Abstract
Native mass spectrometry (nMS) is increasingly used for studies of large biomolecules (>100 kDa), especially proteins and protein complexes. The growth in this area can be attributed to advances in native electrospray ionization as well as instrumentation that is capable of accessing high mass-to-charge (m/z) regimes without significant losses in sensitivity and resolution. Here, we describe modifications to the ESI source of an Agilent 6545XT Q-TOF MS that is tailored for analysis of large biomolecules. The modified ESI source was evaluated using both soluble and membrane protein complexes ranging from ~127 to ~232 kDa and the ~801 kDa protein chaperone GroEL. The increased mass resolution of the instrument affords the ability to resolve small molecule adducts and analyze collision-induced dissociation products of the native complexes.
Keywords: Native mass spectrometry, extended mass range Q-TOF, AmtB mutant, GroEL
GRAPHICAL ABSTRACT
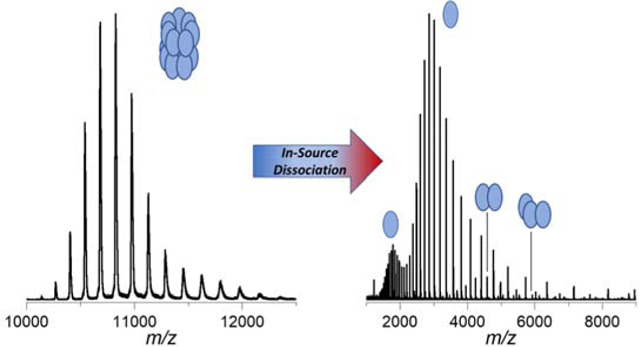
INTRODUCTION
Advancements in biophysical techniques drive greater understanding and deeper interrogation of the structure and function of proteins and protein complexes. Contributions of mass spectrometry (MS) towards studies of protein function have largely been realized through proteomics, including both bottom-up and top-down methods for analysis of protein primary structure.1, 2 These methods are often optimized using denaturing conditions to expose more amino acids to proteolytic digestion in solution or fragmentation in the gas phase. More recently developed methods include those used in studies of intact protein complexes, where solution-phase conformations and noncovalent contacts are retained in the transition from solution to the gas phase.3, 4 Recent developments in these areas are frequently referred to as native MS (nMS) and native ion mobility-MS (nIM-MS). One major focus of nMS has made possible studies of protein complexes with aims to develop better understanding of quaternary structure, including stoichiometry and topology.
Challenges for nMS of intact proteins and protein complexes include developing better understanding of how the solution conditions (e.g. buffer, cosolutes, solvent) influence protein structure and ionization.5–8 Additionally, transmission of ions with high mass-to-charge ratios (m/z) represents a challenge that has been overcome by development of state-of-the-art MS instruments.9–11 Due to the high m/z of large, intact protein complexes (>100 kDa), high resolving power is needed to separate signals with smaller mass shifts. Separation of these signals makes possible the identification of sequence modifications (e.g. truncations, mutations, post-translational modifications (PTM)) and binding of endogenous ligands, salts, and other small molecule adducts.11 Efficient desolvation helps to remove solvent and volatile buffer solutes from droplets formed by electrospray ionization (ESI), leading to narrower ion signals and greater ability to resolve what would otherwise be hidden.11 One method for enhancing desolvation includes the use of small, low flow emitters (1–3 μm inner diameter, nL/min flow) that promote formation of smaller droplets compared to traditional direct infusion nanoESI, and their usage in nMS studies has been invaluable.12, 13 As the transition towards studies of larger (kDa to MDa) biomolecular complexes have progressed, numerous types of ESI emitters and ion source designs have evolved to accommodate static-spray nanoESI.14
Here, we report results obtained using a quadrupole-time-of-flight (Q-TOF) instrument (Agilent 6545XT Q-TOF MS) that has extended mass range (m/z 30,000) and a modified static nanospray ESI emitter (1–10 μm O.D.) for nMS studies of protein complexes. The performance of the instrument is demonstrated using results obtained for several soluble protein complexes, viz. alcohol dehydrogenase (tetramer, 147 kDa), pyruvate kinase (tetramer, 232 kDa), a trimeric 127 kDa membrane protein complex (ammonium transport channel, AmtB), and the tetradecameric 801 kDa protein chaperonin GroEL from E. coli. This study reports enhanced performance (in terms of m/z range and resolution) of a Q-TOF mass spectrometer for large native, intact protein complexes. Additionally, interrogation of low relative abundance and low molecular weight adducts was performed on ions formed by collision-induced dissociation (CID) of the native protein complexes.
EXPERIMENTAL
Materials
Ammonium acetate and lyophilized powders of yeast alcohol dehydrogenase and rabbit muscle pyruvate kinase were purchased from Sigma-Aldrich (St. Louis, MO). Wild type and double mutant (E87C, C312T) AmtB protein complex from E. coli was expressed and purified as previously described.15 The GroEL chaperonin complex was purified as previously described.16 More detailed information for these complexes is provided in Supplemental Table S1. All buffers and lyophilized protein samples were dissolved in 18.2 MΩ·cm water (Barnstead Easy Pure II, Thermo Scientific). All samples were buffer exchanged to 200 mM aqueous ammonium acetate using Micro BioSpin P-6 gel columns (BioRad) at working concentrations of 1–10 μM. For AmtB, the samples were buffer exchanged into 200 mM ammonium acetate supplemented with 2x critical micelle concentration of tetraethylene glycol monooctyl ether (0.5% C8E4).
Mass Spectrometry
The protein samples were analyzed on an Agilent 6545XT Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA). Agilent MassHunter Acquisition (B.09) was used for data acquisition. The Agilent nanoelectrospray ionization (nanoESI) source was adapted using custom parts to enable static spray. Briefly, borosilicate capillary emitter tips were pulled in house using a flaming/brown micropipette puller (P-1000, Sutter Instruments), loaded with aqueous protein solution, and placed in a fabricated capillary tip holder containing a platinum wire as described previously (see Figures S1–S2 for details of the setup and technical drawings of the custom-fabricated tip holder).14 The tip holder was mounted onto the nanoESI capillary holder and positioned to within ~5–10 mm of the nanoESI spray shield. As the emitter solution is maintained at ground, no modification to the application of ESI potentials is required, allowing for the ease of transition between static spray and LC-compatible nanoESI. Capillary voltages are defined as the negative offset between the grounded emitter and the entrance to the ion transfer capillary (see Figure S1). Capillary voltages were adjusted to maximize ionization efficiency for each protein complex. For nMS analyses, capillary exit and skimmer cone potentials were optimized to maximize ionization and ion transmission through the source region while simultaneously minimizing dissociation.
For alcohol dehydrogenase (ADH) and protein kinase (PK), the capillary exit and skimmer potentials were adjusted to optimize transmission of intact protein complex ions or to promote collision-induced dissociation (see Supplemental Figures S3–S6). For example, in-source dissociation (ISD) is performed at high source (capillary exit >240 V; skimmer >260 V), leading to ejection of high charge monomer ions. At low source potentials, overall ion transmission is decreased leading to poor signal-to-noise ratios. The GroEL and AmtB collisional activation and collision-induced dissociation (CID) experiments were performed by increasing potentials in the collision cell (Figure S1), and N2 was used as a bath gas at the recommended backing pressure (22 psi). ISD experiments (for GroEL) were performed as described above by increasing the capillary exit potentials from 200 V to 380 V. Prior to analysis of protein samples, the instrument was tuned in high-mass mode (m/z 20,000) and appropriate mass ranges were selected for different experiments. Sliding scales for different mass ranges are available for m/z 90–10,000 through m/z 6,830–30,000 and were adjusted and recalibrated as needed to optimize for different experiments.
Data Analysis
All mass spectra were compiled using standard vendor procedures (Agilent MassHunter Qualitative Analysis (B.10) and BioConfirm (B.10)). Experimentally measured molecular weights (MW) were determined from peak maxima with average masses and standard deviations calculated from identified charge states. Theoretical MWs were calculated based on the appropriate UniProt sequences using ChemCalc.17, 18 Theoretical isoelectric points (pI) were obtained using the ExPASy web server and are shown in Table S1.19
RESULTS & DISCUSSION
For this study, we selected several large protein complexes ranging in size from 127 kDa to 801 kDa which had been used in prior studies that employed the extended m/z range (20 kDa m/z) Agilent 6560 IM-Q-TOF and homebuilt ion mobility-Orbitrap instruments.6, 10, 20–22 These complexes include two soluble proteins (alcohol dehydrogenase (ADH) and pyruvate kinase (PK)), an integral membrane protein complex (ammonium transport channel (AmtB)), and a chaperonin protein (GroEL) from E. coli.
Soluble Protein Complexes
Optimization of ion source conditions was performed using the homotetrameric protein complexes ADH and PK (see Experimental Section). Tuning the source potentials was necessary in order to optimize desolvation and ionization efficiencies. A compromise between maximizing ion transmission and minimizing dissociation was reached using similar source potentials for both protein complexes (capillary exit: 220 V; skimmer: 140 V).
Native mass spectra of intact ADH and PK are shown in Figure 1. The peaks for the intact ADH homotetrameric complex (Figure 1A) were observed to have a charge-state distribution (CSD) centered at 27+ and a measured MW of 147.5 kDa which is in good agreement with the theoretical MW (146.9 kDa). The discrepancy between the theoretical and measured MW of ADH is attributed to unresolved adducts and/or endogenous ligands; CID of ADH produces monomers having a range of adducted small molecules (see Supplemental Figure S7). The MW of the apo-ADH ejected monomer (36,726 ± 2 Da) is in excellent agreement with the theoretical monomer MW (36,718 Da).
Figure 1.
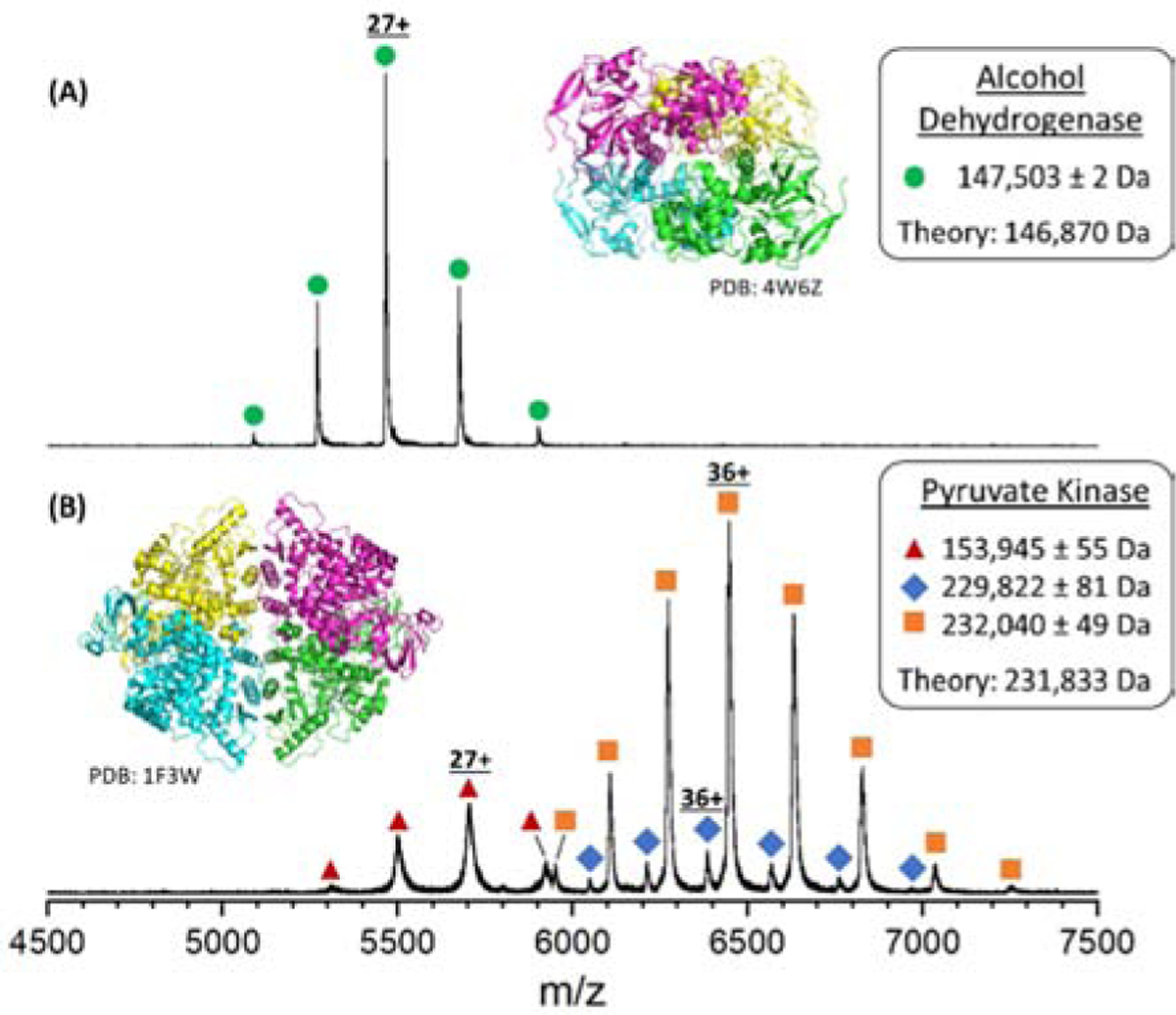
Native mass spectra of (A) alcohol dehydrogenase (ADH) and (B) pyruvate kinase (PK) reveal the intact, native protein complexes. Zero-charge molecular weights determined from the experimental charge-state distributions are shown in the inset boxes along with the theoretical MW. Differences between theoretical and zero-charge molecular weights is attributed to unresolved, nonspecific adducts that are common features of native mass spectra.23 The peaks labelled with red triangles (154 kDa) correspond to an unidentified species; however, these same signals have been reported previously as impurities (see text).
The native mass spectrum of pyruvate kinase (PK) (Figure 1B) contains signals that are assigned to three different complexes. The intact homotetrameric complex shows peaks centered at the 36+ charge state with a measured mass of 232.0 kDa, which agrees well with the MW of PK after removal of the initiator methionine and acetylation of the N-terminal serine (231.8 kDa).24 Low abundance signals centered at 36+, yield a measured MW of 229.8 kDa, tentatively assigned to ions from truncation of the first five N-terminal residues (1MSKSH5) of each subunit with a corresponding theoretical MW of 229.9 kDa for the resulting complex. A third CSD is observed that corresponds to a 153.9 kDa complex centered at 27+. The increased peak widths, much lower MW compared to that of the native tetramer complex, and unidentified stoichiometry suggest that this complex is not a simple sequence modification of the native PK complex. It appears more likely to be a contaminant in the lyophilized preparation. While the specific sequence of this contaminant remains unknown, similar signals have been reported previously from samples that were prepared in a similar manner as was done here.20, 25, 26
Membane protein complexes
Membrane protein complexes have become more important as pharmaceutical drug targets,27 yet their detection by nMS methods has remained challenging due to nonspecific adduction of lipids and their instability in aqueous, hydrophilic environments. Solubilizing of membrane protein complexes in detergent micelles removes the complexes from their supporting lipids without precipitation into solution.28 The detergent micelles are then removed via collisional activation, resulting in well-resolved CSDs. Here, a double mutant variant of the ammonium transport channel complex (DM-AmtB) as well as wild-type (WT-AmtB) are used to evaluate the capabilities of our modified instrument towards characterization of native membrane protein complexes.
Figure 2 contains native mass spectra for the double mutant (E87C, C312T) bacterial ammonium channel (DM-AmtB, 127 kDa) membrane protein complex. Abundant signals for the intact homotrimeric complex are centered at 16+ (Figure 2 A–B). The mass spectrum of the trimer contains a distribution of four species, viz. the apo-AmtB trimer complex and signals for up to three adducts that are shifted by 77 Da. We attribute the mass shifts to covalent disulphide-binding of the β-mercaptoethanol (β-ME) molecule to the cysteine side chain of the E87C mutant. Increasing the collision energy leads to monomer ejection, and the ejected monomer signals provide further evidence for the covalently bound β-ME molecules. The measured mass of the apo-DM-AmtB monomer ions (42,235 Da) is in reasonable agreement with the theoretical MW (42,273 Da). Similar β-ME modifications are not observed in the mass spectra of the wild-type AmtB (see Figure S8). Ejected monomers from WT-AmtB also have measured molecular weights (42,292 Da) in agreement with theoretical MW (42,301 Da). These results further illustrate the utility of the Q-TOF platform for analyzing membrane proteins with sufficient resolving power to identify small molecule adducts with and its potential for future ligand and lipid binding studies.14, 29
Figure 2. Native MS of a trimeric integral membrane protein complex.
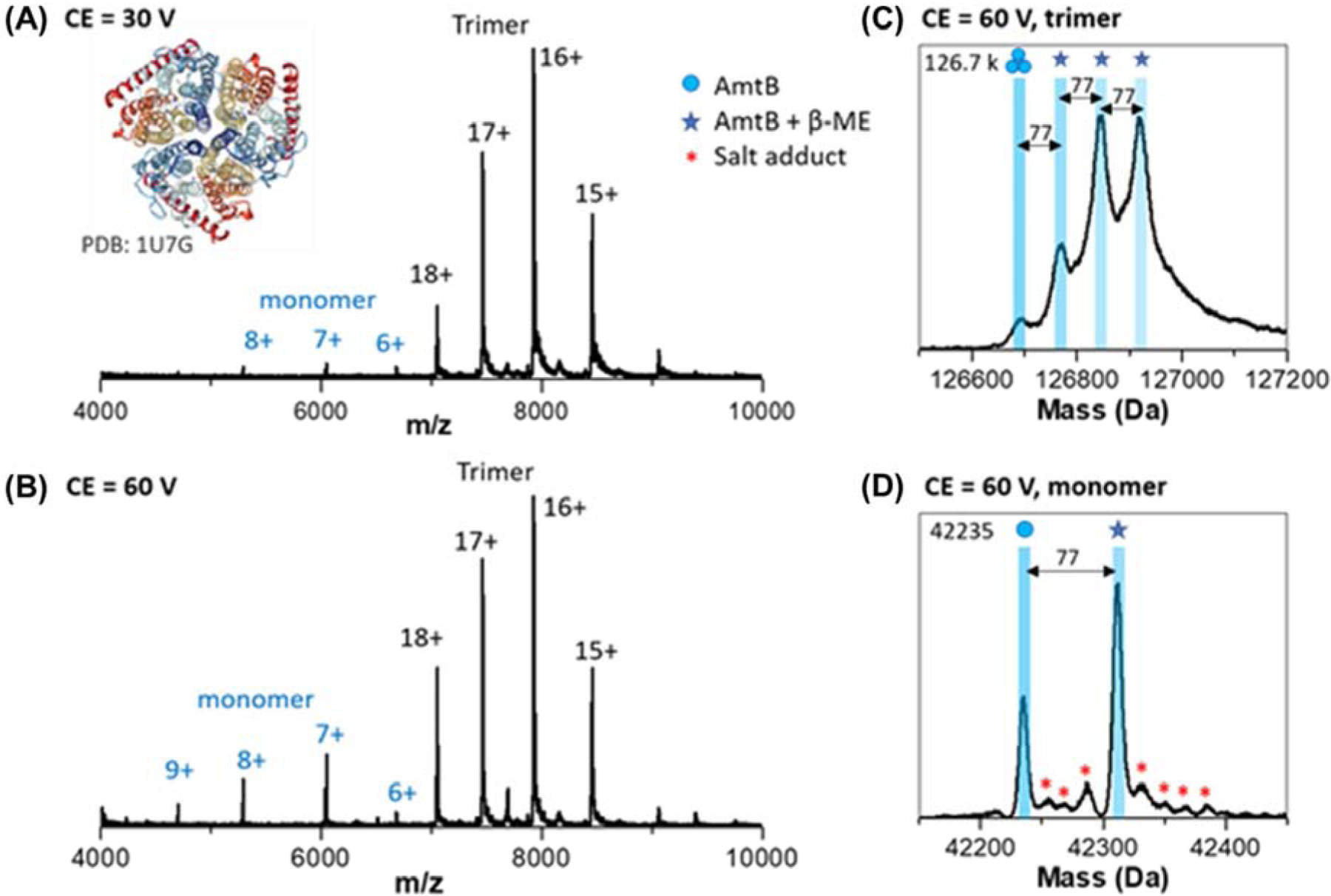
(A) mass spectrum of DM-AmtB at low collision energy (30 V) reveals the native intact trimer complex with a mass of 126.7 kDa. (B) Increasing the collision energy to 60 V leads to ejected monomer ions. (C) The zero-charge mass spectrum reveals the presence of up to three β-mercaptoethanol (β-ME) (77 Da adducts) on the intact trimer ions. (D) Zero-charge mass spectrum of ejected monomer also reveals low abundance salt adducts and a single β-ME adduct.
Chaperonin Complex (GroEL)
Native mass spectrometry of GroEL, a homotetradecameric (14-mer) complex, further illustrates the utility of the modified instrument for studies of high MW, sub-MDa protein complexes.20, 30–32 The native GroEL complex is observed in high relative abundance with well-resolved charge states when electrosprayed using mildly activating source potentials (Figure 3(A)). The measured mass determined under these conditions (801,088 Da) agrees well with the theoretical MW of the chaperonin complex (800,760. Da). The observed differences are consistent with nonspecific binding of salts and/or water molecules.20, 22, 33 The CSD observed under these conditions, centered at 74+, also agrees well with those presented by others using aqueous ammonium acetate solutions.30, 31, 34, 35
Figure 3.

Native mass spectrum for GroEL. (A) Mass spectrum of GroEL obtained using low source potential nESI contains abundance signals for intact 14-mer complex centered at 74+. The inset in (A) contains a narrow m/z range that illustrates the high resolution obtained for the intact 14-mer complex shown and the crystal structure of GroEL (PDB 1SS8). (B) In-source dissociation (ISD) mass spectrum of GroEL contains strong signals for unfolded and native-like monomer ions (charge states 20+ and 32+, respectively) as well as di- and tri-mer ions centered at 25+, and 29+, respectively. The measured MWs for each of the GroEL species are reported in the boxes. Note that the measured mass for the CSD labeled with an * (assigned as 51+ ions with MW of 465.9 kDa) does not agree with any known ions derived from GroEL (see text). It is possible that this species could be an 8-mer of GroEL, as an intact 7-mer with a monomer trapped inside the cavity along with water, salts, and other small molecules.
The measured masses of the intact GroEL 14-mer ions are observed to shift to lower mass by ~644 Da under conditions where ISD occurs (Figure 3B). This is evidence for loss of non-covalent adducts, salts, and/or water bound to the surface or internal cavity of GroEL. ISD of GroEL also leads to ejection of high charge state monomers with a bimodal CSD (centered at 20+ and 32+). The bimodal CSD of the monomer ions also provides evidence of non-uniform dissociation processes and agree well with CSDs previously observed following CID (32+) and SID (20+).30, 32 There are also low abundance signals corresponding to ejected dimer and trimer ions centered at 25+ and 29+, respectively. Dimer and trimer ions have been observed in previous studies of CID and by surface-induced dissociation (SID) of GroEL.31, 32 The subunit MW derived from the CID fragment ions (57,198 Da), is in excellent agreement with the experimental subunit MW of the activated 14-mer (57,173 Da) and the theoretical subunit MW of GroEL (57,197 Da).
Signals corresponding to a possible 8-mer (labeled with * in Figure 3) are observed at both low and high ISD collision energies, suggesting that the ions are not a product of GroEL 14-mer ions. The measured mass of these ions at high energy (465,588 ± 23 Da) would result from an octamer complex with subunit MW of 58,198.5 Da. While octameric ring complexes are common features of Group II chaperonins,36 they are unlikely to coexpress with Group I chaperonins like GroEL. This species may be an octamer of GroEL, wherein an unfolded monomer is bound to a 7-mer ring with the extra mass difference (ca. 1 kDa per subunit, 8 kDa total) due to salt, water, or small molecule binding to the interior and exterior of this complex. Further identification of the stoichiometry and topology of this contaminant species is ongoing.
The native GroEL complex was also examined using different CID energies (Figure 4). Note that increasing the collision cell voltage from 20 to 60 V results in shifts of the measured mass of the GroEL owing to removal of adducted species. At higher CID energies charge stripping of the 14-mer ions is observed as evidenced by shifts in the CSD towards lower charge states. Alternatively, the relative abundance of lower charge state ions (higher m/z) increases at higher CE owing to depletion of higher charge state ions. Note that at CE of 60 V low abundance 13-mer ions are detected, and at CE greater than 80 V 13-mer ions centered at 45+ are present in high abundances. When charge balanced, the CSD of the native 14-mer (CE 0 V, centered at 75+) and charge-stripped 13-mer ions (CE 100 V, centered at 43+) suggest the ejected monomer ions would be centered at 32+ (m/z 1787.6), which agrees well with the results of ISD presented in Figure 3(B) as well as previously published tandem-MS spectra.32, 34 While the monomer signal (centered at m/z 1787.6) is outside the mass range available when sampling up to 24,000 m/z on this instrument, some of the ejected monomer signal can be observed with the charge-stripped 13-mer when the upper m/z limit is reduced to to 20,000 m/z (see Supplemental Figure S9). Overall, these results agree well with the observation of ejected monomer ions centered at 32+ under conditions that promote dissociation (Figure 3(B)). It is interesting to note that no identifiable fragment ion, mass shifts, or charge-stripping product ions associated with the unidentified CSD noted in Figure 3 are observed in Figure 4 (labeled as *). It is unclear as to whether the abundances of these fragment ions are below the detection level, or that these ions are resistant to fragmentation. Also of interest is the detection of ions that are assigned to a GroEL 28-mer, a dimer of native GroEL 14-mers. It is unclear as to whether the 28-mer is a native complex that might be formed by an ESI-induced dimerization. While there is no known biological significance of these GroEL dimers, their transmission, mass analysis, and detection further illustrate the potential of the Agilent 6545XT Q-TOF for studies of megadalton protein complexes.
Figure 4.
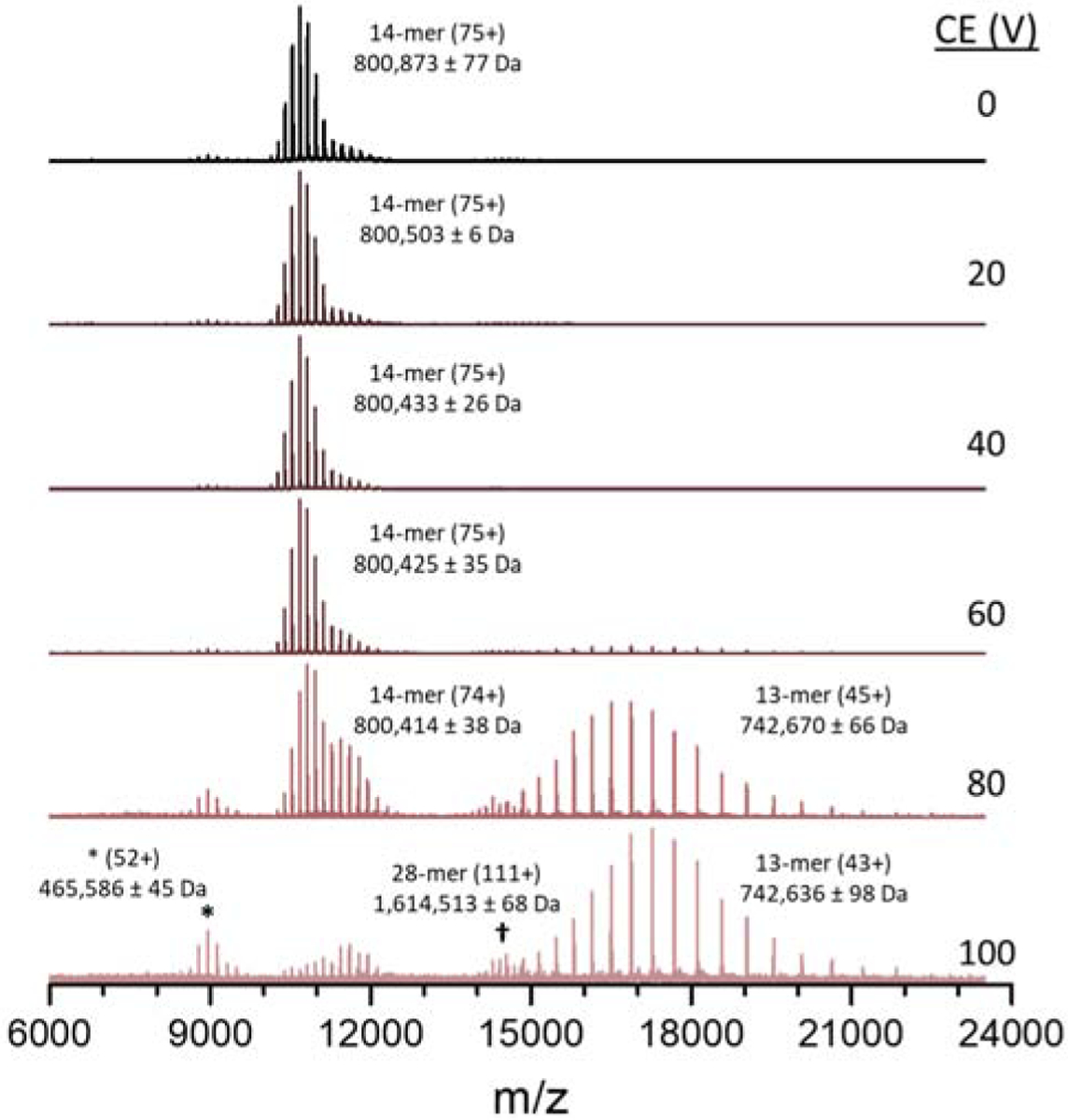
MS spectra of GroEL over a range of collision energy (CE) voltages. As the CE increases, both charge-stripping and CID are observed. Low abundance GroEL 28-mer ions appear at higher relative abundance at high collision voltage due to lower abundance of neighboring signals.
CONCLUSION
MS studies of intact protein complexes require that they retain native contacts and minimal unfolding of the subunits as they undergo the transition from the native solution-phase environment to a solvent-free, gas phase ion. For soluble protein complexes, this transition involves the removal of solvent, whereas for membrane protein complexes both solvent and detergents/buffers must be removed. Great progress has been realized over the past 30 years since Ganem, Li and Henion37 and Katta and Chait38 first reported their studies on soluble, non-covalent protein complexes using ESI-MS. Subsequent developments in the ESI processes, in particular static ESI using nm to μm size emitters,12, 13, 39 and MS instruments are now broadly recognized as native mass spectrometers.3, 4, 40, 41
Recent advances in Orbitrap MS instrumentation has opened new vistas for studies of larger protein complexes (>100 kDa.) and even MDa virus (>10 MDa.);42 however, TOF-MS instruments are still widely used for native MS studies, especially for studies where quantitation is required.43–45 To date, however, the mass resolution of most TOF-MS instruments is not sufficient for accurate determinations of MW of high mass complexes and efficient removal of solvent and salt adducts continues to be a challenge, especially for large protein complexes. The increased mass resolution and mass measurement accuracy of the Agilent 6545XT instrument is achieved by narrowing the pulse width for gating the ions into the TOF analyzer, increasing the length of the TOF, and decreasing the pressure within the TOF analyzer. The improvements in the instrument are most impactful for the analysis of larger ions (i.e. >100 kDa). because these ions are prone to retaining small molecules, salt and/or water adducts during the transition to the gas phase.
Here, these advantages are illustrated by results for soluble and membrane protein complexes as well as the chaperonin GroEL ranging in sizes from ~127 kDa. to ~801 kDa. Increasing MS resolving power allows for analysis of smaller adducts, and the increase in sensitivity allows for detection of low abundance species, which may have biological relevance. The improved mass resolution of advanced TOF analyzers allows for more detailed analysis of multiply-charged ion distributions, thereby expanding characterization of sample heterogeneity.11 In addition, the ability to perform ISD of these complexes affords a means for removal of some small molecule adducts, thereby allowing for more accurate determinations of molecular weight of the complex. ISD can also be used to dissociate the complex to better define the individual subunits of the complex. Collectively, these capabilities can be used to differentiate mass shifts resulting from PTMs and other sequence modifications and/or truncations.37 These improvements will then open new opportunities toward the study and characterization of larger and more complex systems.
Supplementary Material
HIGHLIGHTS.
Development of static nanoESI for extended mass range quadrupole-time-of-flight MS
Native MS of soluble protein complexes, membrane protein complexes, GroEL
Native MS of AmtB double mutant reveals small molecule binding
In-source dissociation of GroEL produces monomer, dimer, and trimer subunits
ACKNOWLEDGEMENTS
The authors wish to thank Noah Goldberg and Ken Newton of Agilent Technologies for their technical support, Dr. Andrew Roth and Prof. Hays Rye of the TAMU Department of Biochemistry and Biophysics for their generous donation of the GroEL samples, and Will Seward and Karl Yeager of the TAMU Chemistry Machine Shop for fabrication of the static spray emitter holder. This work is financially supported by Agilent Technologies, the National Insitutes of Health (grant numbers P41GM128577 (D.H.R), R01GM121751 (D.H.R. and A.L.), DP2GM123486 (A.L.)) and endowment funds from an MDS Sciex Professorship (D.H.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Instrument schematics, MS data of ADH and PK using different conditions, MS spectra of wild type AmtB, and CID spectra of GroEL complex.
Conflict of Interest: none
The authors declare no competing financial interest.
REFERENCES
- 1.Toby TK; Fornelli L; Kelleher NL, Progress in Top-Down Proteomics and the Analysis of Proteoforms. Annu Rev Anal Chem (Palo Alto Calif) 2016, 9 (1), 499–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillet LC; Leitner A; Aebersold R, Mass Spectrometry Applied to Bottom-Up Proteomics: Entering the High-Throughput Era for Hypothesis Testing. Annu Rev Anal Chem (Palo Alto Calif) 2016, 9 (1), 449–72. [DOI] [PubMed] [Google Scholar]
- 3.Leney AC; Heck AJ, Native Mass Spectrometry: What is in the Name? J Am Soc Mass Spectrom 2017, 28 (1), 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heck AJ, Native mass spectrometry: a bridge between interactomics and structural biology. Nat Methods 2008, 5 (11), 927–33. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee M; Mondal J, Osmolyte-Induced Collapse of a Charged Macromolecule. J Phys Chem B 2019, 123 (22), 4636–4644. [DOI] [PubMed] [Google Scholar]
- 6.Lyu J; Liu Y; McCabe JW; Schrecke S; Fang L; Russell DH; Laganowsky A, Discovery of Potent Charge-Reducing Molecules for Native Ion Mobility Mass Spectrometry Studies. Anal Chem 2020, 92 (16), 11242–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrick JW; Laganowsky A, Generation of Charge-Reduced Ions of Membrane Protein Complexes for Native Ion Mobility Mass Spectrometry Studies. J Am Soc Mass Spectrom 2019, 30 (5), 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosgen J; Pettitt BM; Bolen DW, Protein folding, stability, and solvation structure in osmolyte solutions. Biophys J 2005, 89 (5), 2988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worner TP; Snijder J; Bennett A; Agbandje-McKenna M; Makarov AA; Heck AJR, Resolving heterogeneous macromolecular assemblies by Orbitrap-based single-particle charge detection mass spectrometry. Nat Methods 2020, 17 (4), 395–398. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X; Kurulugama RT; Laganowsky A; Russell DH, Collision-Induced Unfolding Studies of Proteins and Protein Complexes using Drift Tube Ion Mobility-Mass Spectrometer. Anal Chem 2020, 92 (10), 7218–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poltash ML; McCabe JW; Shirzadeh M; Laganowsky A; Russell DH, Native IM-Orbitrap MS: Resolving What Was Hidden. Trends Analyt Chem 2020, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Z; Williams ER, Protein-Glass Surface Interactions and Ion Desalting in Electrospray Ionization with Submicron Emitters. J Am Soc Mass Spectrom 2018, 29 (1), 194–202. [DOI] [PubMed] [Google Scholar]
- 13.Kenderdine T; Xia Z; Williams ER; Fabris D, Submicrometer Nanospray Emitters Provide New Insights into the Mechanism of Cation Adduction to Anionic Oligonucleotides. Anal Chem 2018, 90 (22), 13541–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poltash ML; McCabe JW; Patrick JW; Laganowsky A; Russell DH, Development and Evaluation of a Reverse-Entry Ion Source Orbitrap Mass Spectrometer. J Am Soc Mass Spectrom 2019, 30 (1), 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrick JW; Boone CD; Liu W; Conover GM; Liu Y; Cong X; Laganowsky A, Allostery revealed within lipid binding events to membrane proteins. Proc Natl Acad Sci U S A 2018, 115 (12), 2976–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver J; Jiang M; Roth A; Puchalla J; Zhang J; Rye HS, GroEL actively stimulates folding of the endogenous substrate protein PepQ. Nat Commun 2017, 8 (1), 15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patiny L; Borel A, ChemCalc: a building block for tomorrow’s chemical infrastructure. J Chem Inf Model 2013, 53 (5), 1223–8. [DOI] [PubMed] [Google Scholar]
- 18.Desport JS; Frache G; Patiny L, MSPolyCalc: A web-based App for polymer mass spectrometry data interpretation. The case study of a pharmaceutical excipient. Rapid Commun Mass Spectrom 2020, 34 Suppl 2 (n/a), e8652. [DOI] [PubMed] [Google Scholar]
- 19.Gasteiger E; Gattiker A; Hoogland C; Ivanyi I; Appel RD; Bairoch A, ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 2003, 31 (13), 3784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe JW; Mallis CS; Kocurek KI; Poltash ML; Shirzadeh M; Hebert MJ; Fan L; Walker TE; Zheng X; Jiang T; Dong S; Lin CW; Laganowsky A; Russell DH, First-Principles Collision Cross Section Measurements of Large Proteins and Protein Complexes. Anal Chem 2020, 92 (16), 11155–11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poltash ML; McCabe JW; Shirzadeh M; Laganowsky A; Clowers BH; Russell DH, Fourier Transform-Ion Mobility-Orbitrap Mass Spectrometer: A Next-Generation Instrument for Native Mass Spectrometry. Anal Chem 2018, 90 (17), 10472–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe JW; Hebert MJ; Shirzadeh M; Mallis CS; Denton JK; Walker TE; Russell DH, The Ims Paradox: A Perspective on Structural Ion Mobility-Mass Spectrometry. Mass Spectrom Rev 2020, n/a (n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lossl P; Snijder J; Heck AJ, Boundaries of mass resolution in native mass spectrometry. J Am Soc Mass Spectrom 2014, 25 (6), 906–17. [DOI] [PubMed] [Google Scholar]
- 24.Liao YD; Jeng JC; Wang CF; Wang SC; Chang ST, Removal of N-terminal methionine from recombinant proteins by engineered E. coli methionine aminopeptidase. Protein Sci 2004, 13 (7), 1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J AL; S AB; Zhang J, Integrating Native Mass Spectrometry and Top-Down MS for Defining Protein Interactions Important in Biology and Medicine. Mass Spectrom (Tokyo) 2013, 2 (Spec Iss), S0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schachner LF; Ives AN; McGee JP; Melani RD; Kafader JO; Compton PD; Patrie SM; Kelleher NL, Standard Proteoforms and Their Complexes for Native Mass Spectrometry. J Am Soc Mass Spectrom 2019, 30 (7), 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorsam RT; Gutkind JS, G-protein-coupled receptors and cancer. Nat Rev Cancer 2007, 7 (2), 79–94. [DOI] [PubMed] [Google Scholar]
- 28.Laganowsky A; Reading E; Hopper JT; Robinson CV, Mass spectrometry of intact membrane protein complexes. Nat Protoc 2013, 8 (4), 639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y; Cong X; Liu W; Laganowsky A, Characterization of Membrane Protein-Lipid Interactions by Mass Spectrometry Ion Mobility Mass Spectrometry. J Am Soc Mass Spectrom 2017, 28 (4), 579–586. [DOI] [PubMed] [Google Scholar]
- 30.van Duijn E; Simmons DA; van den Heuvel RH; Bakkes PJ; van Heerikhuizen H; Heeren RM; Robinson CV; van der Vies SM; Heck AJ, Tandem mass spectrometry of intact GroEL-substrate complexes reveals substrate-specific conformational changes in the trans ring. J Am Chem Soc 2006, 128 (14), 4694–702. [DOI] [PubMed] [Google Scholar]
- 31.Hogan CJ Jr.; Ruotolo BT; Robinson CV; Fernandez de la Mora J, Tandem differential mobility analysis-mass spectrometry reveals partial gas-phase collapse of the GroEL complex. J Phys Chem B 2011, 115 (13), 3614–21. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M; Jones CM; Wysocki VH, Dissecting the large noncovalent protein complex GroEL with surface-induced dissociation and ion mobility-mass spectrometry. Anal Chem 2013, 85 (17), 8262–7. [DOI] [PubMed] [Google Scholar]
- 33.van Duijn E; Barendregt A; Synowsky S; Versluis C; Heck AJ, Chaperonin complexes monitored by ion mobility mass spectrometry. J Am Chem Soc 2009, 131 (4), 1452–9. [DOI] [PubMed] [Google Scholar]
- 34.Sobott F; Robinson CV, Characterising electrosprayed biomolecules using tandem-MS—the noncovalent GroEL chaperonin assembly. Int J Mass Spectrom 2004, 236 (1–3), 25–32. [Google Scholar]
- 35.Bush MF; Hall Z; Giles K; Hoyes J; Robinson CV; Ruotolo BT, Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal Chem 2010, 82 (22), 9557–65. [DOI] [PubMed] [Google Scholar]
- 36.Klunker D; Haas B; Hirtreiter A; Figueiredo L; Naylor DJ; Pfeifer G; Muller V; Deppenmeier U; Gottschalk G; Hartl FU; Hayer-Hartl M, Coexistence of group I and group II chaperonins in the archaeon Methanosarcina mazei. J Biol Chem 2003, 278 (35), 33256–67. [DOI] [PubMed] [Google Scholar]
- 37.Ganem B; Li YT; Henion JD, Detection of noncovalent receptor-ligand complexes by mass spectrometry. Journal of the American Chemical Society 1991, 113 (16), 6294–6296. [Google Scholar]
- 38.Katta V; Chait BT, Observation of the heme-globin complex in native myoglobin by electrospray-ionization mass spectrometry. Journal of the American Chemical Society 1991, 113 (22), 8534–8535. [Google Scholar]
- 39.Panczyk EM; Gilbert JD; Jagdale GS; Stiving AQ; Baker LA; Wysocki VH, Ion Mobility and Surface Collisions: Submicrometer Capillaries Can Produce Native-like Protein Complexes. Anal Chem 2020, 92 (3), 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loo JA, Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev 1997, 16 (1), 1–23. [DOI] [PubMed] [Google Scholar]
- 41.Rostom AA; Robinson CV, Detection of the intact GroEL chaperonin assembly by mass spectrometry. Journal of the American Chemical Society 1999, 121 (19), 4718–4719. [Google Scholar]
- 42.Fort KL; van de Waterbeemd M; Boll D; Reinhardt-Szyba M; Belov ME; Sasaki E; Zschoche R; Hilvert D; Makarov AA; Heck AJR, Expanding the structural analysis capabilities on an Orbitrap-based mass spectrometer for large macromolecular complexes. Analyst 2017, 143 (1), 100–105. [DOI] [PubMed] [Google Scholar]
- 43.Jones J; Pack L; Hunter JH; Valliere-Douglass JF, Native size-exclusion chromatography-mass spectrometry: suitability for antibody-drug conjugate drug-to-antibody ratio quantitation across a range of chemotypes and drug-loading levels. MAbs 2020, 12 (1), 1682895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W; Yu J; Kane MA, Quantitation of the Noncovalent Cellular Retinol-Binding Protein, Type 1 Complex Through Native Mass Spectrometry. J Am Soc Mass Spectr 2017, 28 (1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L; Vasicek LA; Hsieh S; Zhang S; Bateman KP; Henion J, Top-down LC-MS quantitation of intact denatured and native monoclonal antibodies in biological samples. Bioanalysis 2018, 10 (13), 1039–1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


