Abstract
INTRODUCTION:
Clinical trials are currently investigating whether an extended mesenteric resection for ileocecal resections could reduce postoperative recurrence in Crohn's disease. Resection of the mesorectum, which contains proinflammatory macrophages, during proct(ocol)ectomy, is associated with reduced recurrent inflammation and improved wound healing. We aimed to characterize the macrophages in the ileocecal mesentery, which were compared with those in the mesorectum, to provide a biological rationale for the ongoing trials.
METHODS:
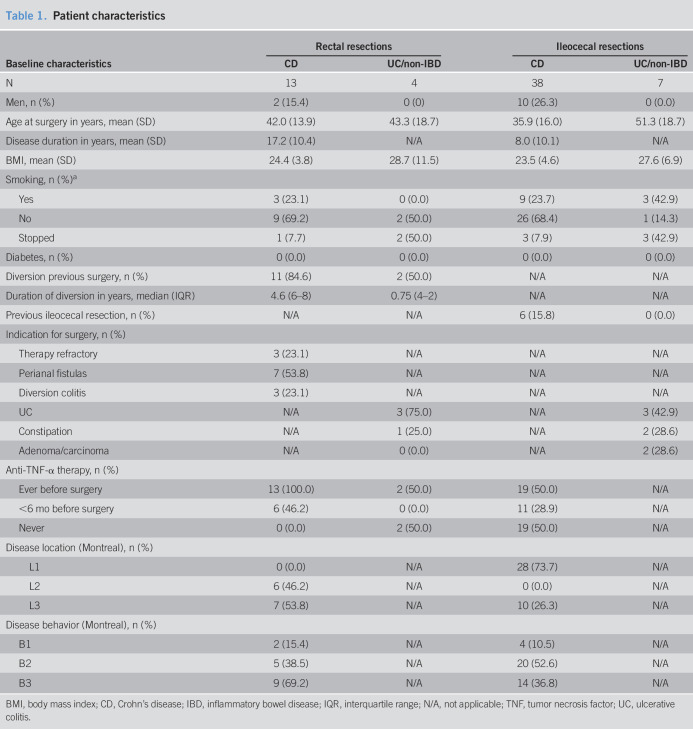
In 13 patients with Crohn's disease and 4 control patients undergoing a proctectomy, tissue specimens were sampled at 3 locations from the mesorectum: distal (rectum), middle, and proximal (sigmoid). In 38 patients with Crohn's disease and 7 control patients undergoing ileocecal resections, tissue specimens also obtained from 3 locations: adjacent to the inflamed terminal ileum, adjacent to the noninflamed ileal resection margin, and centrally along the ileocolic artery. Immune cells from these tissue specimens were analyzed by flow cytometry for expression of CD206 to determine their inflammatory status.
RESULTS:
In the mesorectum, a gradient from proinflammatory to regulatory macrophages from distal to proximal was observed, corresponding to the adjacent inflammation of the intestine. By contrast, the ileocecal mesentery did not contain high amounts of proinflammatory macrophages adjacent to the inflamed tissue, and a gradient toward a more proinflammatory phenotype was seen in the central mesenteric area.
DISCUSSION:
Although the mesentery is a continuous structure, the mesorectum and the ileocecal mesentery show different immunological characteristics. Therefore, currently, there is no basis to perform an extended ileocecal resection in patients with Crohn's disease.
INTRODUCTION
Alterations of the mesentery such as “creeping fat” were already mentioned in the first description of Crohn's disease in 1932 (1). Nonetheless, it was not until recent years that a more prominent role was suggested for the mesentery in Crohn's disease, although the question remains whether this tissue is pathological or regulatory (2–6). Characterization of immune and mesenchymal cells of the mesentery has shown varying results with the presence of both pro- and antiinflammatory factors and cell types (7–16). Because, currently, there is no consensus on the biological function of the mesentery in inflammatory bowel disease and the mesentery is important for the vascularization of the intestine, surgical guidelines advise mesentery-sparing resections for these benign diseases (17,18). By contrast, in patients who undergo proct(ocol)ectomy, recent findings have shown that resection of the rectal mesentery is beneficial to reduce postoperative complication rates (pelvic/perianal abscesses, perineal wound infections, wound dehiscence, persisting fistulas) and promote healing (19). This effect is seen specifically in patients with Crohn's disease but not in patients with ulcerative colitis. In addition, resection of remaining rectal mesentery in patients with Crohn's disease with a persistent presacral sinus that had already undergone a mesentery-sparing proct(ocol)ectomy, helped overcome chronic nonhealing perineal wounds (19). These findings were associated with the presence of considerable numbers of macrophages in the rectal mesentery. Macrophages exist in a spectrum of sub-phenotypes, ranging from highly inflammatory (CD206-low) to immunosuppressive and wound healing (CD206-high). These immunosuppressive macrophages play an important role in dampening disease activity, and are the cells that mediate the therapeutic effect of anti-TNF (20-23). A low ratio of CD206+ vs CD206− cells indicates a relatively proinflammatory phenotype of macrophages (20,21), which was indeed seen in the mesorectal tissue in Crohn's disease (19). In line with the findings in the mesorectum, it has been suggested that a more extended resection including the mesentery in ileocecal resections would lead to fewer surgical recurrences (22). To further investigate this, 2 randomized controlled trials have been initiated in which more extended resections of the mesentery are compared with standard close bowel ileocecal resections in patients with Crohn's disease (NCT02542904 and NCT03172143).
We aimed to determine the distribution of proinflammatory macrophages in the mesentery of the rectum and ileocecal region to guide surgical resection margins. We hypothesized that macrophages reside in the mesentery in a gradient from a proinflammatory to a wound-healing phenotype depending on the proximity to the inflamed intestinal tissue.
METHODS
Tissue collection and analysis
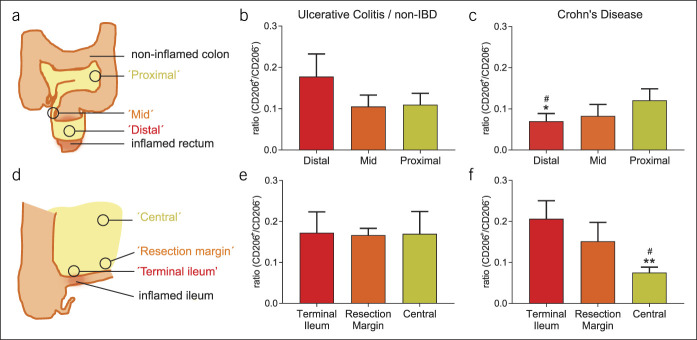
Specimen collection and culturing was approved by the biobank review committee of the Academic Medical Center Amsterdam (number 178#A201470). Rectal mesentery from 13 patients with Crohn's disease and 4 control patients (3 suffering from ulcerative colitis and 1 from refractory constipation) was sampled at 3 locations: distal (adjacent to the inflamed intestine), middle, and proximal (adjacent to least inflamed sigmoid, Figure 1a). Ileocecal mesentery from 38 patients with Crohn's disease and 7 control patients (3 suffering from ulcerative colitis, 2 from refractory constipation, and 2 from a cecal adenoma/carcinoma) was also sampled at 3 locations: adjacent to the inflamed ileum, near the noninflamed ileum (at the ileal resection margin), and centrally (near the base of the ileocecal artery, Figure 1d).
Figure 1.
Mesenteric macrophages show a proinflammatory to regulatory gradient at the rectum, but this is inverted in the ileocecal region. (a) Schematic overview of mesorectal tissue sampling. (b) Mesorectal CD206+/CD206− ratios in control patients (n = 4: 3 suffering from ulcerative colitisand 1 from refractory constipation). (c) Mesorectal CD206+/CD206− ratios in patients with Crohn's disease (n = 13). *P < 0.05 compared with proximal, #P < 0.05 compared with distal ulcerative colitis (UC)/noninflammatory bowel disease (IBD) as calculated by t test. (d) Schematic overview of mesenteric tissue sampling in the ileocecal region. (e) CD206+/CD206− ratios in the ileocecal region of patients with UC or without IBD (n = 7: 3 suffering from ulcerative colitis, 2 from refractory constipation, and 2 from a cecal adenoma/carcinoma). (f) CD206+/CD206− ratios in the ileocecal region of patients with Crohn's disease with terminal ileitis (n = 38). **P < 0.01, compared with terminal ileum; #P < 0.05, compared with central UC/non-IBD as calculated by t-test.
Tissue sections were then cultured on a nontissue culture–treated petri dish using RPMI 1640 medium containing 10% fetal calf serum (Lonza, Verviers, Belgium), 2 mM l-glutamine (Invitrogen, Carlsbad, CA), 100 U/mL penicillin–streptomycin (Invitrogen), 50 μg/mL gentamicin (Lonza), and 50 μg/mL amphotericin B (Thermo Fisher, Landsmeer, the Netherlands). After 48 hours of culture, tissue sections were removed, and cells were harvested by means of 5 mM ethylenediaminetetraacetic acid. Adherent and nonadherent cells were collected separately and both were used for analysis. Cells were then stained using the following antibodies: anti-CD45-AF700 (clone HI30; Biolegend, Uithoorn, the Netherlands), anti-CD3-AF488 (clone OKT3; Biolegend), anti-CD14-PE/Cy7 (clone 61D3; eBioscience, Vienna, Austria), and anti-CD206-APC (Clone 19.2; BD Bioscience, Breda, the Netherlands). Expression was analyzed by flow cytometry using a FACS Fortessa (BD Bioscience) with FlowJo software (Treestar, Ashland, OR).
Statistical analysis
Data are presented as mean and standard error of the mean. Statistical tests used are indicated in figure legends. For statistical analysis, GraphPad Prism (version 8.3.0; GraphPad Software, La Jolla, CA) was used. A P value <0.05 was considered statistically significant. No data imputation was performed.
RESULTS
Samples were collected from the indicated anatomical locations as described in the Methods section and Figure 1a and d. Patient characteristics are summarized in Table 1. In control patients, CD206+/CD206− ratios did not alter along the length of the colon and rectum (Figure 1b). In patients with Crohn's disease, these ratios revealed a gradient from proinflammatory to regulatory macrophages from distal to proximal mesorectum (Figure 1c). The samples collected from the “distal” part of the rectum in patients with Crohn's disease showed a significantly decreased CD206+/CD206−, and thus more proinflammatory, ratio compared with that of the “proximal” samples from the same patients and with that of the samples from the control patients. This was in line with previous findings (19). Confirming our hypothesis, these results indicate that the macrophages in the mesorectum gradually become less proinflammatory further away from the inflamed intestinal tissue.
Table 1.
Patient characteristics
Subsequently, we investigated whether a similar gradient was present in the mesentery of the terminal ileum toward the central part of the mesentery. Again, in control patients, the CD206+/CD206− ratios were consistent throughout the sampled locations (Figure 1e) with a predominantly regulatory phenotype. However, in the patients with Crohn's disease, the opposite was observed of what was found in the mesorectum (Figure 1e): adjacent to the inflamed ileum and the noninflamed resection margin, macrophages displayed a regulatory phenotype comparable with the level of controls (Figure 1e and f). By contrast, the “central” tissue had a low CD206+/CD206− ratio, indicative of a proinflammatory phenotype (Figure 1f). This “central” ratio decreased significantly compared with that of the “terminal ileum” samples from the same patients and with that of the samples from the “central” tissue of the control patients. In brief, although both the rectal and ileal mesentery in patients with Crohn's disease show a gradient in macrophage subphenotype, the direction of the gradient is completely opposite in the 2 different locations of Crohn's disease.
Within the samples acquired from the ileocecal region, we observed variance between the CD206+/CD206− ratios of the patients. Because the patients who underwent ileocecal resections form a heterogeneous patient population, we performed subgroup analysis to investigate patient characteristics that influence mesenteric macrophage phenotypes. This subgroup analysis revealed that the mesentery of patients with L3 disease contained more unfavorable proinflammatory macrophages at the “central” and “resection margin” sites (Figure 2a). This suggests that patients with L3 disease have widespread localization of proinflammatory macrophages in the mesentery, in line with the previous finding that these patients have higher recurrence rates (23). Interestingly, in our cohort, recurrence (defined as a Rutgeerts score ≥ i2b, 6 months after surgery) was associated with the presence of proinflammatory macrophages in the mesentery at the resection margin (Figure 2b). Penetrating disease behavior might be expected to result in a pro-inflammatory environment and, thus, influence the mesenteric macrophage subtypes. However, in our cohort, patients suffering from stricturing and penetrating disease (B2/3) tended to show a more favorable regulatory macrophage profile rather than proinflammatory (Figure 2c).
Figure 2.
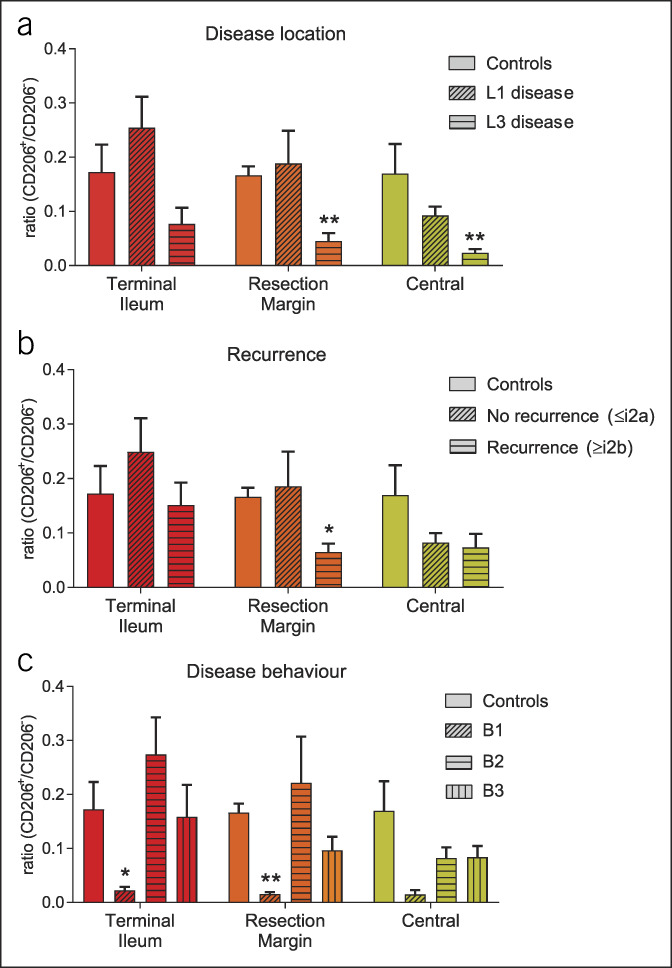
B1 and L3 disease are associated with low CD206+/CD206− ratios throughout the ileocecal mesentery. (a) CD206 ratios in patients with L1 (n = 28) and L3 (n = 10) disease, compared with ulcerative colitis (UC)/non-inflammatory bowel disease (IBD) controls (n = 7). (b) CD206+/CD206− ratios in patients with recurrence, defined as a Rutgeerts score ≥ i2b (n = 9), vs patients without recurrence, defined as a Rutgeerts score ≤ i2a (n = 26), compared with UC/non-IBD controls (n = 7). (c) CD206+/CD206− ratios in patients with B1 (n = 4), B2 (n = 20), and B3 (n = 14) disease, compared with UC/non-IBD controls (n = 7). *P < 0.05; **P < 0.01, compared with UC/non-IBD controls as calculated by t test.
DISCUSSION
In Crohn's disease patients, while in the mesorectum, macrophages are less proinflammatory, the further they are located from the inflamed tissue, this phenomenon was not observed in the ileocecal region. The mesentery adjacent to the inflamed ileum resembled a healthy regulatory phenotype, suggesting an attempt of the immune system to dampen the intestinal inflammatory processes. By contrast, the central area of the mesentery contained fewer regulatory macrophages. Whether this area contributes to disease activity remains to be investigated. Thus, although the mesentery is anatomically a continuous structure, the mesorectum and the mesentery at the ileocecal region show distinctly different immunological characteristics in Crohn's disease. It is well known that, in cases of intestinal perforation, omental fat tissue can migrate over the intestinal tissue to limit the perforation (24). Possibly, the mesenteric fat tissue has a similar function at the ileocecal region in patients with Crohn's disease. The mesorectum is anatomically different from the ileocecal region in the sense that the mesorectum is wrapped around the rectal tissue, whereas the creeping fat at the terminal ileum has to migrate to wrap itself around the intestine. Thus, this tissue might behave differently from the mesentery in the ileocecal region, explaining the differences we find in macrophage phenotypes. On the other hand, this could also be due to the differences between the patient populations. The patients who require a proct(ocol)ectomy often have longstanding therapy-refractory disease, whereas the patients who underwent an ileocecal resection have relatively short disease duration and the disease location is less extensive. Recently, it has been shown that there are differences in adipocyte size, fibrosis, and the T-cell compartment between the ileocecal and colonic mesentery (25). Higher levels of tumor necrosis factor-α and interleukin-1β were measured in the mesocolon, which corresponds to our findings that the mesorectum contains proinflammatory macrophages. However, the ileal mesentery contained higher amounts of infiltrating T-cells, without a significant change in the composition of T-cell subpopulations between the rectal and ileocecal mesentery (25). How this corresponds to our findings remains to be elucidated. In the above-mentioned study and by other studies, it has been suggested that ileal and ileocolonic (L1/L3) and isolated colonic (L2) Crohn's disease are different entities (26,27). In our cohort of patients who underwent proct(ocol)ectomies, we did not observe differences in the mesenteric macrophages of patients with L2 and L3 disease. From our subgroup analyses, we did find that patients with L3 disease shows more proinflammatory macrophages in the ileocecal region compared with patients with L1 disease, which suggests that disease type might influence the mesenteric macrophages. However, numbers are low in our subgroup analyses, so further research is warranted to characterize the mesentery more extensively in various Crohn's disease patient groups, especially considering disease behavior. Additional analysis might also elucidate the role of other cell types that can interact with the macrophages, e.g., adipocytes secreting adipokines, which could influence macrophage polarization (9,10,14–16). A limitation of our study is that we did not investigate other markers than CD206. However, CD206-positive macrophages have been shown to mediate wound healing, (21) and previously we observed that the macrophages with low CD206+/CD206− ratios express high levels of TNFα, which shows that CD206 ratios correspond to functional inflammatory activity (19).
When aiming to provide a biological rationale for altered surgical approaches in which more mesentery is resected, the differences between the mesorectum and the ileocecal mesentery should be taken into account. The current findings undermine the reasoning to resect more mesentery in ileocecal resections because the regulatory macrophages adjacent to the inflamed ileum are likely to mediate a wound-healing response.
CONFLICTS OF INTEREST
Guarantor of the article: Christianne J. Buskens, MD, PhD.
Specific author contributions: Manon E. Wildenberg, PhD, and Christianne J. Buskens, MD, PhD, shared last authorship. J.H.M.v.d.M., K.A.T.G.M.W., M.E.W., and C.J.B. designed the study. J.H.M.v.d.M., K.A.T.G.M.W., J.D.W.v.d.B., M.A.J.B., M.A.B., W.A.B., M.E.W., and C.J.B. collected and/or analyzed the data. J.H.M.v.d.M., K.A.T.G.M.W., J.D.W.v.d.B., M.A.J.B., M.A.B., W.A.B., M.E.W., and C.J.B. interpreted the data. J.H.M.v.d.M., K.A.T.G.M.W., M.E.W., and C.J.B. drafted the manuscript. J.H.M.v.d.B., M.A.J.B., M.A.B., and W.A.B. co-authored the writing of the manuscript. All authors edited the manuscript and read and approved the final manuscript.
Financial support: The International Organisation for the Study of Inflammatory Bowel Disease (IOIBD) Operating grant. European Crohn's and Colitis Organisation (ECCO) grant.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ The mesorectum contains proinflammatory macrophages in patients with Crohn's disease.
✓ Resection of the mesorectum during proct(ocol)ectomy reduces postoperative complications and increases healing rates, specifically in patients with Crohn's disease.
✓ Inclusion of the mesentery in ileocecal resections has been suggested to reduce recurrences and is under investigation.
WHAT IS NEW HERE
✓ We characterized the inflammatory status of mesenteric macrophages in the mesorectum and the ileocecal mesentery in Crohn's disease, compared with non-Crohn's disease.
✓ Proinflammatory macrophages reside in the mesorectum next to the inflamed rectal tissue and display a gradient to a more regulatory phenotype further away from the inflamed rectum.
✓ Macrophages in the ileocecal mesentery adjacent to the inflamed terminal ileum and creeping fat have a regulatory phenotype, whereas macrophages in the central mesentery demonstrate a more proinflammatory phenotype.
TRANSLATIONAL IMPACT
✓ Inclusion of the mesentery in ileocecal resections is not expected to be beneficial based on these findings.
REFERENCES
- 1.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: A pathologic and clinical entity. J Am Med Assoc 1932;99:1323–9. [Google Scholar]
- 2.Schaffler A, Herfarth H. Creeping fat in Crohn's disease: Travelling in a creeper lane of research? Gut 2005;54:742–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn's disease: A pathogenetic hallmark or an innocent bystander? Gut 2007;56:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr MA. The path to Crohn's disease: Is mucosal pathology a secondary event? Inflamm Bowel Dis 2010;16:896–902. [DOI] [PubMed] [Google Scholar]
- 5.Siegmund B. Mesenteric fat in Crohn's disease: The hot spot of inflammation? Gut 2012;61:3–5. [DOI] [PubMed] [Google Scholar]
- 6.Coffey JC, O'Leary DP. The mesentery: Structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1:238–47. [DOI] [PubMed] [Google Scholar]
- 7.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology 1999;117:73–81. [DOI] [PubMed] [Google Scholar]
- 8.Barbier M, Vidal H, Desreumaux P, et al. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol 2003;27:987–91. [PubMed] [Google Scholar]
- 9.Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut 2005;54:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul G, Schaffler A, Neumeier M, et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis 2006;12:471–7. [DOI] [PubMed] [Google Scholar]
- 11.Schaffler A, Furst A, Buchler C, et al. Vascular endothelial growth factor secretion from mesenteric adipose tissue and from creeping fat in Crohn's disease. J Gastroenterol Hepatol 2006;21:1419–23. [DOI] [PubMed] [Google Scholar]
- 12.Schaffler A, Furst A, Buchler C, et al. Secretion of RANTES (CCL5) and interleukin-10 from mesenteric adipose tissue and from creeping fat in Crohn's disease: Regulation by steroid treatment. J Gastroenterol Hepatol 2006;21:1412–8. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut 2012;61:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kredel LI, Batra A, Stroh T, et al. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn's disease. Gut 2013;62:852–62. [DOI] [PubMed] [Google Scholar]
- 15.Sideri A, Bakirtzi K, Shih DQ, et al. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell Mol Gastroenterol Hepatol 2015;1:420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coope A, Pascoal LB, da Silva FAR, et al. Transcriptional and molecular pathways activated in mesenteric adipose tissue and intestinal mucosa of Crohn's disease patients. Int J Inflam 2017;2017:7646859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EC, Dowling BL. Perimuscular excision of the rectum for Crohn's disease and ulcerative colitis. A conservation technique. Br J Surg 1972;59:29–32. [DOI] [PubMed] [Google Scholar]
- 18.Strong S, Steele SR, Boutrous M, et al. Clinical practice guideline for the surgical management of Crohn's disease. Dis Colon Rectum 2015;58:1021–36. [DOI] [PubMed] [Google Scholar]
- 19.de Groof EJ, van der Meer JHM, Tanis PJ, et al. Persistent mesorectal inflammatory activity is associated with complications after proctectomy in Crohn's disease. J Crohns Colitis 2019;13:271–2. [DOI] [PubMed] [Google Scholar]
- 20.Vos AC, Wildenberg ME, Duijvestein M, et al. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology 2011;140:221–30. [DOI] [PubMed] [Google Scholar]
- 21.Vos AC, Wildenberg ME, Arijs I, et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis 2012;18:401–8. [DOI] [PubMed] [Google Scholar]
- 22.Coffey JC, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn's disease is associated with reduced surgical recurrence. J Crohns Colitis 2018;12:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasmann K, van Amesfoort J, van Montfoort ML, et al. The predictive value of inflammation at ileocecal resection margins for postoperative Crohn's recurrence: A cohort study. Inflamm Bowel Dis 2019;2019:izz290. [DOI] [PubMed] [Google Scholar]
- 24.Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am 2000;80:275–93, xii. [DOI] [PubMed] [Google Scholar]
- 25.Kredel LI, Jodicke LJ, Scheffold A, et al. T-cell composition in ileal and colonic creeping fat: separating ileal from colonic Crohn's disease. J Crohns Colitis 2019;13:79–91. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: A systematic review of isolated colonic Crohn's disease: The third IBD? Gut 2017;66:362–81. [DOI] [PubMed] [Google Scholar]
- 27.Dulai PS, Singh S, Vande Casteele N, et al. Should we divide Crohn's disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol 2019;17:2634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]