Abstract
The severe acute respiratory syndrome coronavirus 2 pandemic poses extraordinary challenges. The tremendous number of coronavirus disease 2019 (COVID-19) cases in the United States has resulted in a large population of survivors with prolonged postinfection symptoms. The creation of multidisciplinary post-COVID-19 clinics to address both persistent symptoms and potential long-term complications requires an understanding of the acute disease and the emerging data regarding COVID-19 outcomes. Experience with severe acute respiratory syndrome and Middle East respiratory syndrome, post-acute respiratory distress syndrome complications, and post-intensive care syndrome also informs anticipated sequelae and clinical program design. Post-COVID-19 clinical programs should be prepared to care for individuals previously hospitalized with COVID-19 (including those who required critical care support), nonhospitalized individuals with persistent respiratory symptoms following COVID-19, and individuals with preexisting lung disease complicated by COVID-19. Effective multidisciplinary collaboration models leverage lessons learned during the early phases of the pandemic to overcome the unique logistical challenges posed by pandemic circumstances. Collaboration between physicians and researchers across disciplines will provide insight into survivorship that may shape the treatment of both acute disease and chronic complications. In this review, we discuss the aims, general principles, elements of design, and challenges of a successful multidisciplinary model to address the needs of COVID-19 survivors.
Key Words: clinical outcomes, COVID-19, multidisciplinary clinic design, post-COVID-19 programs, SARS-CoV-2
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; HRQoL, health-related quality of life; MERS, Middle East respiratory syndrome; PFT, pulmonary function test; PICS, post-intensive care syndrome; RECOVERY, Comprehensive Post-COVID Center at Yale; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has devastated patients, communities, and health-care systems. More than 170 countries have been affected by the COVID-19 pandemic, with over 6.8 million cases and 200,000 deaths in the United States as of September 23, 2020.1 , 2 Although efforts to manage the ongoing pandemic must remain a priority, our clinical response must also address the needs of a large COVID-19 survivorship.3 Health-care systems must develop clinical infrastructures to address the complex needs of COVID-19 survivors experiencing significant persistent respiratory symptoms and must anticipate potential long-term pulmonary and nonpulmonary sequelae. In this review, we touch on individual risk factors and features of acute disease that impact post-COVID-19 care, explore potential post-COVID-19 complications, and propose a clinical model for the multidisciplinary care of COVID-19 survivors.
Features of Acute Disease Impacting Post-COVID-19 Clinic Design
Risk Factors and Comorbidities
Our understanding of risk factors is evolving rapidly. In the United States, significant health-care disparities have been documented during the pandemic. SARS-CoV-2 infection and associated mortality are disproportionately high in Black and Latinx patients, many of whom are essential workers.4 , 5 Within the health-care industry, women and nonphysician staff are disproportionately affected.6
Current Centers for Disease Control and Prevention (CDC) guidelines identify the following risk factors for severe disease and complications: older age, cancer, COPD, chronic kidney disease, immunocompromised state from solid organ transplant, obesity, serious heart conditions, sickle cell disease, and type 2 diabetes.7 Although COPD is currently the only pulmonary disease identified as a risk factor by the CDC, 18% of infected individuals have chronic lung disease,8 and those with moderate to severe asthma, cystic fibrosis, pulmonary fibrosis, or active smoking are considered at potentially increased risk pending further study.7 In addition, investigators have postulated that the levels of the angiotensin-converting enzyme 2 receptor, the viral entry point into the cell, and its variable expression in patients with underlying lung disease or smoking exposure may impact susceptibility and disease severity.9 , 10
Acute Manifestations
The primary pulmonary manifestations of SARS-CoV-2 infection include hypoxemia, dyspnea, and cough. Extrapulmonary symptoms vary, with fever, fatigue, headache, myalgia, and diarrhea commonly reported.11 Although most infections are mild (81%), a subset of individuals develop severe disease manifestations.12, 13, 14 Hallmarks of severe disease include hypoxemic respiratory failure, ARDS, sepsis, septic shock, and multiorgan dysfunction.15
The predominant reason for hospitalization is hypoxemia. Although many patients with hypoxemic respiratory failure are successfully treated by noninvasive strategies,16 those with severe respiratory failure require mechanical ventilation along with adjunctive paralytics, sedation, prone positioning, and, in selected cases, extracorporeal membrane oxygenation.17 , 18 Patients surviving these advanced life support measures are anticipated to have long-term sequelae similar to ARDS survivors and will require post-COVID-19 evaluation and longitudinal care.
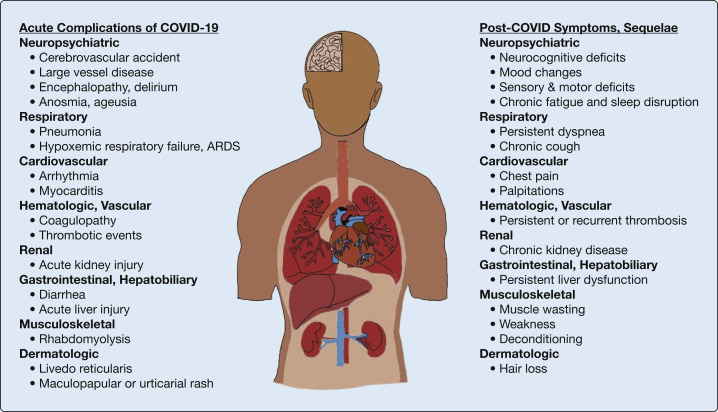
Significant acute extrapulmonary manifestations can occur in virtually any organ (Fig 1 ). This could be due to direct viral injury via the angiotensin-converting enzyme-2 receptor or due to nonspecific systemic insults such as poor perfusion, treatment toxicities, or systemic inflammation.12 , 19, 20, 21 The dysregulated proinflammatory responses induced by SARS-CoV-2 infection can lead to a maladaptive “cytokine storm,” which can contribute to multiorgan dysfunction and death.22 , 23 There is also growing appreciation for thrombotic complications in patients with COVID-19. Autopsy series have revealed microthrombus formation in the pulmonary macro- and microvasculature, as well as in the skin, cardiac, and renal microvasculature.24, 25, 26 The involvement of multiorgan vascular beds may represent a different unifying mechanism of multiorgan injury in COVID-19.
Figure 1.
Model of acute pulmonary and extrapulmonary complications of coronavirus disease 2019 (COVID-19) with projected post-COVID-19 symptoms and end-organ sequelae.
Although the full spectrum of extrapulmonary manifestations is beyond the scope of this article, acute cardiac, neurologic, neuromuscular, and hematologic complications have significant implications for post-COVID-19 clinical program design. For example, cardiac complications including arrhythmias, acute coronary syndrome, myocarditis, and heart failure have been described.27, 28, 29, 30 Neurologic and neuromuscular manifestations are another area of significant concern. An early cohort study reported frequent occurrence of acute cerebrovascular disease (6% in severe infection vs 1% in nonsevere), impaired consciousness (15% vs 2%), and skeletal muscle injury (19% vs 5%).20 Coagulopathies and thrombotic complications have also been described.31 High rates of VTE have been observed despite appropriate prophylaxis, and both the ideal approach to acute events as well as implications for chronic disease remain uncertain.32
Acute Treatment
In addition to supportive therapy, individuals with COVID-19 have received antiviral agents, immunomodulatory agents, and convalescent plasma as part of their care.33, 34, 35 Remdesivir shortens recovery in severely ill hospitalized adults, received emergency authorization by the US Food and Drug Administration, and has been used extensively in patients with COVID-19.36 Similarly, as of late June 2020, the National Institutes of Health guidelines recommend corticosteroids, specifically dexamethasone when available, in patients with COVID-19 who are mechanically ventilated or require supplemental oxygen.37 A number of other immunomodulatory therapies are under investigation for treatment of the hyperinflammatory response in severely ill patients, and potential adverse effects of these therapies must be a consideration in the care of COVID-19 survivors.
Outcomes of Acute Infection
Data regarding COVID-19 outcomes are evolving, significantly impacted by both methodologic variability and access to confirmatory testing. Of 1.3 million laboratory-confirmed US cases as of May 30, 2020, 14% required hospitalization, 2% required ICU care, and 5% died.8 Protracted hospitalizations and prolonged convalescence have been noted. A Seattle-based study of hospitalized patients demonstrated a median length of ICU stay of 14 days and a median length of hospital stay of 17 days.38 Preexisting lung disease may increase risk for poor COVID-19 outcomes. In a meta-analysis of seven studies with 1,592 patients, individuals with COPD demonstrated a nearly sixfold increased risk for ICU care, mechanical ventilation, or death.39 Although patients with asthma have not consistently demonstrated an increased risk of SARS-CoV-2 infection or severe disease, a recent review of 10,926 COVID-19-related deaths identified an increased risk of death (hazard ratio, 1.55) among patients with asthma requiring oral corticosteroids in the past year.40 , 41 Current evidence suggests that patients with cystic fibrosis are not at increased risk for COVID-19 or for increased disease severity.42 Patients with preexisting underlying interstitial lung disease are at increased risk of death from COVID-19 and may be at particularly high risk for the thromboembolic complications associated with COVID-19.43 , 44
Anticipating Potential Post-COVID-19 Complications
The clinical trajectory and long-term outcomes for COVID-19 survivors are unknown. Predictions about post-COVID-19 complications are based on emerging data in COVID-19 survivors, established complications in survivors of prior respiratory virus and zoonotic coronavirus outbreaks, and evidence of long-term sequelae following ARDS and other forms of critical illness.
Available studies of COVID-19 survivors suggest a spectrum of persistent respiratory dysfunction. An observational study of 110 Chinese patients demonstrated that diffusion impairment was present at the time of discharge in 30% of those with mild and 84% of those with severe COVID-19.45 In addition, 25% of patients demonstrated reductions in total lung capacity, and 5% demonstrated obstructive physiology.45 Other case series have revealed that 44% to 83% of individuals, particularly those with higher inflammatory markers, longer hospitalizations, or initial interstitial changes, have residual radiographic findings of ground-glass opacities and fibrotic changes suggesting persistent interstitial disease.46 , 47 Investigators have inferred that a subset of patients may be at risk for developing either persistent fibrotic lung disease or secondary organizing pneumonia.48, 49, 50, 51 However, with limited longitudinal follow-up, it is difficult to distinguish whether these patterns represent a secondary process or ongoing recovery from the initial infectious insult. There is also the potential for persistent airway disease. Bronchial wall thickening and bronchiectasis have been observed on CT scans in 10% to 23% of patients with COVID-19.52 In a separate case series, 42% of patients had small-airway dysfunction on spirometry, and obstruction was observed in up to 25% of patients.46 Finally, limited autopsy studies have demonstrated diffuse alveolar damage, fibrosis, and extensive microthrombi. The long-term implications of this in survivors remain to be seen but may increase risk for the development of chronic thromboembolic pulmonary hypertension.24 , 53 , 54
Although the 2003 severe acute respiratory syndrome (SARS) and 2012 Middle East respiratory syndrome (MERS) coronavirus outbreaks affected fewer patients, insight from survivor cohorts may help to predict long-term outcomes in COVID-19.55 , 56 Like SARS-CoV-2, these viruses were frequently associated with severe hypoxemic respiratory failure and ARDS.57 A longitudinal study of 97 SARS survivors found persistent radiographic abnormalities and diffusion capacity impairments in 28% and 24% of patients, respectively, at 1 year.58 These findings were associated with reductions in functional capacity and health-related quality of life (HRQoL).58 Similarly, high-resolution CT studies of 258 survivors with diffusion capacity impairment revealed fibrotic changes in 80%, particularly in older patients and in those who had required ICU care.59 These findings are echoed in studies of MERS survivors, who frequently demonstrate fibrotic changes, abnormal spirometry, impaired diffusion, and reduced exercise capacity.60, 61, 62
The post-ARDS literature provides important insights as well. Longitudinal studies of ARDS survivors demonstrate heterogeneous lung function impairments at short-term follow-up that generally resolve by 1 year.63 However, up to 5 years later, many survivors continue to demonstrate significant physical function impairments, suggesting that the legacy of severe lung injury extends beyond the direct impact on lung function.63 , 64 Pulmonary fibrosis is also a well-described ARDS complication, and the presence of persistent radiologic abnormalities correlates with both persistent restrictive physiology and decreased HRQoL.65 Importantly, many of the early studies in ARDS survivors describe patients with subjective dyspnea and protracted physical function limitations out of proportion to the degree of pulmonary function impairment. This suggests a contribution by nonpulmonary factors such as critical illness neuromyopathy.63 , 64 , 66 The finding of dyspnea out of proportion to pulmonary function impairment is anecdotally reported in many COVID-19 survivors, even those with milder disease. Whether post-COVID-19 symptoms correlate more closely with physical function impairments than persistent lung function decrement remains to be seen, but merits exploration in the care of post-COVID-19 patients.
Last, COVID-19 survivors who require ICU care are expected to have a high prevalence of post-intensive care syndrome (PICS). PICS encompasses impairments in physical, cognitive, and mental health function after any critical illness.67 Nearly all ICU survivors are impaired in one or more of these domains at discharge, and co-occurrence of impairments in the year after a critical illness is common. Although not formally part of PICS, persistent sleep disruption is also common among critical illness survivors and likely impairs recovery in all PICS domains.68 , 69 Supporting concerns for PICS in COVID-19 survivors, a meta-analysis of SARS and MERS survivors highlighted high prevalence of post-traumatic stress disorder (39% of survivors), depression (33%), and anxiety (30%), as well as low HRQoL.62 Regarding anticipated physical function impairments, it is reasonable to expect the prolonged functional deficits related to muscular weakness and neuromyopathy observed in other causes of critical illness and ARDS.63 , 64 In fact, 19% of patients with COVID-19 have demonstrated skeletal muscle injury during acute illness.20
We recognize that not all patients with significant post-COVID-19 symptoms will have had severe disease or have required hospitalization. Anecdotal reports and patient interactions indicate that even those classified as having had mild disease continue to have protracted symptoms that overlap considerably with those recovering from more severe disease. In a single-center study of 143 patients recovering from COVID-19, 44% reported decreased quality of life and 87% of patients reported persistent symptoms including dyspnea, chest pain, cough, fatigue, and joint pain.70 In sum, a comprehensive, longitudinal approach to post-COVID-19 care will require strategies and resources to meet the common and divergent needs of these populations, addressing both pulmonary and extrapulmonary concerns and complications.
Blueprint for RECOVERY
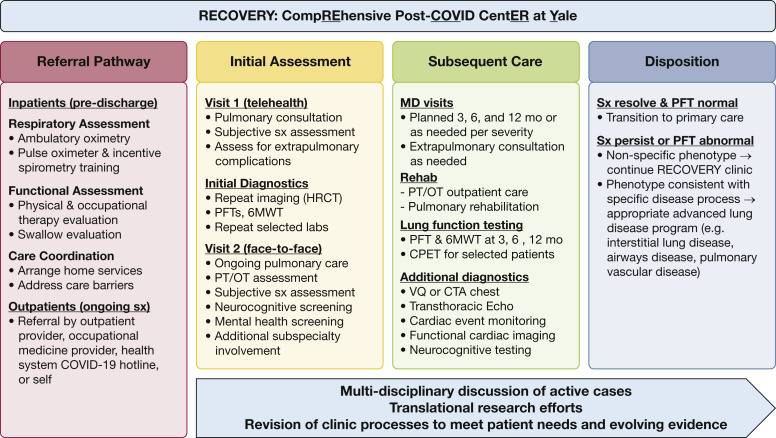
Multiple centers across the United States are creating multidisciplinary ambulatory programs for the post-COVID-19 population. These programs may differ based on local resources and needs but will share common goals, challenges, and design elements. The Yale New Haven Health system, which spans five hospitals across Connecticut and southern Rhode Island, discharged more than 3,500 patients with moderate to severe COVID-19 from March to mid-August. In an observational study of 2,154 patients with COVID-19 admitted to Yale New Haven Health, 76% were discharged alive within the study period (March 1 to April 30, 2020); among these survivors, 12% required mechanical ventilation during their admission.71 The impact of COVID-19 survivorship in our community is substantial. Below, we outline our specific approach to caring for these patients as part of the RECOVERY (Comprehensive Post-COVID Center at Yale) program developed within our academic pulmonary practice (Fig 2 ).
Figure 2.
The RECOVERY clinic model. 6MWT = 6-min walk test; COVID-19 = coronavirus disease 2019; CPET = cardiopulmonary exercise testing; CTA = CT angiogram; Echo = echocardiogram; HRCT = high-resolution CT; OT = occupational therapy; PFT = pulmonary function test; PT = physical therapy; RECOVERY = Comprehensive Post-COVID Center at Yale; sx = symptoms; VQ = ventilation-perfusion scan.
Principles and Design of a Multidisciplinary Model
The primary goals of RECOVERY are to (1) provide a comprehensive evaluation of post-COVID-19 complications, (2) characterize and mitigate pulmonary sequelae of COVID-19, and (3) address persistent symptoms experienced by post-COVID-19 survivors. Given the multisystemic and heterogeneous nature of COVID-19, we will also identify and address nonpulmonary sequelae, including extrapulmonary organ dysfunction, physical rehabilitation needs, cognitive impairments, and psychosocial vulnerabilities. Key populations of interest to RECOVERY include individuals hospitalized with moderate to severe disease, nonhospitalized individuals with persistent respiratory symptoms, and individuals with established preexisting lung disease.
Traditional multidisciplinary models incorporate multiple specialists in a centralized location to facilitate patient visits. However, pandemic-related inpatient service demands, disrupted ambulatory practices, social distancing, and physical space limitations require creative adaptation of this model. Given the predominance of pulmonary symptoms among survivors and the potential for long-term pulmonary disease, we have centered this model within our pulmonary practice. Targeted collaborations with multidisciplinary stakeholders leverage a combination of coordinated in-clinic evaluations, hub-and-spoke external consultations (including e-consults and telehealth consults), and case conferences to facilitate implementation of a patient-centered treatment program. Importantly, this model conserves space and staff resources for sustainability. Although our local transmission rates have declined, future surges may necessitate simultaneous care for patients newly infected with SARS-CoV-2 and post-COVID-19 survivors.
As above, the RECOVERY program is designed to accommodate post-COVID-19 patients across the spectrum of severity. For hospitalized patients, standardized predischarge assessment bundles facilitate the transition to ambulatory evaluation, identifying priorities for initial assessment. For nonhospitalized patients, prioritized concerns are identified by the referring physician and the patient. Given logistical challenges, including infection control measures and physical space restrictions, we have employed expedited telehealth visits, followed shortly thereafter by an in-clinic comprehensive evaluation. This initial telehealth visit allows the physician and patient to review individual case details and identify prominent symptoms or concerns. This information guides selection of subsequent diagnostics, targeted subspecialty referrals, and prioritization of in-clinic assessments. In our experience, most patients, regardless of disease severity, describe persistent dyspnea and exertional limitations. As such, the standard initial diagnostic evaluation includes comprehensive pulmonary symptom assessment, pulmonary function tests (PFTs, including spirometry, lung volumes, diffusion capacity, and 6-min walk test), physical function assessment with a physical therapist, and repeat imaging (with chest radiograph or high-resolution CT scan). When extrapulmonary issues are identified, subspecialists are engaged through multidisciplinary case review, electronic/telehealth consults, or formal in-clinic consultations as appropriate. Our program has cultivated COVID-19-specific collaboration with multiple specialties including cardiology, neurology, psychiatry, hematology, otolaryngology, and sleep medicine to date. Additional laboratory and radiologic studies are tailored to individual patient course, prior laboratory abnormalities, and active symptoms. For example, we anticipate that cardiopulmonary exercise testing will help differentiate causes of dyspnea in those with otherwise normal PFT results.
The optimal role of bronchoscopy and/or surgical lung biopsy in individuals with persistent or evolving infiltrates after SARS-CoV-2 infection also remains to be defined and has been approached on a case-to-case basis. The decision to institute corticosteroids for presumed secondary organizing pneumonia and/or defining the length of therapy for those already receiving corticosteroids remains uncharted territory. For the subset of patients with evidence of fibrosis, it is unclear if this will persist or progress; as such, the role of antifibrotic therapy remains speculative.
We emphasize physical therapy in our initial evaluation model based on the symptoms and deficits reported by our earliest patients, and our perception that rehabilitation will play a crucial role in pulmonary and nonpulmonary recovery. Social distancing and ambulatory rehabilitation closures during the COVID-19 pandemic have compounded the sedentarism of acute illness, thereby increasing the risk of skeletal muscle dysfunction in recovering patients. Addressing rehabilitation needs across all illness severity can improve physical function and other aspects of HRQoL.72 Programs can be implemented using a combination of at-home exercise plans, individual outpatient therapy sessions, or enrollment in pulmonary rehabilitation. Efforts are underway to incorporate telemedicine and wearable devices into pulmonary rehabilitation practice to improve the efficacy of these modalities within COVID-19 pandemic constraints.
On the basis of prior experiences with all-cause ARDS, SARS, and MERS, we expect that a subset of patients will require longitudinal pulmonary care, and plan to monitor patients regularly for a minimum of 1 year. Over subsequent visits, patients with persistent symptoms and respiratory pathology may diverge into follow-up care within programs in interstitial lung disease, airway disease, and pulmonary vascular disease, depending on phenotypic differentiation. Those with complete resolution of pulmonary symptoms and radiologic abnormalities with normal PFT results would ultimately continue under the long-term guidance of primary care.
Adapting Ambulatory Care to Pandemic Practice
The realities of “pandemic practice” have required creativity and allowed innovation. Although telehealth’s success in pulmonary care predates COVID-19, the initial COVID-19 surge accelerated broad-based telehealth visit adoption to overcome physical space limitations, infection control concerns, isolation protocol measures, and workforce redeployment. Telehealth has allowed for frequent monitoring of recently discharged patients with active symptoms, facilitated triage, and promoted involvement of appropriate subspecialty services.73 , 74 Our institution has also leveraged remote monitoring during the pandemic. At discharge, patients with COVID-19 with baseline or exertional hypoxemia are given pulse oximeters and enrolled in a 2-week monitoring program supervised by a primary care nurse care coordinator. Finally, we have optimized patient and provider use of the electronic health record and communication portal. This facilitates previsit completion of patient-reported symptom measures across multiple domains, improving clinic efficiency and increasing patient engagement.
Ongoing infection control is, of course, of paramount concern for both patients and staff. The provision of nonemergency clinical care is guided by the CDC time- and symptom-based approaches to discontinuation of isolation. The CDC recommends against test-based strategies, which require documenting viral clearance to discontinue isolation except in special circumstances. This is because tests detecting virus may remain positive for several weeks after acute infection, and it is unclear if this suggests the presence of infectious virus.75 , 76 However, as an aerosol-generating procedure, PFTs at our institution require a negative COVID-19 test within 72 h of the procedure. Finally, as the presence of short-term or long-term immunity in post-COVID-19 patients is unknown, it is not our practice to routinely order serologic testing, and serologic status does not have a bearing on infection control approaches.
Opportunities for Research and Collaboration
Given limited knowledge regarding the trajectory of COVID-19, collaborations between physicians and researchers are essential. The RECOVERY program affords the opportunity to develop a longitudinal cohort of convalescent patients to prospectively identify the incidence, prevalence, and persistence of pulmonary and extrapulmonary complications that are associated with SARS-CoV-2 infection. Variables to be explored include demographic characteristics, clinical parameters such as medical comorbidities, COVID-19-related treatment regimens, and biological variables such as inflammatory markers and coagulation parameters. COVID-19 survivors are also at risk for adverse psychosocial and socioeconomic consequences, including an increased risk for disability. Patient-reported outcomes, including measures of HRQoL, mental health, dyspnea, and cough, should be monitored over time. Investigators should also focus on how the intersection of race, socioeconomic status, occupation, and differential health-care access may contribute to the disproportionate impact of COVID-19 on Black and Latinx communities.
RECOVERY program patients may also participate in serial collection of biospecimens to foster understanding of the long-term trajectory of COVID-19. Studies of such specimens may support biomarker discovery and support efforts to predict the development of chronic disease. Furthermore, collecting and analyzing specimens suitable for multi-omics, microbiome, and virome studies can catalyze scientific discovery across disciplines. These efforts can lead to collaborations between academic, industry, governmental, and patient advocacy organizations. This enhances the power of any individual or institutional effort and allows for a more nuanced understanding of the impact of regional, national, and international practices post-COVID-19.
Conclusion
The COVID-19 pandemic has posed unprecedented challenges to the medical community. Although much focus necessarily remains on reducing transmission, case detection, and management of acute COVID-19, we must work in parallel to address the needs of those recovering from this illness. Effective and sustainable care models will need to leverage the successes of telehealth and remote monitoring, employ creative strategies for multidisciplinary engagement, and adapt to the shifting logistical landscape of the ongoing pandemic. Comprehensive evaluation of survivors will refine our understanding of the clinical course of COVID-19 and facilitate the development of care plans to mitigate symptoms and complications for survivors. Ultimately, post-COVID-19 programs like RECOVERY are poised to play a crucial role in pandemic response for years to come.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. P. K. is supported by the NHLBI [grant K23 HL138229] and the American Academy of Sleep Medicine Foundation. G. C. is supported by grants UH2HL123876-01, UM1AI114271, and U23HL138998, and by grants from CureVac AG and GlaxoSmithKline. C. S. D. C. was supported by RO1HL126094 and VA Merit BX004661, and by a Department of Defense grant [PR181442]. L. E. F. is supported by a Paul B. Beeson Emerging Leaders Career Development Award in Aging from the National Institute on Aging [grant K76AG057023] and by the Yale Claude D. Pepper Older Americans Independence Center [grant P30AG021342]. E. L. H. was supported by grants R01HL15267701, R01HL109233, R01HL125850, and U01HL112702, and by grants from the Gabriel and Alma Elias Research Fund and the Greenfield Foundation. C. L. R. has participated on COPD scientific advisory boards for GlaxoSmithKline Pharmaceuticals and Boehringer Ingelheim Pharmaceuticals and has participated in clinical research funded by AstraZeneca. C. R. was supported by grants from the Parker B. Francis Foundation, the Foundation for Sarcoidosis Research, and Boehringer Ingelheim. M. G. is supported by a grant from the NHLBI [grant R01 HL12580-01A1], Boehringer Ingelheim, and the Pulmonary Fibrosis Foundation. None declared (D. D. L., D. E. A.-O., L. C., J. K., I. S., M. T., V. W., J. D. P.).
Footnotes
Drs Lutchmansingh and Knauert contributed equally.
Drs Gulati and Possick contributed equally.
References
- 1.World Health Organization (WHO) Coronavirus Disease (COVID-19): Situation Report—138. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200606-covid-19-sitrep-138.pdf?sfvrsn=c8abfb17_4
- 2.Centers for Disease Control and Prevention (CDC) CDC COVID Data Tracker: United States COVID-19 Cases and Deaths by State. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 3.Connecticut Department of Public Health Connecticut COVID-19 Data Tracker. https://portal.ct.gov/Coronavirus
- 4.Centers for Disease Control and Prevention (CDC) Coronavirus Disease (COVID-19): Health Equity Considerations and Racial and Ethnic Minority Groups. http://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
- 5.Lancet The plight of essential workers during the COVID-19 pandemic. Lancet. 2020;395(10237):1587. doi: 10.1016/S0140-6736(20)31200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai X., Wang M., Qin C., et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Network Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Coronavirus Disease 2019 (COVID-2019): People With Certain Medical Conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 8.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance: United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung J.M., Yang C.X., Tam A., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai G., Bossé Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201(12):1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 12.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed]
- 13.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Severe Outcomes Among Patients With Coronavirus Disease 2019 (COVID-19): United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Clinical Management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 [PubMed]
- 16.Damarla M., Zaeh S., Niedermeyer S., et al. Prone positioning of nonintubated patients with COVID-19. Am J Respir Crit Care Med. 2020;202(4):604–606. doi: 10.1164/rccm.202004-1331LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 18.Falcoz P.E., Monnier A., Puyraveau M., et al. Extracorporeal membrane oxygenation for critically ill patients with COVID-19-related acute respiratory distress syndrome: worth the effort? Am J Respir Crit Care Med. 2020;202(3):460–463. doi: 10.1164/rccm.202004-1370LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2020 Jun 27. 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed]
- 23.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranard L.S., Fried J.A., Abdalla M., et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13(7) doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung I.F.N., To K.K.W., Lee C.K., et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A (H1N1) infection. Chest. 2013;144(2):464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 34.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortegiani A., Ippolito M., Greco M., et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2020;27(1):52–66. doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19: preliminary report [reply] N Engl J Med. 2020;383(10):994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health (NIH) COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/#:∼:text=On%20the%20basis%20of%20the,who%20are%20mechanically%20ventilated%20(AI) [PubMed]
- 38.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19 death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosgriff R., Ahern S., Bell S.C., et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19(3):355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprunger D.B., Olson A.L., Huie T.J., et al. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012;39(1):125–132. doi: 10.1183/09031936.00041411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake T.M., Docherty A.B., Harrison E.M., et al. ISARIC4C Investigators Outcome of hospitalization for COVID-19 in patients with interstitial lung disease: an international multicenter study. Am J Respir Crit Care Med. 2020;202(12):1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You J., Zhang L., Ni-Jia-Ti M.Y., et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020;81(2):e150–e152. doi: 10.1016/j.jinf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu M., Liu Y., Xu D., Zhang R., Lan L., Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchiori E., Zanetti G., Ferreira Francisco F.A., Hochhegger B. Organizing pneumonia as another pathological finding in pandemic influenza A (H1N1) Med Intensiva. 2013;37(1):59. doi: 10.1016/j.medin.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson S., Kay F.U., Abbara S., et al. Radiological Society of North America Expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA-Secondary Publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spagnolo P., Balestro E., Aliberti S., et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmud E., Madani M.M., Kim N.H., et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol. 2018;71(21):2468–2486. doi: 10.1016/j.jacc.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Klok F.A., Couturaud F., Delcroix M., Humbert M. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Eur Respir J. 2020;55(6):2000189. doi: 10.1183/13993003.00189-2020. [DOI] [PubMed] [Google Scholar]
- 55.Peiris J.S., Lai S.T., Poon L.L., et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 57.Hui D.S. Epidemic and emerging coronaviruses (severe acute respiratory syndrome and Middle East respiratory syndrome) Clin Chest Med. 2017;38(1):71–86. doi: 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hui D.S., Wong K.T., Ko F.W., et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie L., Liu Y., Xiao Y., et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das K.M., Lee E.Y., Singh R., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park W.B., Jun K.I., Kim G., et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci. 2018;33(24):e169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 63.Herridge M.S., Cheung A.M., Tansey C.M., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 64.Herridge M.S., Tansey C.M., Matte A., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 65.Burnham E.L., Janssen W.J., Riches D.W., Moss M., Downey G.P. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43(1):276–285. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orme J., Jr., Romney J.S., Hopkins R.O., et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167(5):690–694. doi: 10.1164/rccm.200206-542OC. [DOI] [PubMed] [Google Scholar]
- 67.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 68.Altman M.T., Knauert M.P., Murphy T.E., Ahasic A.M., Chauhan Z., Pisani M.A. Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: an observational cohort study. Ann Intensive Care. 2018;8(1):63. doi: 10.1186/s13613-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Altman M.T., Knauert M.P., Pisani M.A. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457–1468. doi: 10.1513/AnnalsATS.201702-148SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carfì A., Bernabei R., Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McPadden J, Warner F, Young HP, et al. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. medRxiv. DOI: 10.1101/2020.07.19.20157305. [DOI] [PMC free article] [PubMed]
- 72.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Portnoy J.M., Waller M., De Lurgio S., Dinakar C. Telemedicine is as effective as in-person visits for patients with asthma. Ann Allergy Asthma Immunol. 2016;117(3):241–245. doi: 10.1016/j.anai.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27(7):1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention (CDC) Discontinuation of Isolation for Persons with COVID-19 Not in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html
- 76.Park S.Y., Yun S.G., Shin J.W., et al. Persistent severe acute respiratory syndrome coronavirus 2 detection after resolution of coronavirus disease 2019-associated symptoms/signs. Korean J Intern Med. 2020;35(4):793–796. doi: 10.3904/kjim.2020.203. [DOI] [PMC free article] [PubMed] [Google Scholar]