein assay (Fisher Scientific) and stored promptly at −20 °C until use.
Hydrogen peroxide survival assay
H. pylori were grown for 24 h on blood agar and then suspended to OD600 0.1 in Iso-Sensitest broth (Oxoid) + 5 % (v/v) FCS (Sigma Aldrich). Bacteria were mixed with a final concentration of 0–50 µg OMVs ml−1 or 0.1 % (w/v) bovine catalase in triplicate in sterile 96-well plates and then H2O2 was added to all wells to a final concentration of 1 mM. After 2.5 h incubation at 37 °C, samples were taken from each well and diluted into 1 % (w/v) bovine catalase to inactivate any residual H2O2 before Miles and Misra determination of the number of c.f.u. ml−1.
Growth inhibition assay
H. pylori were grown for 24 h on blood agar and then suspended in BHI broth + 0.2 % β-cyclodextrin to OD600 0.1, supplemented with either 50 µg OMVs ml−1 in PBS or an equal volume of PBS without OMVs. Bacteria with and without OMVs were then incubated with 0.25–1.0 µg amoxicillin ml−1 (Sigma Aldrich) or 1.25–5.0 µg LL-37 ml−1 (InvivoGen) in 96-well plates under microaerobic conditions, and bacterial growth was monitored by measuring the OD600 at 24 h intervals for 1 week.
Antimicrobial survival assays
Bacteria were adjusted to OD600 0.1 in BHI broth + 0.2 % β-cyclodextrin and treated with the antimicrobial peptide LL-37 (InvivoGen) or the antibiotics amoxicillin, clarithromycin, metronidazole or levofloxacin (all from Sigma Aldrich) at the concentrations indicated in the figures, in the presence of 0–25 µg purified OMVs ml−1. Survival assays were set up in triplicate wells in sterile 96-well plates, in a total volume of 100 µl per well. After incubation in microaerobic conditions at 37 °C for 3 h, the surviving bacteria were quantified by serial dilution and plating out. Data were expressed as c.f.u. ml−1 and as percentage survival compared to the bacterial c.f.u. ml−1 in untreated control wells.
Heat treatment of OMVs
Heat-treated OMVs were prepared by heating purified OMVs at 80 °C for 10 min, cooling to room temperature and then briefly centrifuging the heated OMV suspension to bring all liquid to the bottom of the tube.
Statistical analysis
GraphPad Prism 8.1.2 was used for statistical analysis and figure generation. The tests used are indicated in each figure.
Results
Membrane vesicles can protect H. pylori against hydrogen peroxide-mediated killing in a dose-dependent manner
H. pylori OMVs are enriched with catalase [23], which is thought to contribute to bacterial survival of oxidative stress in the human stomach. We treated H. pylori with 1 mM H2O2 and measured bacterial survival using colony counts after 2.5 h. H2O2 treatment caused greater than 8-log reduction in c.f.u. ml−1 compared to a control that included 0.1 % bovine catalase to inactivate the H2O2 (Fig. 1).
Fig. 1.
Viability of H. pylori in the presence of 1 mM H2O2 is restored by supplementation with OMVs. Bacteria were incubated at 37 °C in Iso-Sensitest broth, 5 % (v/v) FCS with 1 mM H2O2 and viability determined after 2.5 h by the Miles and Misra method. Triplicate means ± sd are shown. Bacterial viability was reduced by H2O2 treatment compared to the control in which H2O2 was inactivated using 0.1 % (w/v) bovine catalase (bKatA). Bacterial viability was restored to control levels by the addition of purified OMVs, in a dose-dependent manner. Asterisks indicate statistically significant differences compared to H2O2-treated bacteria without the addition of bKatA or OMVs (non-parametric Kruskal–Wallis test with multiple comparisons by Dunn’s test; multiplicity corrected P values reported; *P<0.05).
To assess potential protective effects of OMVs against oxidative stress, OMVs were purified from stationary phase serum-free H. pylori culture supernatant by filtration and ultracentrifugation, suspended in PBS and confirmed free of viable bacteria by incubating samples on blood agar plates under microaerobic conditions for several days. Addition of OMVs protected H. pylori against H2O2-mediated killing in a dose-dependent manner (Fig. 1; P<0.05). Concentrations of OMVs at or above 12.5 µg ml−1 provided similar levels of protection against H2O2 to 0.1 % bovine catalase.
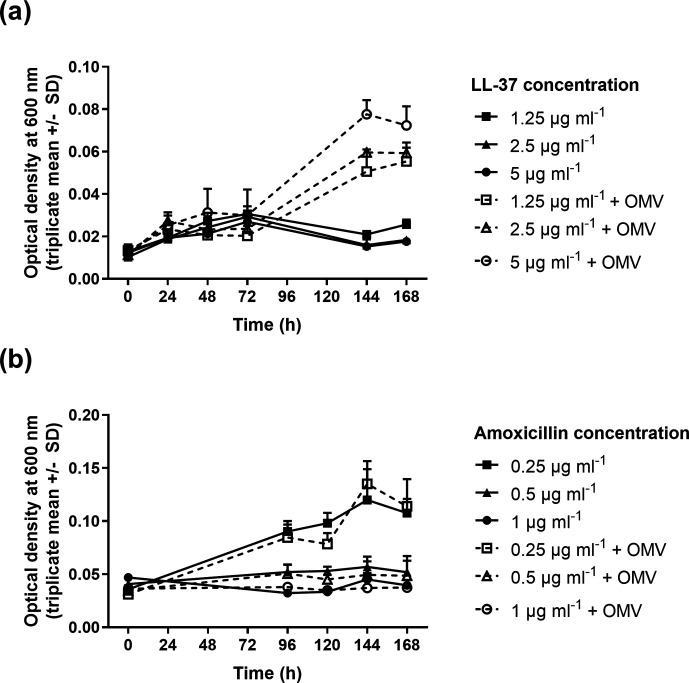
Supplementation with membrane vesicles allows H. pylori to grow in the presence of the antimicrobial peptide LL-37
LL-37 is a cationic antimicrobial peptide involved in mucosal immune defence, and OMVs have previously been shown to protect Vibrio cholerae against this peptide [26]. LL-37 inhibited the growth of H. pylori when included in the growth media at concentrations between 1.25 and 5 µg ml−1, but growth in the presence of all concentrations of LL-37 was significantly enhanced (P<0.05) when the cultures were supplemented with 50 µg OMVs ml−1 (Fig. 2a). OMV-mediated growth promotion was greatest at the highest LL-37 concentration. OMVs were not able to enhance the growth of H. pylori in the presence of amoxicillin (Fig. 2b).
Fig. 2.
OMVs can protect H. pylori against the antimicrobial peptide LL-37, but not against amoxicillin. Bacteria were grown in different concentrations of LL-37 (a) or amoxicillin (b) as indicated by the keys, with (dashed lines) or without (solid lines) supplementation with 50 µg purified OMVs ml−1 . Growth of H. pylori 60190 in BHI + 0.2 % β-cyclodextrin was inhibited by 1.25–5 µg LL-37 ml−1 . When the bacteria were supplemented with 50 µg purified OMVs ml−1, growth was significantly enhanced (endpoint P<0.05, two-way ANOVA with Tukey post hoc tests) (a). OMVs did not enhance the growth of H. pylori in the presence of amoxicillin (b).
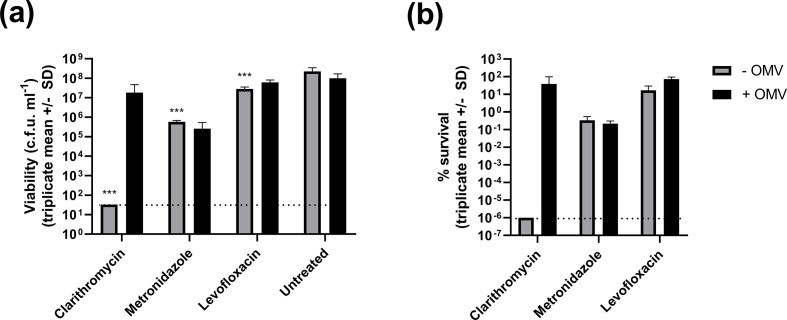
Membrane vesicles can promote H. pylori survival of antibiotic treatment
H. pylori incubated for 3 h in the presence of clarithromycin, metronidazole or levofloxacin had significantly reduced survival compared to untreated control bacteria (P<0.001 for each antibiotic, two-way ANOVA with Dunnett’s multiple comparisons tests). Addition of 25 µg purified OMVs ml−1 improved bacterial survival in the presence of clarithromycin and levofloxacin, but not metronidazole (Fig. 3).
Fig. 3.
Protective effects of OMVs against clarithromycin treatment. H. pylori were incubated for 3 h in the presence of 10 µg clarithromycin ml−1, 100 µg metronidazole ml−1 or 10 µg levofloxacin ml−1. These concentrations of drug were sufficient to significantly reduce bacterial survival (***P<0.001, two-way ANOVA with Dunnett’s multiple comparisons tests versus the untreated control group). The limit of detection, 33 c.f.u. ml−1, is indicated by dashed lines. Supplementation with 25 µg purified OMVs ml−1 improved bacterial survival of clarithromycin and levofloxacin treatment, but not metronidazole. After OMV supplementation, bacterial survival was not significantly different to the untreated control group. Data shown are mean c.f.u. ml−1 ± sd (a) and percentage survival compared to the untreated control group (b) for three independent replicates.
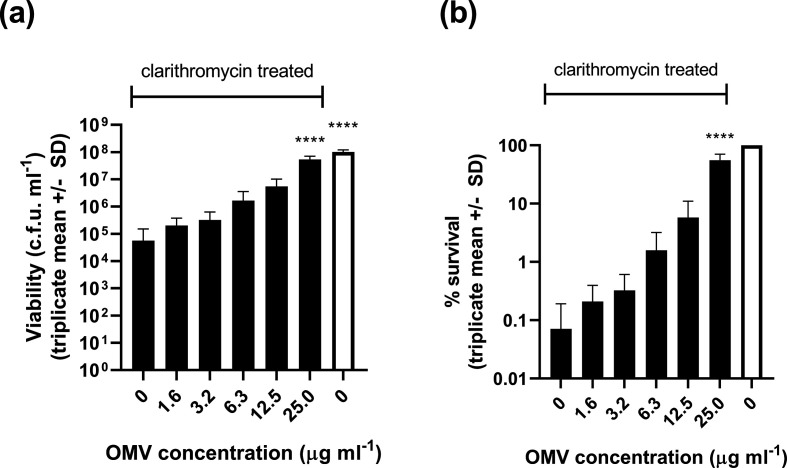
Since the protective effect of OMVs was most dramatic for clarithromycin treatment, we studied it in more detail. We reduced the clarithromycin concentration from 10 to 5 µg ml−1 (sufficient to cause a several log reduction in bacterial viability) and tested a range of OMV concentrations for potential protective effects. OMVs protected H. pylori against clarithromycin-mediated killing in a dose-dependent manner (Fig. 4) and the protective effect of OMVs was not ablated by pre-heating them to 80 °C for 10 min to inactivate any OMV-associated enzymes (Fig. 5).
Fig. 4.
OMV-mediated protection of H. pylori against clarithromycin treatment is dose-dependent. Bacteria were suspended in BHI broth and exposed to 5 µg clarithromycin ml−1 (black bars) with and without the addition of the indicated concentrations of purified OMVs. After 3 h incubation at 37 °C under microaerobic conditions, the surviving bacteria were quantified by serial dilution and plating. Data are expressed as c.f.u. ml−1 (a) and percentage survival compared to untreated bacteria (b). Untreated bacteria (white bars) were incubated in BHI broth without clarithromycin and OMVs. Mean values ± sd from three independent replicates are shown. Addition of 25 µg OMVs ml−1 significantly protected H. pylori against clarithromycin treatment (****P<0.0001, one-way ANOVA with Dunnett’s multiple comparison test versus the clarithromycin-treated control without addition of OMVs).
Fig. 5.
Heat treatment does not prevent OMV-mediated protection of H. pylori against clarithromycin. Bacteria were suspended in BHI broth and exposed to 5 µg clarithromycin ml−1 (black bars) with and without 25 µg purified OMVs ml−1. The OMV label indicates that normal purified OMVs were added. OMV + heat indicates that the OMVs were pre-treated with heat (80 °C for 10 min) before use in the assay. After 3 h incubation at 37 °C under microaerobic conditions, the surviving bacteria were quantified by serial dilution and plating. Data are expressed as c.f.u. ml−1 (a) and percentage survival compared to untreated bacteria (b). Untreated bacteria (white bar) were incubated in BHI broth without clarithromycin and OMVs. Mean values ± sd from three independent replicates are shown. The limit of detection, 33 c.f.u. ml−1, is indicated by a dashed line. Addition of 25 µg OMVs ml−1 significantly protected H. pylori against clarithromycin treatment (**P<0.01, one-way ANOVA with Tukey’s multiple comparison tests) and there was no significant difference in the protective effects of heat-treated versus non-heat-treated OMVs. ns, No significant difference.
Discussion
Constitutive production of OMVs during growth is now understood to be a well-characterized and highly conserved feature of all Gram-negative bacteria studied to date. Continuous packaging and export of cellular components is energetically expensive, so OMV secretion must perform some important beneficial functions. Given that OMV production is upregulated in response to stress in some species, the contribution of vesiculation to bacterial survival of environmental stress has been proposed as one such universal benefit of OMV production [17–19].
H. pylori is able to persist in the harsh environment of the human stomach for decades, despite a vigorous immune response by the host [1]. Consistent with the recent report by Lekmeechai et al. [23], but using a different H. pylori strain, we found that addition of purified OMVs could enhance H. pylori survival of hydrogen peroxide treatment in a dose-dependent manner. Using a katA mutant, Lekmeechai et al. [23] showed that the protective effects of H. pylori OMV against hydrogen peroxide were mediated by the catalase enzyme.
We also found that OMVs were protective against the antimicrobial peptide LL-37 that is produced by human gastric epithelial cells in response to infection and is bactericidal to H. pylori [24]. This protective effect is presumably due to sequestration of LL-37 by OMVs, as previously shown for other membrane active antimicrobial peptides in other bacterial species, for example E. coli and P. syringae OMVs versus colistin and melittin [20, 22], and V. cholerae versus polymyxin B and LL-37 [26].
Addition of OMVs alleviated the LL-37-mediated inhibition of H. pylori growth at all LL-37 doses tested but, curiously, growth of OMV-supplemented H. pylori was greatest at the highest concentrations of LL-37. It is unclear how OMV addition might have caused this observed trend, but it is possible that the lysing of OMVs by LL-37 resulted in the dispersal of packaged nutrients for the surviving H. pylori cells. Alternatively, proteolytic enzymes in the OMVs might have digested LL-37, effectively supplementing the culture media with amino acids to promote bacterial growth. Further mechanistic studies will be needed to characterize the interactions between bacterial OMVs and LL-37.
Treatment of H. pylori and other bacterial infections is becoming increasingly difficult due to the development of antibiotic resistance. Improved understanding of bacterial mechanisms of antibiotic resistance and tolerance could help inform the design of new treatments, so we investigated the potential for OMVs to protect H. pylori against exposure to antibiotics. Although OMV-mediated protection against β-lactam antibiotics has been reported for other species, for example Staphylococcus aureus [27] and Acinetobacter baumannii [28], this protection was dependent on the activity of β-lactamase enzyme exported with or inside the vesicles. Since the development of amoxicillin resistance in H. pylori does not depend on β-lactamase production [29], it is unsurprising that H. pylori-derived OMVs were not directly protective against amoxicillin.
We observed a modest protective effect of OMVs against levofloxacin treatment, but not against metronidazole. In contrast, OMV-mediated protection of H. pylori against exposure to clarithromycin was dramatic and dose-dependent. OMVs were still able to protect H. pylori against clarithromycin treatment after heat treatment at 80 °C, indicating that a heat-labile enzymatic activity was not likely to be responsible for this protective effect, although more comprehensive analysis will be required to definitely rule out an enzyme-based mechanism. Clarithromycin is a macrolide antibiotic that inhibits protein synthesis by targeting the 23S rRNA region in the 50S ribosomal subunit. Mutations A2143G and A2142G/C in the target region of the 23S rRNA are the most common causes of clarithromycin resistance in H. pylori in Europe (reviewed by Xuan et al. [30]). The mechanism driving OMV-mediated protection of H. pylori against clarithromycin in our study has not yet been elucidated. Simple sequestration is one possible explanation – clarithromycin is a hydrophobic antibiotic and enters bacterial cells via lipid-mediated passive diffusion [31, 32], and macrolide antibiotics can bind directly to lipid membranes [33], so OMVs may have acted as a decoy taking up clarithromycin that would otherwise have diffused into bacterial cells. However, levofloxacin has intermediate lipophilicity and metronidazole is also lipophilic, so a comprehensive study of drug–OMV interactions is needed to assess the potential for vesicles to adsorb each drug. The presence or absence of potential molecular targets for clarithromycin in OMVs, and their binding affinities for the drug, should also be determined. Recent genomic analysis of clarithromycin-sensitive and -resistant H. pylori strains has identified additional mutations associated with clarithromycin susceptibility, including membrane proteins [34], and the expression of some outer-membrane proteins is upregulated in clarithromycin-resistant strains [35].
Further work is needed to elucidate the mechanisms by which H. pylori OMVs can protect the bacteria against LL-37 and clarithromycin, and to assess whether OMV-mediated survival and growth promotion are biologically relevant in vivo. It is not yet clear what concentrations of OMVs are present in vivo during bacterial infections, but it is possible that local OMV concentrations could become relatively high in the context of biofilm and/or thick mucus layers. Further study of in vivo OMV production is needed.
In some other bacterial species, exposure to stressors has been shown to cause upregulation of vesiculation [19] and/or modulation of OMV contents [26], and it would be useful to determine which antimicrobial treatments might modulate the rate of vesiculation by H. pylori . Comprehensive mapping of the OMV biogenesis pathways of H. pylori is also needed, to identify candidate targets for novel anti-vesiculation drugs. If such drugs could be developed, they might reduce bacterial stress survival and virulence by disabling OMV production, in turn potentiating the antibacterial activities of conventional antibiotics and the host immune response.
Funding information
The authors received no specific grant from any funding agency. L.M.A. contributed to this work while studying for postgraduate qualifications, sponsored by the Government of the State of Kuwait, represented by the Kuwait Cultural Office of the Embassy of the State of Kuwait in London.
Acknowledgements
We thank John Atherton and team at the University of Nottingham for providing H. pylori strain 60190.
Author contributions
Conceptualization, supervision and writing original draft: J.A.W. Investigation, formal analysis, review and editing: all authors.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviation: OMV, outer-membrane vesicle.
Edited by: J. Cavet and K. Robinson
References
- 1.Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 4.Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 6.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miendje Deyi VY, Lare MS, Burette A, Ntounda R, Elkilic O, et al. Update of primary Helicobacter pylori resistance to antimicrobials in Brussels, Belgium. Diagn Microbiol Infect Dis. 2019;95:114875. doi: 10.1016/j.diagmicrobio.2019.114875. [DOI] [PubMed] [Google Scholar]
- 8.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 9.Keenan J, Day T, Neal S, Cook B, Perez-Perez G, et al. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol Lett. 2000;182:259–264. doi: 10.1111/j.1574-6968.2000.tb08905.x. [DOI] [PubMed] [Google Scholar]
- 10.Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188:220–226. doi: 10.1002/(SICI)1096-9896(199906)188:2&lt;220::AID-PATH307&gt;3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullaney E, Brown PA, Smith SM, Botting CH, Yamaoka YY, et al. Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori . Proteomics Clin Appl. 2009;3:785–796. doi: 10.1002/prca.200800192. [DOI] [PubMed] [Google Scholar]
- 13.Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539–1555. doi: 10.1111/j.1365-2958.2010.07307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olofsson A, Nygård Skalman L, Obi I, Lundmark R, Arnqvist A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. mBio. 2014;5:e00979-14. doi: 10.1128/mBio.00979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner L, Bitto NJ, Steer DL, Lo C, D'Costa K, et al. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front Immunol. 2018;9:1466. doi: 10.3389/fimmu.2018.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163:607–618. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa . J Bacteriol. 2013;195:2971–2981. doi: 10.1128/JB.02267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwechheimer C, Kuehn MJ. Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli . J Bacteriol. 2013;195:4161–4173. doi: 10.1128/JB.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram‐negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni HM, Nagaraj R, Jagannadham MV. Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol Res. 2015;181:1–7. doi: 10.1016/j.micres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni HM, Swamy CVB, Jagannadham MV. Molecular characterization and functional analysis of outer membrane vesicles from the Antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J Proteome Res. 2014;13:1345–1358. doi: 10.1021/pr4009223. [DOI] [PubMed] [Google Scholar]
- 23.Lekmeechai S, Su Y-C, Brant M, Alvarado-Kristensson M, Vallström A, et al. Helicobacter pylori outer membrane vesicles protect the pathogen from reactive oxygen species of the respiratory burst. Front Microbiol. 2018;9:1837. doi: 10.3389/fmicb.2018.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori . Gastroenterology. 2003;125:1613–1625. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Winter J, Letley D, Rhead J, Atherton J, Robinson K. Helicobacter pylori membrane vesicles stimulate innate pro- and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect Immun. 2014;82:1372–1381. doi: 10.1128/IAI.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duperthuy M, Sjöström AE, Sabharwal D, Damghani F, Uhlin BE, et al. Role of the Vibrio cholerae matrix protein BAP1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 2013;9:e1003620. doi: 10.1371/journal.ppat.1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Lee E-Y, Kim S-H, Kim D-K, Park K-S, et al. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob Agents Chemother. 2013;57:2589–2595. doi: 10.1128/AAC.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y-T, Kuo S-C, Chiang M-H, Lee Y-T, Sung W-C, et al. Acinetobacter baumannii extracellular OXA-58 is primarily and selectively released via outer membrane vesicles after Sec-dependent periplasmic translocation. Antimicrob Agents Chemother. 2015;59:7346–7354. doi: 10.1128/AAC.01343-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Co E-MA, Schiller NL. Resistance mechanisms in an in vitro-selected amoxicillin-resistant strain of Helicobacter pylori . Antimicrob Agents Chemother. 2006;50:4174–4176. doi: 10.1128/AAC.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xuan S-H, Wu L-P, Zhou Y-G, Xiao M-B. Detection of clarithromycin-resistant Helicobacter pylori in clinical specimens by molecular methods: a review. J Glob Antimicrob Resist. 2016;4:35–41. doi: 10.1016/j.jgar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Doucet-Populaire F, Capobianco JO, Zakula D, Jarlier V, Goldman RC. Molecular basis of clarithromycin activity against Mycobacterium avium and Mycobacterium smegmatis . J Antimicrob Chemother. 1998;41:179–187. doi: 10.1093/jac/41.2.179. [DOI] [PubMed] [Google Scholar]
- 32.Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosol S, Schrank E, Krajačić MB, Wagner GE, Meyer NH, et al. Probing the interactions of macrolide antibiotics with membrane-mimetics by NMR spectroscopy. J Med Chem. 2012;55:5632–5636. doi: 10.1021/jm300647f. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Ye L, Jin L, Xu X, Xu P, et al. Application of next-generation sequencing to characterize novel mutations in clarithromycin-susceptible Helicobacter pylori strains with A2143G of 23S rRNA gene. Ann Clin Microbiol Antimicrob. 2018;17:10. doi: 10.1186/s12941-018-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smiley R, Bailey J, Sethuraman M, Posecion N, Showkat Ali M. Comparative proteomics analysis of sarcosine insoluble outer membrane proteins from clarithromycin resistant and sensitive strains of Helicobacter pylori . J Microbiol. 2013;51:612–618. doi: 10.1007/s12275-013-3029-5. [DOI] [PubMed] [Google Scholar]