Abstract
Background
Corona Virus Disease 2019 (COVID-19) cases continue to increase around the World. Typical symptoms include fever and respiratory illness but a constellation of multisystem involvement including central nervous system (CNS) and peripheral nervous system (PNS) have been reported with COVID-19. Acute ischemic strokes (AIS) have also been reported as a complication.
Methodology
We analyzed patient characteristics, clinical outcomes, laboratory results and imaging results of four patients with COVID-19 who had AIS.
Results
All four patients were =< 60 years, had hypoxemic respiratory failure secondary to pneumonia, elevated D-dimer and inflammatory markers.
Conclusion
Ischemic strokes are known complications in patients with severe COVID-19.
Keywords: Stroke, Covid-19
Highlights
-
•
There is an association between acute ischemic stroke and COVID-19 infection.
-
•
COVID-19 related strokes can be multifocal.
-
•
Hypercoagulability and vasculitis due to intracranial cytokine storm are among the proposed mechanisms for AIS in COVID-19.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first reported in Wuhan, China in December 2019 (Avula et al., 2020a). Since that time, 44 million people tested positive and more than 1,100,000 have died worldwide by the end of October 2020 (D-19D). The clinical presentation varies between asymptomatic infection, mild respiratory infection, severe pneumonia with respiratory failure and death (ZhouTing et al., 2020). Common symptoms of patients admitted with COVID-19 include fever, cough, sputum production and fatigue (ZhouTing et al., 2020). Older age, elevated D-dimer and higher Sequential Organ Failure Assessment (SOFA) score on presentation are associated with higher odds of in hospital death (ZhouTing et al., 2020). Elevated interleukin 6 (IL-6), troponin I and lactate dehydrogenase (LDH) as well as lymphopenia were associated with severe infection (ZhouTing et al., 2020). Coagulopathy has been reported in 19% of patients in a retrospective cohort study from China (ZhouTing et al., 2020). Multiple case reports and case series of COVID-19 patients presenting with stroke have emerged (Avula et al., 2020a, 2020b; Oxley et al., 2020; Carod-Artal, 2020; Whittaker et al., 2020; Goldberg et al., 2020; Ahmad and Rathore, 2020; Tunç et al., 2020; Yaghi et al., 2020). Cerebral venous thrombosis has also been reported in a patient who eventually tested positive for COVID-19 (Hughes et al., 2020). The proposed mechanisms for AIS in COVID-19 include hypercoagulability, vasculitis secondary to intracranial cytokine storm, new onset atrial fibrillation and the infection by the virus itself (Oxley et al., 2020; Jasti et al., 2020; Mishra et al., 2020). Here, we report 4 cases of AIS associated with COVID-19 infection.
2. Methods
Institutional Review Board (IRB) has been waived for case series at the University of Arkansas for Medical Sciences and Southern Illinois University. Chart review was performed by physicians in the neurology departments of both institutions.
28 patients presenting with COVID-19 and neurological symptoms at the University of Arkansas for Medical Sciences and 18 in the Southern Illinois University were reviewed. Four patients with COVID-19 and confirmed AIS were identified, two patients from the University of Southern Illinois Hospital and two from the University of Arkansas for Medical Sciences. Three of the four patients were admitted to medical intensive care unit and one patient was admitted to the neurology service. All four patients tested positive for SARS CoV-2 polymerase chain reaction (PCR) and had AIS. Retrospective data was collected from the electronic medical record including patient characteristics, laboratory results and imaging results.
3. Results
Details of four cases of AIS in patients who presented with symptoms related to COVID-19 infection (Table 1, Table 2).
Table 1.
Characteristics, imaging and echocardiogram of the four reported patients.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age | 60 | 59 | 55 | 59 |
| Sex | Female | Male | Male | Male |
| Risk factors for stroke | Diabetes mellitus type 2, hypertension | No known Risk Factors | Diabetes mellitus type 2, hypertension | Hypertension, DM and cardiac mass |
| Medications | Aspirin | – | Aspirin | – |
| NIHSS initial | 6 | 7 | 4 | 30 |
| NIHSS 24 h | 4 | 6 | 4 | 28 |
| NIHSS discharge | 3 | – | 4 | – |
| Outcome | Rehab | Deceased | Rehab, for cervical spinal stenosis | Deceased |
| Stroke symptom and signs | Right sided facial droop and weakness | Right sided facial droop and weakness | Presented with quadriparesis, has severe cervical spine stenosis | Right sided weakness |
| Imaging result | MRI Brain: bilateral embolic tiny cortical infarcts in different vascular territories. | CT head: Embolic infarcts in the left parieto-occipital area | MRI brain: multiple small infarcts in the centrum semiovale of the bilateral frontoparietal lobes suggestive of watershed infarcts | MRI brain: Multiple infarcts in bilateral medullar, left occipital lobe and right parahippocampal region |
| Vessel imaging | No evidence of LVO or ICAD | No evidence of LVO or ICAD | Intracranial atherosclerosis | Occlusion of the right vertebral artery |
| Acute treatment of stroke | TPA | No tPA or endovascular therapy | No tPA or endovascular therapy | No tPA or endovascular therapy |
| Treatment of COVID | Azithromycin Toclizumab | Tocilizumab | Hydroxychloroquine and azithromycin | Remdesivir and dexamethasone |
| COVID-19 symptoms | Fever, cough and shortness of breath | cough, dyspnea and confusion | Fever, cough and shortness of breath | Shortness of breath |
| ICU admission | Yes | Yes | No | Yes |
| Transthoracic Echocardiogram | Normal | Not done | Pending | Not done |
Table 2.
Laboratory results of the four reported patients.
| Laboratory finding | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| White blood count (3.6–9.5 K/μL) | 2.7 | 2.8 | 4.03 | 13.4 |
| Neutrophils (1.4–6 K/μL) | 2.o | 1.6 | 2.9 | 11.6 |
| Lymphocytes (1.2–3.4 K/μL) | 0.6 | 1.1 | 0.83 | 0.68 |
| Platelet count (K/uL) | 179 | 96 | 163 | 287 |
| Hemoglobin (13–17 g/dL) | 11.9 | 9.1 | 12.2 | 11.6 |
| Albumin (3–5 g/dL) | 3.8 | 2.4 | 3 | 3.8 |
| Alanine aminotransferase ALT (4–45 IU/L) | 28 | 387 | 39–96 | 45 |
| Aspartate aminotransferase AST (15–41 IU/L) | 29 | 226 | 49–113 | 77 |
| Lactate dehydrogenase (100–248 IU/L) | 721 | 750 | 268–452 | 359 |
| Troponin I | 0.03 | 21.3 | 1.1 | 0.23 |
| INRI (0.9–1.1) | 1.4 | 1.3 | 1.1 | |
| Activated partial thromboplastin time | 44.7 | 15.8 | 33.5 | |
| D-Dimer ng/ml | >4000 | >4000 | 378–1195 | 1498 |
| Ferritin (10.0–300 ng/ml) | 602 | >7500 | 436–1084 | 192 |
| Procalcitonin (0.00–0.10 ng/ml) | 4.2 | 12.26 | 0.16–0.90 | 11.48 |
| Fibrinogen (200–393 mg/dL) | 125 | 299 | 499–998 | 567 |
| IL-6 (0.0–6.3 pg/ml) | – | 52–115 | 38.5 | |
| C-reactive protein (less than 10) | 160 | 125.5 | 28–144 | 197.0 |
| Creatine kinase (49–397 IU/L) | 169 | 432 | 568.0 | |
| Creatinine (0.6–1.3 mg/dL) | 0.7 | 1.2 | 1.5 | 4.0 |
| Hemoglobin A1C (4–6%) | 8.6 | 6 | 6.1% | 13.2 |
| ESR (0–20 mm/h) | – | 49 | 98.0 |
3.1. Patient 1
60-year-old female with Past history of obstructive sleep apnea (OSA), hypertension and uncontrolled type 2 diabetes mellitus (DM) presented to the emergency department with cough, shortness of breath and fever after exposure to with a COVID-19 patient. She was obese with a Body mass index (BMI) of 36. Computed tomography (CT) of the chest revealed multifocal predominantly peripheral ground-glass opacities. She tested positive for COVID-19, and was admitted for further management. One day after admission she developed sudden onset right facial droop, right-sided weakness and slurred speech with National Institutes of Health Stroke Scale (NIHSS) of 6 (moderate stroke). CT head without contrast showed no intracranial hemorrhage (ICH) and intravenous tissue plasminogen activator (IV tPA) was given. CT Angiogram showed no large vessel occlusion. She eventually improved and Magnetic resonant imaging (MRI) of the brain performed 24 h later showed punctate bilateral embolic cortical infarcts (Fig. 1). She was still febrile up to 38.9 C, and her labs showed low white blood cells (WBC) 2.7 K/μL with lymphopenia 0.6 K/CUMM, Platelets 179 K/μL. She had elevated hypercoagulability markers including D-Dimer > 4000 ng/ml and fibrinogen 125 mg/dL (normal 200–393 mg/dL), in addition to elevated inflammatory markers including C-reactive protein (CRP) of 160 mg/L (normal less than 10), ferritin of 602 ng/ml (normal 10.0–300) and procalcitonin of 4.2 ng/ml (normal 0.00–0.10). Transthoracic echocardiogram (TTE) was normal with no evidence of left ventricular thrombus, normal ejection fraction and left atrial index (LAI). Patient improved to NIHSS of 3 (minor stroke). She was treated with Aspirin and Atorvastatin for secondary stroke prevention, and was discharged to inpatient rehabilitation. Event monitor showed no arrhythmias.
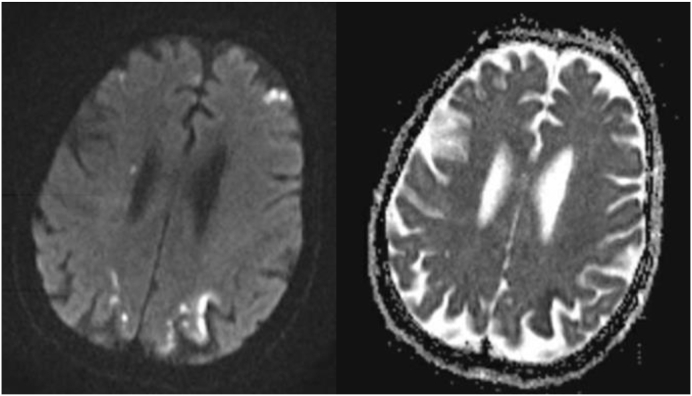
Fig. 1.
MRI brain DWI showing tiny bilateral cortical infarcts.
The hypercoagulability markers and inflammatory markers were elevated in this patient and the cause of stroke is likely COVID-19 causing hypercoagulability.
3.2. Patient 2
59-year-old man with no known vascular risk factors and diagnosed with COVID-19 (tested due to contact with SARS-CoV-2 positive sibling) who was in self quarantine presented to the hospital with progressive cough, dyspnea and confusion for 2 days. He was febrile, hypoxic and hypotensive, and quickly developed respiratory failure requiring intubation in the emergency room. CT chest showed peripheral and basilar predominant ground-glass opacities with patchy areas of consolidation. On day 3 of hospitalization he was noted to have right facial weakness with reduced movement of the right side for which stroke team was consulted. CT head showed embolic infarcts in the left parieto-occipital area (Fig. 2). His labs showed low WBCs of 2.8 K/μL, platelets 96 K/μL, in addition to elevated D-Dimer > 4000 ng/ml, CRP 125.5 mg/L, procalcitionin 12.26 ng/ml and ferritin >7500 ng/ml.
Fig. 2.
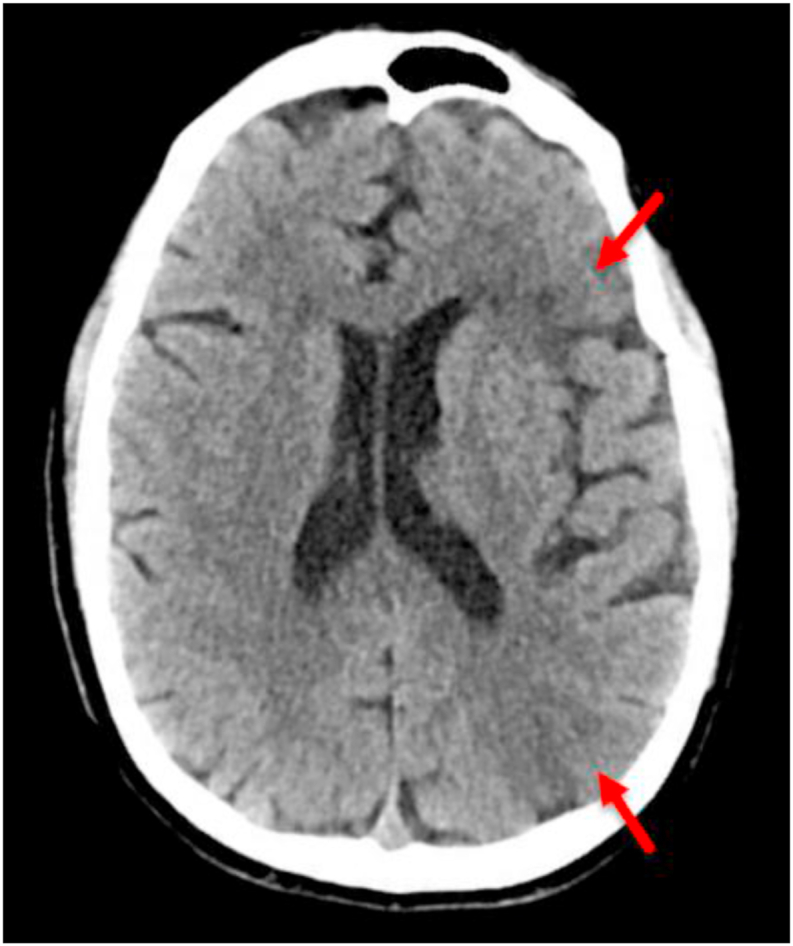
CT Head showing multiple left MCA territory parieto-occipital infarcts (Arrows).
CT Angiogram showed no evidence of large vessel occlusion or atherosclerosis. MRI brain and TTE were requested but were not done because of rapid deterioration. Hospital course was complicated with septic shock and multiorgan failure, and despite aggressive management with maximum vasopressors, and continuous renal replacement therapy,he passed away.
COVID-19 is a likely contributing factor to AIS in this case. The inflammatory and hypercoagulability markers were elevated. Other contributing factors in this patient include critical care illness and septic shock.
3.3. Patient 3
55-year-old man with history of hypertension and type 2 diabetes mellitus presented to the emergency department with fever and cough. He tested positive for SARS-CoV-2 on the day of admission. He fell four days prior to admission when his “knees gave away”. He reported two-month history of lower extremity weakness. Examination showed a mild generalized weakness which was more evident in his lower extremities with exaggerated knee reflex and extensor plantar reflexes. Rest of neurological examination was normal. MRI of the cervical spine showed degenerative changes with moderate spinal stenosis. His labs showed elevated CRP of 28 mg/L on admission that eventually went up to 144 mg/L by day 8, procalcitonin 0.16 ng/ml on day 1 and 0.90 on day 5, ferritin 436 ng/ml on day 1 and 1084 ng/ml on day 6, fibrinogen 499 mg/dL on day 1 and 998 mg/dL on day 7 (200–393), and D-Dimer 779 ng/ml on day 2 and 1195 ng/ml on day 8. CT scan of the head done on day 1 showed chronic ischemic changes and MRI of the brain on day 3 revealed multiple small infarcts in the centrum semiovale of the bilateral frontoparietal lobes suggestive of borderzone vs embolic infarcts (Fig. 3). MR Angiogram of the head and neck on day 3 showed mild atheromatous irregularity in the proximal intracranial vessels of the anterior circulation, fetal origin of the bilateral posterior cerebral arteries with diminutive size of the basilar artery and small bilateral vertebral arteries. He was treated with Aspirin and was discharged to a rehab facility on day 8 of admission with plan to perform TTE and 30-day event monitor as an outpatient and follow up in stroke clinic.
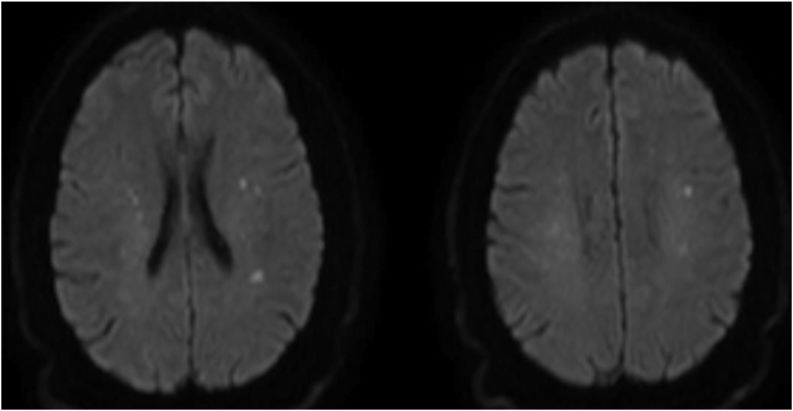
Fig. 3.
MRI brain DWI showing multiple small infarcts in the centrum semiovale of the bilateral frontoparietal lobes.
COVID-19 is likely the cause of stroke in this patient because of elevated hypercoagulability and inflammatory markers. On the other hand, the presence of possible borderzone infarcts in the setting of history of falls may point towards other causes of AIS like hypotension. Blood pressure was not low at any point during hospitalization.
3.4. Patient 4
59-year-old male with history of hypertension, diabetes millets and atrial mass was admitted with shortness of breath and hypertensive urgency. Shortly after admission, he became diaphoretic, dizzy and was falling to the right side. That was followed by hypoxemic respiratory failure and cardiac arrest. He tested positive for COVID 19. The patient was intubated emergently. On examination he was not responsive, had right sided weakness and absent right corneal response. MRI of the brain showed ischemic strokes in the entire right medulla, left medulla, left posterior occipital lobe and right posterior para hippocampal gyrus (Fig. 4). MRA showed occlusion of the right vertebral artery. Ferritin peaked at 192 ng/ml, fibrinogen went up to 567, C-reactive protein 197 and D-dimer 1498. He eventually passed away on day 7 after compassionate extubation.
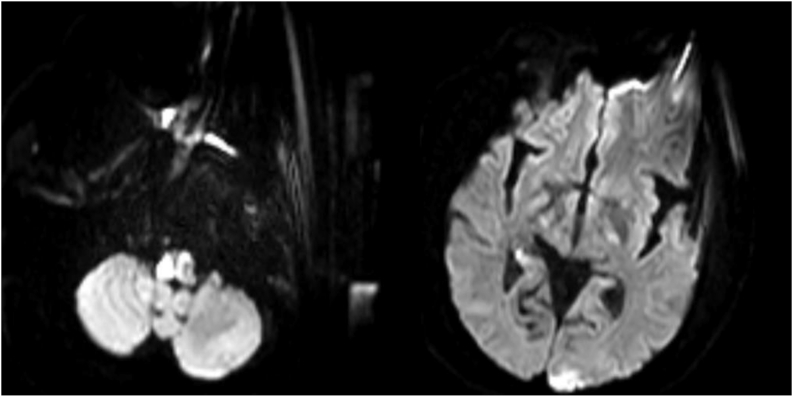
Fig. 4.
MRI brain DWI showing ischemic strokes in the entire right medulla, left medulla, left posterior occipital lobe and right posterior para hippocampal gyrus.
In this case the hypercoagulable effect of both COVID-19 and the cardiac mass likely contributed to AIS.
4. Discussion
Acute ischemic strokes in patients with COVID-19 have been reported in several case series. There have been several proposed mechanisms for AIS in COVID-19 and these include hypercoagulability leading to large vessel occlusion(especially in young patients), vasculitis secondary to intracranial cytokine storm, new onset atrial fibrillation and the infection by the virus itself (Oxley et al., 2020; Jasti et al., 2020; Mishra et al., 2020).
4.1. Hypercoagulability with large vessel occlusion
COVID-19 patients with moderate to severe AIS are noted to have a high prevalence of LVO. A case series by Oxley et al. reported five patients with large vessel ischemic stroke and COVID 19 infection in young patients (Oxley et al., 2020). Another series by Avula et al. reported four patients with ischemic stroke and COVID two of them had large vessel occlusion (Avula et al., 2020a). Patients with severe COVID-19 infection have noted to develop a hyperinflammatory state followed by a prothrombotic state that is frequently complicated by both venous and arterial thromboembolism (Klein et al., 2020; Tang et al., 2020). Simultaneous multiple LVO of different vascular territories has been consistently reported and was postulated to be secondary to the prothrombotic state induced by COVID-19 (Kaesmacher et al., 2018).
A study compared the stroke characteristics of COVID-19 positive patients with COVID-19 negative patients and historical controls. In this it was observed that patients with concomitant COVID-19 suffered severe strokes, with a higher NIHSS score with a greater proportion of large vessel occlusion (Yaghi et al., 2020). AIS in COVID-19 patients have worse outcomes in the form of higher hemorrhagic transformation and all-cause mortality. Embolic events as noted by elevated d-dimer, serum cardiac markers including high-sensitivity troponin and proBNP have been consistently reported (Thachil, 2020).
4.2. Vasculitis secondary to intracranial cytokine storm
COVID-19 has been very well reported to cause endothelial cell inflammation, apoptosis and dysfunction within arteries, arterioles, capillaries, venules and veins which in turn lead to tissue hypoperfusion, thrombosis and vascular dysfunction (Becker, 2020, 2020b). Patients with severe COVID-19 infection have noted to develop a hyperinflammatory state secondary to a cytokine storm which produced a vasculitic picture causing strokes. The exaggerated and uncontrolled activation of the immune system causing excessive cytokine release has been consistently reported in patients with severe pulmonary disease. Cytokine storm is diagnosed with the presence of elevated plasma markers of inflammation like C-reactive protein, erythrocyte sedimentation rate, procalcitonin, ferritin, interleukins (IL2, IL6, IL-7), granulocyte - colony-stimulating factor and tumor necrosis alpha (Sahu et al., 2020).
Accumulation of inflammatory cells and viral inclusions occurred within the vascular endothelium of the heart, small bowel, brain, kidneys, and lungs (Varga et al., 2020).
Hanafi et al. reported a case of SARS-CoV-2–induced vasculitis/endotheliitis. They reported extensive cerebral small-vessel ischemic lesions resembling cerebral vasculitis in a characteristic imaging pattern of ischemia, hemorrhage, and punctuate postcontrast enhancement (Hanafi et al., 2020).
Pinot et al. reported a case of central nervous system vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in a patient with COVID-19. There was rapid clinical improvement following immunomodulating treatment (Pinto et al., 2020).
4.3. New onset atrial fibrillation
Cardiac involvement is common in patients with COVID-19. Myocardial involvement mostly presents as an acute cardiac injury. This has been defined by elevated serum cardiac biomarkers and abnormal findings in the echocardiogram of an infected patient (Clerkin Kevin et al., 2019).
Patients are also reported to be at a higher risk of arrhythmia secondary to myocarditis possibly precipitating embolism(Clerkin Kevin et al., 2019; Mao et al., 2020; Klok et al., 2020; Inciardi et al., 2019).
Arrhythmias, such as atrial fibrillation, are more frequent in COVID-19 cardiomyopathy as inflammation is a substrate for atrial arrhythmias. Ventricular arrhythmias are also observed and may accompany cardiac arrest in these patients (Wang et al., 2020). It is very well known historically that Afib poses an increased risk of Cardio-embolic strokes.
4.4. Infection by the virus
Historically, viral infections like influenza have been shown to have a slightly increased risk of stroke in the past. In similar lines, severe infection with COVID-19 has been shown to have an increased risk of AIS. A study showed that the risk of acute ischemic stroke in patients admitted with COVID-19 was 1.6% versus 0.2% for patients admitted with influenza (Merkler et al., 2020). The mechanism of the apparent increase in the risk of stroke in COVID-19 patients is not clear.
5. Conclusion
Ischemic stroke can be seen as complication of COVID-19 in severe illness. More studies are needed to identify the mechanism of hypercoagulability and the increased risk of stroke in COVID-19 patients.
Declaration of competing interest
None.
References
- Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review [published online ahead of print, 2020 May 6] J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.017. S0967-5868(20)31078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., Glaser A., Elsayegh D. COVID-19 presenting as stroke. Brain Behav. Immun. 2020 Apr 28 doi: 10.1016/j.bbi.2020.04.077. S0889-1591(20)30685-1. Epub ahead of print. PMID: 32360439; PMCID: PMC7187846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A., Gill A., Nassar R., Nalleballe K., Siddamreddy S., Chalhoub M. Locked-in with COVID-19. J. Clin. Neurosci. 2020;79:80–83. doi: 10.1016/j.jocn.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis. 2020;50(3):499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Complicaciones neurológicas por coronavirus y COVID-19. Rev. Neurol. 2020;70(9):311-322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- Clerkin Kevin J., Fried Justin A., R. Jayant, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 0 (0). doi:10.1161/CIRCULATIONAHA.120.046941.
- COVID-19 Coronavirus pandemic. https://www.worldometers.info/coronavirus/ [online]. Available at:
- Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular Disease in COVID-19 [published online ahead of print, 2020 May 14] AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6588. 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafi R., Roger P.-A., Perin B., Kuchcinski G., Deleval N., Dallery F., Michel D., Hacein-Bey L., Pruvo J.-P., Outteryck O., Constans J.-M. COVID-19 neurologic complication with CNS vasculitis-like pattern. American Journal of Neuroradiology June. 2020;41(8):1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5) doi: 10.12890/2020_001691. Published 2020 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online March 27, 2020]. JAMA Cardiol. doi: 10.1001/jamacardio.2020.1096. https://jamanetwork.com/journals/jamacardiology/fullarticle/2763843. [DOI] [PMC free article] [PubMed]
- Jasti M., Nalleballe K., Dandu V. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J. Neurol. 2020;3:1–6. doi: 10.1007/s00415-020-09950-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaesmacher J., Mosimann P.J., Giarrusso M., El-Koussy M., Zibold F., Piechowiak E. Multivessel occlusion in patients subjected to thrombectomy. Stroke. 2018;49(6):1355–1362. doi: 10.1161/strokeaha.118.021276. [DOI] [PubMed] [Google Scholar]
- Klein D.E., Libman R., Kirsch C., Arora R. Cerebral venous thrombosis: atypical presentation of COVID-19 in the young. J. Stroke Cerebrovasc. Dis. 2020;29(8):104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F., Kruip M., van der Meer N. Incidence of thrombotic complications in critically ill ICU patients with COVID19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza [published online ahead of print, 2020 jul 2] JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A.K., Sahu K.K., George A.A., Lal A. A review of cardiac manifestations and predictors of outcome in patients with COVID - 19 [published online ahead of print, 2020 May 3] Heart Lung. 2020 doi: 10.1016/j.hrtlng.2020.04.019. S0147-9563(20)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.A., Carroll L.S., Nar V., Varatharaj A., Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm. 2020;7:e813. doi: 10.1212/NXI.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu K.K., Mishra A.K., Lal A. Comprehensive update on current outbreak of novel coronavirus infection (2019-nCoV) Ann. Transl. Med. 2020;8:393. doi: 10.21037/atm.2020.02.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. 2020. https://onlinelibrary.wiley.com/doi/full/10.1111/jth.14817 [DOI] [PMC free article] [PubMed]
- Thachil J. The versatile heparin in COVID-19. J. Thromb. Haemostasis. 2020;18(5):1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunç A., Ünlübaş Y., Alemdar M., Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases [published online ahead of print, 2020 May 6] J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.018. S0967-5868(20)31081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Hulme O.L., Khurshid S., Weng L.C., Choi S.H., Walkey A.J., Ashburner J.M., McManus D.D., Singer D.E., Atlas S.J. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A., Anson M., Harky A. Neurological Manifestations of COVID-19: a review [published online ahead of print, 2020 May 15] Acta Neurol. Scand. 2020 doi: 10.1111/ane.13266. 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J. SARS2-CoV-2 and Stroke in a New York Healthcare System [published online ahead of print, 2020 May 20] Stroke. 2020 doi: 10.1161/STROKEAHA.120.030335. STROKEAHA120030335. [DOI] [Google Scholar]
- Zhou Fei, Ting Yu, Ronghui Du, Guohui Fan, Ying Liu, Zhibo Liu, Jie Xiang, Yeming Wang, Bin Song, Xiaoying Gu, Lulu Guan, Yuan Wei, Hui Li, Xudong Wu, Jiuyang Xu, Shengjin Tu, Yi Zhang, Hua Chen, Bin Cao. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Published Online March 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]