Abstract
Purpose of review:
This review examines emerging neuroimaging research in pediatric obsessive compulsive disorder (OCD) and explores the possibility that developmentally sensitive mechanisms may underlie OCD across the lifespan.
Recent findings:
Diffusion tensor imaging (DTI) studies of pediatric OCD reveal abnormal structural connectivity within frontal-striato-thalamic circuity (FSTC). Resting-state functional magnetic resonance imaging (fMRI) studies further support atypical FSTC connectivity in young patients, but also suggest altered connectivity within cortical networks for task-control. Task-based fMRI studies show that hyper- and hypo-activation of task control networks may depend on task difficulty in pediatric patients similar to recent findings in adults.
Summary:
This review suggests that atypical neurodevelopmental trajectories may underlie the emergence and early course of OCD. Abnormalities of structural and functional connectivity may vary with age, while functional engagement during task may vary with age and task complexity. Future research should combine DTI, resting-state fMRI and task-based fMRI methods and incorporate longitudinal designs to reveal developmentally sensitive targets for intervention.
Keywords: Pediatric OCD, DTI, resting-state, fMRI, executive function, task-control network
Introduction
Obsessive-compulsive disorder (OCD), characterized by intrusive thoughts (obsessions) and related behavioral rituals (compulsions), is a disabling psychiatric illness that begins during childhood or adolescence in 50% of patients [1]. The prevalence of OCD in pediatric samples is 1–3% [2], similar to estimates in adults [3]. Among pediatric patients who receive treatment for OCD, approximately half continue to experience full-blown illness into adulthood [4] and, in patients with adult onset illness, many report subclinical symptoms beginning in childhood [2, 3]. Yet, despite the apparent origin of OCD in early life, neuroimaging studies designed to elucidate the neural underpinnings of the disorder are mostly derived from research with adults. Understanding brain abnormalities in pediatric, compared to adult OCD, may help to elucidate unique features of illness across the lifespan, and ultimately guide the design of therapies most appropriate for different patients at different ages.
FSTC in OCD: a neuroanatomical model
Neuroimaging research has consistently demonstrated abnormalities of fronto-striato-thalamic circuitry (FSTC) in adult OCD [5] and accumulating research in pediatric patients provides evidence for FSTC abnormalities at early stages of illness. The FSTC system is comprised of parallel, segregated “loops” between distinct portions of the cortex, striatum, and thalamus [6]. FSTC loops of functional relevance for OCD include those passing through dorsal and ventral striatum into the medial dorsal thalamus via topographically organized projections from cortical centers for cognitive control (e.g., anterior cingulate cortex, dorsolateral prefrontal cortex; [7]) and for emotionally driven evaluative functions, including reward processing and internal mood states (e.g., ventral medial prefrontal cortex; [8]).
The first neuroimaging evidence of FSTC abnormality in OCD came from positron emission tomography (PET) studies showing increased metabolic uptake of radiotracers marking glucose and oxygen metabolism in the anterior cingulate cortex (ACC), orbital frontal portion of the ventral medial preferontal cortex (vmPFC), striatum and thalamus in adult patients compared to healthy controls [9]. Initial studies were conducted while patients lay awake in the PET scanner, not performing any particular tasks, thereby demonstrating hyperactivity of FSTC at rest. Follow-up work showed that symptom provocation further increased metabolic hyperactivity in FSTC and that treatment resolved FSTC hypermetabolism [9]. Taken together, these findings suggested a neuroanatomical model of OCD in which excessive signaling through FSTC was hypothesized to underlie symptoms.
An important element of the FSTC system, which is likely relevant to its role in OCD, involves the splitting of each loop into direct and indirect pathways at the level of the basal ganglia (i.e., striatum, globus pallidum, subthalamic nucleus; Figure 1). In general, the direct pathway facilitates neuronal activity through FSTC, whereas the indirect pathway inhibits it [10]. Neuroanatomical models of OCD suggest that greater direct pathway activity through vmPFC-based loops for emotion processing and lower indirect pathway activity through dACC- and dorsolateral prefrontal cortex-based loops for cognitive control may underlie intrusive thoughts, ritualistic behaviors and related anxiety in OCD [5, 9, 11]. In other words, hyperactivity in neural circuitry underlying the affective valuation of thoughts and behaviors (i.e., vmPFC-based FSTC) may couple with hypoactivity in neural substrate underlying capacity for task control (i.e., dACC-, dorsolateral prefrontal-based FSTC). A resulting imbalance in FSTC substrate for emotion-processing, relative to task control, could lead to the intrusion of distressing, obsessional thoughts and the repetition of compulsive behaviors to reduce distress, despite insight that such thoughts and behaviors “do not make sense”. Task-based neuroimaging research has supported this possibility, demonstrating deficits of dACC activation during cognitive tasks requiring behavioral adjustment and hyperactivity of vmPFC during emotion-laden evaluative processing in patients, even when OCD symptoms are not directly provoked [5].
Figure 1.
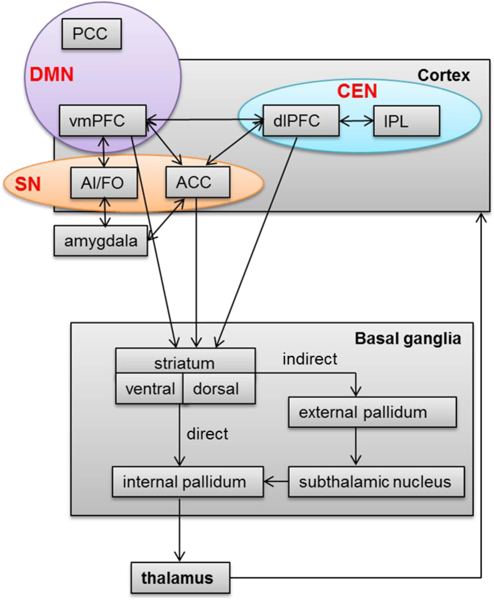
Simplified illustration of fronto-striato-thalamic circuitry (FSTC) and its overlap with salience network (SN), central executive network (CEN) and default mode network (DMN), adapted from Brem et al [12]. FSTC model adapted from prior reviews [5, 10, 11, and 12]. ACC, anterior cingulate cortex; AI/FO, anterior insula/frontal opercular; dlPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; vmPFC, ventromedial prefrontal cortex; PCC, posterior cingulate cortex.
MRI-based Technology Enables Study of FSTC in Pediatric OCD
Despite the pediatric onset of OCD in at least half of all patients [1], the FSTC model of OCD was first developed based on studies conducted in adults. Initial focus on adult patients was largely due to technical characteristics of PET, the first widely available tool for neuroimaging research, which requires injection of radioactive tracer to reveal brain activity. With the advent of non-invasive magnetic resonance imaging (MRI) technologies, neuroimaging research in children became more feasible and MRI-based neuroimaging studies of pediatric OCD began to emerge. Initial evidence for FSTC abnormality in young patients came from MRI studies showing altered volume of FSTC nodes, including ACC, striatum and thalamus, but also superior parietal lobule and precuneus (for a review, see [13]). In addition, task-based functional MRI studies began to propagate, revealing abnormalities of activation in FSTC regions during tasks designed to engage OCD-relevant psychological processes (see next section).
Advances in MR-based technology also produced diffusion tensor imaging and resting state functional MRI methods, enabling the measurement of FSTC structural and functional connectivity, respectively, in young patients. Diffusion tensor imaging (DTI) measures the direction and magnitude of water diffusion within white matter tracts [14, 15]. The most commonly studied DTI measure is fractional anisotropy (FA), an index of white matter coherence and thus, the integrity of white matter tracts [16–19]. Resting state functional connectivity MRI (rsfcMRI) measures fluctuations of blood oxygen level dependent (BOLD) MRI signal. During rsfcMRI data collection, subjects are instructed to “allow your mind to wander” to induce a so-called “resting state” during which low frequency BOLD signal oscillations throughout the brain are measured. Correlations between oscillations in different brain regions are then calculated to produce a metric of resting state connectivity. Greater resting state connectivity is believed to reflect a history of co-activation, providing evidence of a functional circuit [20, 21].
Diffusion Tensor Imaging Research in Pediatric OCD
The literature on DTI in pediatric OCD has provided evidence of white matter involvement in the FSTC from early in the course of illness (Table 1). White matter tracts of particular relevance to FSTC include the anterior corpus callosum (CC), anterior cingulum bundle (CB) and anterior limb of the internal capsule (ALIC). The anterior corpus callosum contains white matter fibers connecting the right and left prefrontal cortex [22]; the anterior cingulum bundle contains fibers that connect emotion processing regions such as the amygdala to ACC [23]; and, the ALIC contains white matter pathways connecting the frontal lobe and thalamus. Several DTI studies have found increased FA and/or axial diffusivity (another DTI metric of white matter integrity) in these tracts in OCD-affected youth compared to healthy controls [24–26], while other researchers have found the reverse [27, 28]. Interestingly, the largest DTI study of pediatric OCD [29··] found no overall differences in FA but, rather, demonstrated steeper age-related increases of FA in FSTC white matter in patients compared to controls across the ages of 8 to 19. After subdividing the sample into child, early adolescent, and late adolescent groups, lower FA was demonstrated in 8- to 11-year-old child patients, but higher FA was found in 16- to 19-year-old adolescent patients relative to same-aged controls in the anterior corpus callosum and anterior cingulum bundle. These results suggest a possible interaction between FA and age, a finding that may help clarify the discrepant reports of lower FA in OCD compared to healthy youth [27, 28]; the examination of FA in older as compared to younger participants may increase the likelihood of finding abnormally increased or decreased FA in FSTC white matter tracts in pediatric samples (Table 1).
Table 1.
Diffusion tensor imaging and resting state connectivity in pediatric obsessive compulsive disorder
| Study | Subject Characteristics | Design | Analytic Method | Main results |
|---|---|---|---|---|
| Diffusion tensor imaging | ||||
| Rosso et al, 2014 [28] | Range: 10 – 19 OCD: 14.1 ± 2.6, n=17, 11M HC: 13.6 ± 2.1, n=19, 13M |
OCD v HC | TBSS |
↓FA in genu, body, splenium of CC, ATR, UF, IFOF, forceps minor, anterior corona radiate. ↑ RD in body of CC, ATR, anterior corona radiata, forceps minor. Correlation of lower FA in right thalamus, greater RD in right body of CC with earlier age of onset No correlation with OCD severity on CYBOCs. |
| Fitzgerald et al, 2014[29··] | Range: 8 – 19 OCD: 14.1 ± 2.9, n=36, 16M HC: 14.7 ± 3.1, n=27, 9M |
OCD v HC Group x age interaction |
TBSS, ROI |
↑FA with increasing age in anterior CB, ALIC, anterior CC, right splenium, right PLIC, right PTR, bilateral external capsule, anterior & superior corona radiata, right SLF. Correlation of greater FA in anterior CB with greater OCD severity on CYBOCs. |
| Silk et al, 2013[27] | Range: 8 – 18 OCD: 12.8 ± 2.8, n=16, 6M HC: 11.2 ± 2.1, n=22, 16M |
OCD v HC | TBSS |
↓AD in genu and splenium. No FA differences. Correlation lower AD in left cingulate, left SLF, bilateral PLIC with greater OCD severity on CBCL-OCS. |
| Gruner et al, 2012[25] | Range: 9 – 17 OCD: 14.3 ± 2.1, n=23, 13M HC: 14.2 ± 2.2, n=23, 12M |
OCD v HC | FSL, SPM8 |
↑FA in the anterior CB, splenium of CC, right corticospinal tract and left IFOF. Correlation of greater FA in splenium with greater obsession severity in drug-naïve patient subgroup. Correlation of greater FA in anterior CB with better response inhibition and cognitive control performance. |
| Jayarajan et al, 2012[26] | Range: less than 18 OCD: 14.1 ± 1.8, n=15, 8M HC: 14.3 ± 2.2, n=15, 8M |
OCD v HC | TBSS |
↑AD in genu, body, and splenium of CC, bilateral cingulum, bilateral ALIC, bilateral ATR, bilateral SLF, left ILF, left PLIC, and middle cerebellar peduncle. ↑ RD in genu of CC, bilateral SLF, left ILF, bilateral UF, bilateral ATR, bilateral IFOF, left PLIC, right superior, middle & right inferior cerebellar peduncle. No correlation with OCD severity on CYBOCs. |
| Zarei et al, 2011[24] | Range: 8 – 17 OCD: 16.6 ± 1.5, n=26, 14M HC: 16.5 ± 1.4, n=26, 14M |
OCD v HC | TBSS |
↑FA in genu and splenium, left cingulum, left inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus, right inferior fronto-occipital fasciculus, bilateral corticospinal tract, bilateral forceps major, bilateral forceps minor, and right uncinate fasciculus. Correlation of greater FA in ALIC (and other tracts) with OCD severity on CYBOCs. |
| Resting state connectivity | ||||
| Fitzgerald et al,2010[50] | Range: 8 – 19 OCD: 13.9 ± 2.6, n=18, 6M HC: 14.1 ± 2.7, n=18, 6M |
OCD v HC | Seed |
↓ dACC – right AI/FO , ↓vmPFC – PCC connectivity. No correlation with OCD severity on CYBOCs. |
| Fitzgerald et al,2011[45] |
Child range: 8 – 12 OCD: 11 ± 1.3, n=11, 6M HC: 10.7 ± 1.7, n=13, 6M Adolescent range: 13 – 17 OCD: 16 ± 1.4, n=11, 5M HC: 15.3 ± 1.3, n=13, 8M Young adult range: 18 – 25 OCD: 20 ± 1.4, n=18, 10M HC: 21 ± 2.3, n=15, 7M Adult range: 26 – 40 OCD:32 ± 6.0, n=13, 6M HC: 32.3 ± 5.9, n=17, 7M |
OCD v HC Group x age interaction: |
Seed |
↓ dorsal striatum – rACC, left medial dorsal thalamus – left dACC in child group. ↑ right dorsal striatum – vmPFC across age groups. Correlation of lower dorsal striatum-rACC connectivity with greater OCD severity on CYBOCs in child OCD. |
| Weber et al,2014[46] | Range: 8 – 16 OCD: 13.0± 2.9,n=11, 6M HC: 12.7± 3.2,n=9, 5M |
OCD v HC | ICA |
↓BA 8 (dorsomedial prefrontal cortex) and BA40 (inferior parietal cortex) within cingulate network. ↑BA 43(inferior post-central gyrus) within auditory network. No correlation with OCD severity on CYBOCs. |
, greater in pediatric obsessive compulsive disorder (OCD) than healthy controls (HC)
, lower in OCD than HC.
M, male; F, female; TBSS, Tract-Based Spatial Statistics; ROI, region of interest; ICA, independent component analysis; FA, fractional anisotropy; AD, axial diffusivity; RD, radial diffusivity; ATR, anterior thalamic radiations; UF, uncinated fasiculus; IFOF, inferior fronto-occipital fasciculus; CC, corpus callosum; CB, cingulum bundle; ALIC, anterior limb internal capsule; PLIC, posterior limb internal capsule; PTR, posterior thalamic radiations; SLF, superior longitudinal fasciculus; ILF, inferior longitudinal fasciculus; CYBOCs, Child Yale Brown Obsessive Compulsive Scale [30]; CBCL-OCS, Child Behavior Checklist Obsessive Compulsive Subscale [31]; dACC, dorsal anterior cingulate cortex; AI/FO, anterior insula/frontal operculum; vmPFC, ventral medial prefrontal cortex; rACC, rostral anterior cingulate cortex; BA, brodmann area.
NOTE: AD measures diffusion in parallel with the primary direction of white matter fibers; as with FA, AD reflects white matter integrity. RD measures diffusion along the radius of a white matter tract, perpendicular its primary direction; in contrast to FA and AD, RD is thought to reflect deficient myelination.”
If steeper age-related increases in FSTC structural connectivity in OCD relative to healthy youth [29··] continues beyond adolescence, then abnormally increased FA might be expected in adult patients. Greater FA has been reported in adult OCD in the CC [32, 33], CB [33, 34], ALIC [32, 34] and in other white matter tracts, including superior longitudinal fasciculus (SLF) and anterior corona radiata in some reports (for a review, see [35]). However, other studies have found decreased FA in these regions in adult patients compared to healthy controls (for a review, see [35]). A meta-analysis of DTI research in adult OCD suggests that conflicting results across studies may derive from sample heterogeneity due to demographics, medication status, illness chronicity, and imaging methodology [35] and the same may be said of DTI research in pediatric OCD with the added complexity of developmental stage. In typically developing individuals, most white matter tracts (e.g., internal capsule, CC, CB) exhibit curvilinear trajectories (i.e., inverted ‘U’ shaped), with age-related increases found during childhood and adolescence followed by decreases in adulthood [36]. DTI research in patients compared to matched controls from childhood into older adulthood will be needed to assess whether shifts in the timing of this curvilinear trajectory (e.g., earlier peaks for healthy, later peaks for OCD) may best describe developmental differences in FSTC structural connectivity over the lifespan.
In summary, the bulk of DTI research in pediatric OCD suggests that increased white matter in FSTC and other white matter tracts occurs in young patients by the time of adolescence and that, from childhood into adolescence, structural connectivity within these tracts may increase at faster rates in patients compared to age-matched healthy youth. Critically, longitudinal research is needed to understand when white matter abnormalities in pediatric OCD emerge, to map changes in white matter abnormalities over time, and to determine how these changes associate with course of illness. Moreover, combining DTI and other MR-based imaging methods may elucidate the functional significance of atypical white matter development in pediatric OCD to aid identification of DTI measures as potential targets for intervention and/or intermediate outcomes. Finally, FA abnormalities outside of FSTC have been demonstrated in pediatric (e.g., SLF, corona radiata, posterior limb of internal capsule, see Table 1) and adult patients (for a review, see [37]), prompting a reevaluation of the FSTC model originally theorized to underlie symptoms.
Resting State Functional Connectivity in Pediatric OCD: from FSTC to Cortical-cortical Networks
Myelinated neuronal projections (i.e., white matter connections) between FSTC regions were first revealed by chemical tracer studies in laboratory animals [6], but the advent of task-based fMRI and rsfcMRI methods has revealed that functional connectivity between regions can exist in the absence of direct structural connections [38, 39]. These “functional” networks are identified by regions that co-activate in response to task demands and exhibit connectivity at rest [40]. For example, cortical targets of FSTC, particularly dACC, vmPFC and the dorsolateral frontal cortex (dlPFC), are now realized as critical nodes within such networks. As depicted in Figure 1, functional connectivity of the dACC-bilateral anterior insula, dlPFC-parietal cortex, and vmPFC-posterior cingulate cortex define canonical networks that are now widely believed to support, respectively: salience detection (salience network, SN), executive functions (central executive network, CEN), and “default” mode processes such as self-reflection, internally-directed mentation, and episodic memory requiring task control (default mode network, DMN) [41].
Building from rsfcMRI research in adult patients with OCD [42, 43], rsfcMRI research in pediatric OCD initially focused on FSTC. This work tested for temporal correlations of fMRI BOLD signal between anatomically defined regions or “seeds” placed in the striatum and thalamus with voxels across the rest of the brain. Replicating work in adults [42, 44], evidence for distinguishable FSTC loops was demonstrated for seeds placed in the ventral striatum, dorsal striatum and medial dorsal thalamus [45]. Functional connectivity for each seed was then compared for patients and healthy individuals by developmental stage (child, adolescent and adult), demonstrating excessive connectivity of dorsal striatum with medial frontal pole, a subregion of vmPFC, across the age span. By contrast, the youngest patients exhibited reduced connectivity of dorsal striatum with rostral ACC and of medial dorsal thalamus with dorsal ACC. These child-specific abnormalities of functional connectivity have since been partially replicated in a study of patients with pediatric OCD compared to healthy youth, ages 8 to 16 years; within a “cingulate network” defined by resting state correlations of striatum, bilateral dlPFC, and dorsal medial prefrontal cortex (dmPFC), patients exhibited reduced connectivity of dmPFC [46].
The relevance of abnormal functional connectivity within FSTC loops in pediatric OCD remains poorly understood, but can be interpreted in the context of task-based literature. For instance, FSTC running through vmPFC is associated with the processing of emotionally salient stimuli to motivate behavior [8, 47], whereas the maturation of ACC-based FSTC plays a critical role in the development of cognitive control [48]. Thus, excessive connectivity of the FSTC loop running through vmPFC in child, adolescent and adult patients could drive excessive worry about errors and related attempts at corrective behavior in OCD in patients across the lifespan. By contrast, premature reduction in the connectivity of ACC-based FSTC for cognitive control may contribute to an inability to suppress the contextually inappropriate thoughts and behaviors near illness onset and perhaps, at a critical period of development, give rise to the emergence and progression of OCD in young patients.
In conclusion, interpretation of DTI and rsfcMRI research in pediatric OCD can be informed by neuroimaging work in typically developing youth showing that development of ACC-associated cognitive control and vmPFC-associated emotion processing functions depends not only on the maturation of structural connections between FSTC nodes, but also on the developing connectivity of these regions within large scale, neural networks for salience detection (SN), central executive processes (CEN) and default mode function (DMN) [20, 41, 49]. Indeed, preliminary work in pediatric OCD shows that hyperactivation of dACC and failure to deactivate vmPFC during a simple cognitive task occurs in the context of reduced functional connectivity within the salience (dACC-anterior insula, SN) and default mode (vmPFC-posterior cingulate, DMN) networks [50]. These task-based and rsfcMRI findings extend historical models of altered FSTC connectivity in OCD to include abnormalities in overlapping, resting state networks in young patients (Figure 1). In addition, atypical functional connectivity between dACC and vMPFC in pediatric OCD suggests inappropriate interactions of SN and DMN in young patients [50]. In adult OCD, rsfcMRI research has shown that the normally inverse relationship between task positive networks, namely the SN and CEN, with DMN is attenuated in patients with OCD compared to healthy controls [51, 52]. These findings suggest a failure to segregate between networks that could lead to deficits in task-control processes due to intrusion of emotional and introspective function of DMN. Task-based fMRI studies may aide examination of the functional significance of altered SN and CEN connectivity and will be further reviewed in the next section.
Functional Activation during Task Control Demands
OCD has long been theorized to stem from core deficits of task control [53, 54]. Task control is a broadly defined term that encompasses a variety of cognitive tasks including: interference control, response inhibition, working memory and cognitive flexibility [55, 56]. Collectively, task control processes enable the selection of appropriate behavior across a myriad of internal and external inputs and, when impaired, may associate with the repetitive thoughts and behaviors characteristic of OCD. For instance, recurrent intrusive obsessions might be related to an inability to inhibit and select certain stimuli (interference control), and/or an inability to switch attention from one aspect of a stimuli to another depending on environment and context (cognitive flexibility), whereas the repetitive compulsive behavior of OCD might stem from failure to inhibit certain prepotent, but inappropriate, response sets (response inhibition) and/or a deficit in working memory prompting repeated urges to check.
Task control demands are known to engage “task positive” salience and central executive networks [55, 57]. These networks were originally defined by task positive co-activations and later found to remain functionally connected, even at rest [40, 41, 57]. As noted above, preliminary evidence suggests reduced functional connectivity between task positive regions during rest in pediatric OCD [50]. Below, we will review accumulating research from task-based fMRI studies demonstrating abnormal SN and CEN function in both adult (for reviews, see [58·, 59] ) and pediatric patients with OCD. Taken together, these studies suggest that altered function of canonical networks for task control in adults with OCD may develop at the early stages of illness.
To frame an understanding of task-based fMRI studies of OCD, it is important to consider the relationship of brain activation to behavioral performance during tasks which, in turn, relates to task difficulty. For example, tasks tapping interference control (e.g., the flanker task) might be less difficult than tasks requiring motor response inhibition (e.g., stop-signal task) since interference control requires inhibition of a potential response through the focusing of attention on task-relevant over -irrelevant stimulus features, whereas motor response inhibition requires the suppression of a behavioral response that has already been triggered and is closer to actually being produced [60]. In some tasks, difficulty can be manipulated by varying parameters within a task; for example, the 3-back working memory task is harder than the 2-back working memory task. Other complex tasks, such as the Wisconsin Card Sorting Test (WCST) and Tower of London (TOL), entail relatively high levels of difficulty by requiring the coordination of multiple task control processes to produce correct performance [61]. In fMRI research, hyperactivation in the context of normal performance in a patient compared to a control group has been interpreted to reflect compensation for underlying neural inefficiency and may be most likely to occur on less difficult tasks [62]. By contrast, as task difficulty increases, hypoactivation may occur as capacity for compensatory activation is exceeded and performance deficits emerge [58·, 63].
Task-based fMRI research in adult OCD
Functional MRI studies of adult OCD have revealed altered activation of SN and CEN during task control demands [58·, 59], and provide context for interpreting the fMRI literature in pediatric patients. In adults with OCD, increased activation in the dlPFC and dACC has been demonstrated during a relatively simple interference control task relative to healthy controls[64]; in this study, patients maintained normal performance relative to controls, consistent with the interpretation that hyperactivation may enable compensation for underlying inefficiency of task control networks [58·, 64]. By contrast, a more difficult task requiring response inhibition elicited decreased activation in the inferior frontal gyrus (IFG) and parietal regions in OCD relative to HC adults; hypoactivation occurred in the context of performance deficits in patients [65]. The notion that task difficulty impacts the nature of task control network function in OCD is further supported by fMRI research showing increased activation of dlPFC under low-cognitive demand (e.g, 1- and 2-back working memory task), but decreased-to-normative levels of activation in dACC under increased task demand for patients compared to controls [66–68]. On the more complex tasks of self-shifting/task switching, adult OCD patients showed decreased activation in the dlPFC, dACC, parietal and caudate regions in cognitive flexibility relative to HC [69–71]. Similarly, on the Tower of London task, OCD patients showed decreased activation in the dlPFC and parietal lobe during planning, relative to controls [72, 73].
Thus, whether task-related brain areas are hypo- or hyper-activated in patients compared with healthy controls appears to depend largely on the difficulty of the task and whether compensatory mechanisms are enlisted [58·, 59]. During less difficult tasks, OCD patients may recruit additional neural resources in SN and CEN, possibly to compensate for an underlying inefficiency of these task control networks. This hyperactivation may explain why individuals with OCD are able to maintain normal behavioral performance, relative to healthy controls, during less complex tasks (e.g., the Flanker task and the Simon task). However, with increasing task demand (e.g. the Go/no-go and the Stop-signal task), these compensatory mechanisms may fail in individuals with OCD, such that behavioral impairments and decreased activity in task control networks emerge.
Task-based fMRI research in pediatric OCD
Modelling after recent reviews of the adult literature, we consider whether altered activation of task-control networks in pediatric populations also depends on task complexity and/or relates to performance. In contrast to the fMRI literature in adult OCD, only a few studies have examined task control processing in pediatric samples (Table 2). During simple cognitive tasks with relatively low levels of difficulty (e.g. simplified serial reaction time, interference, 1–2 back working memory tasks), fMRI studies in pediatric OCD reveal increased activation in patients compared to healthy youth in task control regions, including dACC, dlPFC, and parietal cortex [50, 74, 75]. As with adult studies, hyperactivation of task control networks in pediatric patients occurred in the context of normal performance relative to controls, suggesting that increased engagement of task control regions may reflect a compensatory function by which patients maintain appropriate behavioral output. Review of the pediatric OCD literature on brain activation during more difficult/complex cognitive tasks (e.g., motor inhibition, set-shifting, planning) demonstrated that, relative to healthy controls, pediatric patients with OCD showed decreased activation in task control regions including dlPFC, dACC, IFG and parietal [76–78].
Table 2.
Task control function in pediatric obsessive compulsive disorder
| Study | Subject Characteristics | Task(s) | Behavior Findings | Main Imaging Findings |
|---|---|---|---|---|
| Lazaro et al,2008[74] | Range: 7 – 18 OCD: 13.1 ± 2.7, n=12, 7M HC: 13.7 ± 2.8, n=12, 7M |
Simplified SRTT, pre- to post-treatment (lower difficulty) |
not reported |
Before treatment: ↑bilateral dlPFC activation for more v less complex response options. After treatment: ↑right IPL activation for more v less complex response options. Before v After: ↓left insula and left putamen activation for more v less complex response options within OCD group. Correlation of greater Nacc and RSPL activation with greater OCD severity on CYBOCs and greater rSPL and left posterior cingulate activation with greater obsession severity before treatment. |
| Fitzgerald et al,2010[50] | Range: 8 –19 OCD: 13.9 ± 2.6, n=18, 6M HC: 14.1 ± 2.7, n=18, 6M |
MSIT (lower difficulty) |
no group difference |
↑dACC activation for high v. low interference. ↑vmPFC activation for error v correct. ↑dACC–vmPFC connectivity for high vs. low interference (psychophysiological interaction analysis). No correlation with OCD severity on CYBOCs |
| Fitzgerald et al,2013[80] | Range: 8 –19 OCD: 12.9 ± 3.0, n=21 HC: 14.1 ± 3.1, n=25 (all F) |
MSIT (lower difficulty) |
no group difference | OCD = HC for high v low interference. ↓left dlPFC activation for error v correct. No correlation with OCD severity on CYBOCs. |
| Huyser et al,2011[79] | Range: 8 –19 OCD: 14.0 ± 2.5, n=25, 9M HC: 13.7 ± 2.9, n=25, 9M |
Flanker, pre- to post-treatment (lower difficulty) |
no group difference before or after treatment |
Before treatment:
↓dlPFC and posterior insula activation for high v low interference. ↑ bilateral insula activation with age for high v low interference (OCD relative to HC, group x age interaction). ↑rACC and bilateral insula activation with age for error v correct (OCD relative to HC, group x age interaction). After treatment: ↑bilateral dlPFC and right premotor activation for high v low interference. ↑rACC and right insula activation with age for error v correct (OCD relative to HC, group x age interaction). Correlation of greater left dlPFC activation to interference with OCD severity on CYBOCs before treatment. Correlation of greater right insula activation to errors with OCD severity on CYBOCs before treatment. Correlation of increase in bilateral dlPFC, right dmPFC and left premotor cortex to interference with decrease in OCD severity on CYBOCs before-to-after treatment. |
| Diwadkar et al,2015[75] | Range: 11 –21 OCD: 17.2 ± 3.3, n=18, 11M HC: 17.4 ±3.1, n=27, 18M |
n-back (lower difficulty) |
not reported |
↑ dlPFC, dACC and parietal activation to both 1- and 2-back tasks. ↑dACC connectivity with parietal, middle frontal and BG to 1-back. ↑dACC connectivity with parietal, dmPFC/dACC to 2-back. |
| Wooley et al,2008[77] | Range: 12 –16 OCD: 14 ± 1.7, n=10 HC: 14.5 ± 1.1, n=9 (all M) |
Stroop (lower difficulty) |
no group difference | ↓right middle temporal gyrus and bilateral cerebellar vermis activation to high v low interference. |
| Wooley et al, 2008[77] | Range: 12 –16 OCD: 14 ± 1.7, n=10 HC: 14.5 ± 1.1, n=9 (all M) |
Stop-signal (higher difficulty) |
no group difference |
↓right OFC/insula, thalamus, basal ganglia activation to response inhibition. ↓dmPFC/dACC and dlPFC activation to error v correct. |
| Wooley et al,2008[77] | Range: 12 –16 OCD: 14 ± 1.7, n=10 HC: 14.5 ± 1.1, n=9 (all M) |
Set-shifting/ Switch (higher difficulty) |
no group difference |
↓ precentral cortex, IFG, TPJ and cerebellar activation to task switch v no switch. |
| Britton et al,2010[76] | Range: 10 –17 OCD: 13.5 ± 2.4, n=15, 9M HC: 13.6 ± 2.4, n=20, 13M |
Set-shifting/ Switch (higher difficulty) |
no group difference |
↓IFG activation to task switch v no switch. ↓right caudate activation with shift costs. IFG–Caudate connectivity only in HC, but not in OCD. |
| Huyser et al,2010[78] | Range: 8 –19 OCD: 14.0 ± 2.5, n=25, 9M HC: 13.7 ± 2.9, n=25, 9M |
Tower of London, pre- to post-treatment (higher difficulty) |
OCD slower than HC before treatment, but no group difference after treatment |
Before treatment: ↓ left dlPFC/premotor and right parietal cortex activation to planning. ↑dlPFC, left dACC, right dmPFC, left insula with increased task load. After treatment: No group difference; no group x task load interaction. Correlation of before-to-after treatment decrease in the left dlPFC and parietal to planning with decrease in OCD severity on CYBOCs. |
, greater in pediatric obsessive compulsive disorder (OCD) than healthy controls (HC)
, lower in OCD than HC
M, male; F, female; SRTT, Serial Reaction Time Task; MSIT, Multi-Source Interference Task; dlPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule; Nacc, nucleus accumbens ;rSPL, right superior parietal lobule; dACC, dorsal anterior cingulate cortex; vmPFC, ventral medial prefrontal cortex; rACC, rostral anterior cingulate cortex; dmPFC, dorsal medial prefrontal cortex; IFG, inferior frontal gyrus; TPJ, temporoparietal junction; CYBOCs, Child Yale Brown Obsessive Compulsive Scale [30] .
Thus, consistent with adult OCD literature, a pattern of increased activation during tasks requiring lower cognitive load, and decreased activation during tasks with higher level demand for control characterizes pediatric patients with OCD compared to healthy youth. In line with this notion, Huyser and colleague [79] found decreased activation in conjunction with impaired performance (slower reaction times) in pediatric OCD participants relative to healthy youth on a task requiring higher levels of control (Tower of London). However, this study stands out as an exception since, in other fMRI studies of pediatric OCD, patients performed as well as healthy youth on relatively difficult tasks (e.g., [76, 77]), despite decreased activation in task-control network. Finally, patients with pediatric OCD have been found to exhibit normal performance in the context of decreased dlPFC activation during incorrect [80] and correct [79]trials on relatively low load, interference tasks. These findings are in conflict with the theory that, during less difficult tasks, hyperactivation of task control regions is necessary to support the maintenance of performance in OCD [58·].
Several factors may contribute to the discrepancies observed in the pediatric OCD fMRI literature. First, most of the pediatric OCD fMRI studies employed small sample sizes (ranging from 10 to 25 pediatric OCD participants). Small samples in neuroimaging studies often yield low reproducibility of results [81] and may contribute to the observed inconsistencies. Future studies should include larger sample sizes to increase the external validity of the findings. Second, fMRI studies in pediatric OCD have typically included children and adolescents across a wide age range, spanning 8 to 19 years. The function and connectivity of task-control networks develop dramatically from early childhood into adolescence and early adulthood [20, 49]. Thus, different studies may produce different findings depending on specific ages of each study sample. That is, developmental variability within age groups may outweigh the between group (OCD versus healthy) variability in brain function and/or performance. Future studies should further stratify by age and recruit more subjects at each age, differentiating effects for young children from adolescents [82].
Conclusions
In conclusion, there is strong evidence demonstrating abnormalities of both FSTC and canonical networks for task control (SN, CEN) in pediatric OCD. Emerging works suggests that these abnormalities may vary with age and performance in young patients. Understanding this variation will be important for elucidating the neurodevelopmental trajectories that may underlie the emergence and early course of OCD. Additional research combining DTI and rsfcMRI studies with task-based fMRI methodologies will also be needed to elucidate the relationships between developing connectivity and interactive cognitive and emotional functions served by FSTC and cortical-cortical networks. Such knowledge would guide efforts to develop brain stimulation (e.g., transcranial magnetic stimulation [TMS] or transcranial direct-current stimulation [tDCS]) to potentiate/modulate activity in the relevant neural circuits or cognitive training paradigms to target the brain regions involved in cognitive and emotional dysfunction specific to pediatric OCD. Longitudinal imaging designs will be especially important in reaching these goals. By following patients over time, neuroimaging research may reveal developmentally sensitive MR metrics, as well as functional activation and connectivity patterns, to serve as targets or intermediate outcomes, by which to measure the effect of cognitive training and neuromodulatory therapies. Ultimately, this line of research may identify personalized strategies for adjusting neurodevelopment to treat (and even prevent) OCD in different patients, at different ages.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Yanni Liu, Dr. Emily L. Bilek, and Dr. Kate D. Fitzgerald declare that they have no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kessler RC, et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 2005. 62(6): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 2.Pauls DL, et al. , A family study of obsessive-compulsive disorder. Am J Psychiatry, 1995. 152(1): p. 76–84. [DOI] [PubMed] [Google Scholar]
- 3.Ruscio AM, et al. , The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry, 2010. 15(1): p. 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart SE, et al. , Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand, 2004. 110(1): p. 4–13. [DOI] [PubMed] [Google Scholar]
- 5.Menzies L, et al. , Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev, 2008. 32(3): p. 525–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander GE, DeLong MR, and Strick PL, Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci, 1986. 9: p. 357–81. [DOI] [PubMed] [Google Scholar]
- 7.Ridderinkhof KR, et al. , The role of the medial frontal cortex in cognitive control. Science, 2004. 306(5695): p. 443–7. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN and Knutson B, The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 2010. 35(1): p. 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter LR Jr., et al. , Brain Mediation of Obsessive-Compulsive Disorder Symptoms: Evidence From Functional Brain Imaging Studies in the Human and Nonhuman Primate. Semin Clin Neuropsychiatry, 1996. 1(1): p. 32–47. [DOI] [PubMed] [Google Scholar]
- 10.Albin RL, Young AB, and Penney JB, The functional anatomy of basal ganglia disorders. Trends Neurosci, 1989. 12(10): p. 366–75. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel OA, et al. , Frontal-striatal abnormalities underlying behaviours in the compulsive-impulsive spectrum. J Neurol Sci, 2010. 289(1–2): p. 55–9. [DOI] [PubMed] [Google Scholar]
- 12.Brem S, et al. , Neuroimaging of cognitive brain function in paediatric obsessive compulsive disorder: a review of literature and preliminary meta-analysis. J Neural Transm (Vienna), 2012. 119(11): p. 1425–48. [DOI] [PubMed] [Google Scholar]
- 13.Radua J and Mataix-Cols D, Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry, 2009. 195(5): p. 393–402. [DOI] [PubMed] [Google Scholar]
- 14.Dell’Acqua F and Catani M, Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol, 2012. 25(4): p. 375–83. [DOI] [PubMed] [Google Scholar]
- 15.Hagmann P, et al. , Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics, 2006. 26 Suppl 1: p. S205–23. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu C, The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed, 2002. 15(7–8): p. 435–55. [DOI] [PubMed] [Google Scholar]
- 17.Bonekamp D, et al. , Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage, 2007. 34(2): p. 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paus T, Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn, 2010. 72(1): p. 26–35. [DOI] [PubMed] [Google Scholar]
- 19.Walhovd KB, Johansen-Berg H, and Karadottir RT, Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies. Neuroscience, 2014. 276: p. 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair DA, et al. , Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A, 2007. 104(33): p. 13507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst M, et al. , fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol, 2015. 11: p. 361–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer S and Frahm J, Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage, 2006. 32(3): p. 989–94. [DOI] [PubMed] [Google Scholar]
- 23.Jones DK, Knosche TR, and Turner R, White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage, 2013. 73: p. 239–54. [DOI] [PubMed] [Google Scholar]
- 24.Zarei M, et al. , Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry, 2011. 70(11): p. 1083–90. [DOI] [PubMed] [Google Scholar]
- 25.Gruner P, et al. , White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology, 2012. 37(12): p. 2730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayarajan RN, et al. , White matter abnormalities in children and adolescents with obsessive-compulsive disorder: a diffusion tensor imaging study. Depress Anxiety, 2012. 29(9): p. 780–8. [DOI] [PubMed] [Google Scholar]
- 27.Silk T, et al. , White matter abnormalities in pediatric obsessive-compulsive disorder. Psychiatry Res, 2013. 213(2): p. 154–60. [DOI] [PubMed] [Google Scholar]
- 28.Rosso IM, et al. , Brain white matter integrity and association with age at onset in pediatric obsessive-compulsive disorder. Biol Mood Anxiety Disord, 2014. 4(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.·· Fitzgerald KD, et al. , Atypical frontal-striatal-thalamic circuit white matter development in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry, 2014. 53(11): p. 1225–1233 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scahill L, et al. , Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry, 1997. 36(6): p. 844–52. [DOI] [PubMed] [Google Scholar]
- 31.Nelson EC, et al. , Obsessive-compulsive scale of the child behavior checklist: specificity, sensitivity, and predictive power. Pediatrics, 2001. 108(1): p. E14. [DOI] [PubMed] [Google Scholar]
- 32.Yoo SY, et al. , White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand, 2007. 116(3): p. 211–9. [DOI] [PubMed] [Google Scholar]
- 33.Li F, et al. , Microstructural brain abnormalities in patients with obsessive-compulsive disorder: diffusion-tensor MR imaging study at 3.0 T. Radiology, 2011. 260(1): p. 216–23. [DOI] [PubMed] [Google Scholar]
- 34.Cannistraro PA, et al. , A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety, 2007. 24(6): p. 440–6. [DOI] [PubMed] [Google Scholar]
- 35.Piras F, et al. , Brain circuitries of obsessive compulsive disorder: a systematic review and meta-analysis of diffusion tensor imaging studies. Neurosci Biobehav Rev, 2013. 37(10 Pt 2): p. 2856–77. [DOI] [PubMed] [Google Scholar]
- 36.Westlye LT, et al. , Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex, 2010. 20(9): p. 2055–68. [DOI] [PubMed] [Google Scholar]
- 37.Radua J, et al. , Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology, 2014. 39(7): p. 1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honey CJ, et al. , Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A, 2009. 106(6): p. 2035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Reilly JX, et al. , Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci U S A, 2013. 110(34): p. 13982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox MD, et al. , The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 2005. 102(27): p. 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon V, Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci, 2011. 15(10): p. 483–506. [DOI] [PubMed] [Google Scholar]
- 42.Harrison BJ, et al. , Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry, 2009. 66(11): p. 1189–200. [DOI] [PubMed] [Google Scholar]
- 43.Sakai Y, et al. , Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Di Martino A, et al. , Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex, 2008. 18(12): p. 2735–47. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald KD, et al. , Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry, 2011. 50(9): p. 938–948 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber AM, Soreni N, and Noseworthy MD, A preliminary study of functional connectivity of medication naive children with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry, 2014. 53: p. 129–36. [DOI] [PubMed] [Google Scholar]
- 47.Etkin A, et al. , Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 2006. 51(6): p. 871–82. [DOI] [PubMed] [Google Scholar]
- 48.Rubia K, et al. , Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp, 2006. 27(12): p. 973–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uddin LQ, et al. , Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci, 2011. 31(50): p. 18578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzgerald KD, et al. , Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry, 2010. 68(11): p. 1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beucke JC, et al. , Default mode network subsystem alterations in obsessive-compulsive disorder. British Journal of Psychiatry, 2014. 205(5): p. 376–382. [DOI] [PubMed] [Google Scholar]
- 52.Stern ER, et al. , Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One, 2012. 7(5): p. e36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlain SR, et al. , The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev, 2005. 29(3): p. 399–419. [DOI] [PubMed] [Google Scholar]
- 54.Snyder HR, et al. , Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clin Psychol Sci, 2015. 3(2): p. 301–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dosenbach NU, et al. , A core system for the implementation of task sets. Neuron, 2006. 50(5): p. 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diamond A, Executive functions. Annu Rev Psychol, 2013. 64: p. 135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sridharan D, Levitin DJ, and Menon V, A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A, 2008. 105(34): p. 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.· van Velzen LS, et al. , Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci, 2014. 8: p. 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eng GK, Sim K, and Chen SH, Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci Biobehav Rev, 2015. 52: p. 233–57. [DOI] [PubMed] [Google Scholar]
- 60.Sebastian A, et al. , Disentangling common and specific neural subprocesses of response inhibition. Neuroimage, 2013. 64: p. 601–15. [DOI] [PubMed] [Google Scholar]
- 61.Miyake A, et al. , The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol, 2000. 41(1): p. 49–100. [DOI] [PubMed] [Google Scholar]
- 62.Eysenck MW, et al. , Anxiety and cognitive performance: attentional control theory. Emotion, 2007. 7(2): p. 336–53. [DOI] [PubMed] [Google Scholar]
- 63.Manoach DS, Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res, 2003. 60(2–3): p. 285–98. [DOI] [PubMed] [Google Scholar]
- 64.Marsh R, et al. , Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry, 2014. 75(8): p. 615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Wit SJ, et al. , Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry, 2012. 169(10): p. 1100–8. [DOI] [PubMed] [Google Scholar]
- 66.Koch K, et al. , Aberrant anterior cingulate activation in obsessive-compulsive disorder is related to task complexity. Neuropsychologia, 2012. 50(5): p. 958–64. [DOI] [PubMed] [Google Scholar]
- 67.de Vries FE, et al. , Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry, 2014. 76(11): p. 878–87. [DOI] [PubMed] [Google Scholar]
- 68.Nakao T, et al. , Working memory dysfunction in obsessive-compulsive disorder: a neuropsychological and functional MRI study. J Psychiatr Res, 2009. 43(8): p. 784–91. [DOI] [PubMed] [Google Scholar]
- 69.Gu BM, et al. , Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain, 2008. 131(Pt 1): p. 155–64. [DOI] [PubMed] [Google Scholar]
- 70.Page LA, et al. , A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res, 2009. 174(3): p. 202–9. [DOI] [PubMed] [Google Scholar]
- 71.Han JY, et al. , Altered brain activation in ventral frontal-striatal regions following a 16-week pharmacotherapy in unmedicated obsessive-compulsive disorder. J Korean Med Sci, 2011. 26(5): p. 665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Heuvel OA, et al. , Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry, 2005. 62(3): p. 301–9. [DOI] [PubMed] [Google Scholar]
- 73.den Braber A, et al. , An fMRI study in monozygotic twins discordant for obsessive-compulsive symptoms. Biol Psychol, 2008. 79(1): p. 91–102. [DOI] [PubMed] [Google Scholar]
- 74.Lazaro L, et al. , Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: a functional MRI study. J Psychiatr Res, 2008. 42(13): p. 1051–9. [DOI] [PubMed] [Google Scholar]
- 75.Diwadkar VA, et al. , Dysfunctional Activation and Brain Network Profiles in Youth with Obsessive-Compulsive Disorder: A Focus on the Dorsal Anterior Cingulate during Working Memory. Front Hum Neurosci, 2015. 9: p. 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Britton JC, et al. , Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry, 2010. 49(9): p. 944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woolley J, et al. , Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br J Psychiatry, 2008. 192(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 78.Huyser C, et al. , Functional magnetic resonance imaging during planning before and after cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry, 2010. 49(12): p. 1238–48, 1248 e1–5. [DOI] [PubMed] [Google Scholar]
- 79.Huyser C, et al. , Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT. J Child Psychol Psychiatry, 2011. 52(12): p. 1251–60. [DOI] [PubMed] [Google Scholar]
- 80.Fitzgerald KD, et al. , Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry, 2013. 52(11): p. 1183–1191 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Button KS, et al. , Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci, 2013. 14(5): p. 365–76. [DOI] [PubMed] [Google Scholar]
- 82.Abramovitch A, et al. , Neuroimaging and neuropsychological findings in pediatric obsessive-compulsive disorder: a review and developmental considerations. Neuropsychiatry, 2012. 2(4): p. 313–329. [Google Scholar]


