Abstract
In a survey of household cats and dogs of laboratory-confirmed COVID-19 patients, we found a high seroprevalence of SARS-CoV-2 antibodies, ranging from 21% to 53%, depending on the positivity criteria chosen. Seropositivity was significantly greater among pets from COVID-19+ households compared to those with owners of unknown status. Our results highlight the potential role of pets in the spread of the epidemic.
Keywords: SARS-CoV-2, COVID-19, Pets, Seroprevalence, One health, Luminex, Neutralization assay
1. Introduction
Since its emergence in December 2019, in Wuhan, China, Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has spread throughout the world, probably exclusively through human-to-human transmission. However, the existence of hundreds of millions of companion animals living closely with humans raises the question of their susceptibility to infection and potential role in the outbreak.
Cats and dogs are known to be infected by Alphacoronaviruses and Betacoronavirus (Feline CoVs, Canine CoVs) [1], and thus may be susceptible to SARS-CoV-2, which also belongs to the Betacoronavirus group. In Europe, the prevalence of canine coronavirus infection is low [2]. Feline coronavirus prevalence is higher [[3], [4], [5]], with typical seroprevalence ranging from 50% in healthy Swiss cats to 37% in Japan. Additionally, epidemiological, biological, and virological characteristics of coronaviruses, mainly based on Spike-protein plasticity, suggest species barriers to infection may be easily crossed. Thus, pet contamination by sick owners is not only likely but perhaps expected, given the numerous opportunities for spillover [[6], [7], [8]]. The observation of several cases of mild infections in dogs and cats of infected owners, and serological surveys of pet populations reporting infection rates ranging from 0% to 15.8% [[9], [10], [11], [12]], highlight this risk. Yet, despite these observations, studies continue to suggest that the risk of contamination of pets by their owners is low and that the role of pets in the spread of the outbreak is trivial or nonexistent.
Presently there is no published study accurately assessing the contamination levels in household pets. Here we present results from a serological survey of pets conducted between May and June 2020 in two neighbouring regions of eastern France: Franche-Comté and Rhône-Alpes. Both regions had similar epidemiological characteristics and health management policies, with the first hospitalised deaths registered in March 2020 (https://www.gouvernement.fr/info-coronavirus/carte-et-donnees). The first group of pets, from the Franche-Comté region, were living in homes where at least one person expressed respiratory symptoms and tested positive for SARS-CoV-2 at the University Hospital of Besançon (COVID-19+ household group). The second group, from the Rhône-Alpes, were pets from households where exposure was unknown (unknown status household group). Lastly, we included a control group of animals sampled in 2018 and early 2019 before the outbreak, including hyperimmune sera from ten cats with feline infectious peritonitis virus (FIPV), (Control group). Inclusion FIPV-infected cat sera in the control group allows us to exclude possible cross-reactivity of antibodies generated in response to non-SARS-CoV-2 coronaviruses.
2. Materials and methods
2.1. COVID-19+ household group
The COVID-19+ household group was recruited from a cohort of 825 patients diagnosed with SARS-CoV-2 infection by reverse-transcriptase–polymerase-chain-reaction testing of nasopharyngeal swabs in the infectious tropical disease department at the University Hospital of Besançon between March 1 to April 25. From May 11 to 22, 384 patients were contacted and 84 reported owning dogs and/or cats. Thirty-one people gave us their informed consent to sample their pets (47 pets total). Whole blood samples were collected from 13 dogs and 34 cats between June 7 and June 12, 2 to 3 months after the owners were diagnosed. Clinical examination at the time of sampling indicated that all animals were healthy. More complete information about animals and households can be found in supplementary Table S1.
2.2. Unknown status household group
The unknown status household group recruited volunteers among staff and students at VetAgro Sup (Lyon's National Veterinary School). Twenty-two dogs and 16 cats (38 animals in total) from all volunteers were included. The COVID-19 status of the pet owners was unknown. Blood samples were obtained from each animal (no selection) from 14th of May to 4th of June 2020. Before blood sample collection, all animals were clinically examined using standard physical examination procedures revealing no signs of animal disease.
2.3. Neutralization activity measurement
To measure the neutralizing activity in sera, we developed a MLV-based pseudoparticle carrying a GFP reporter pseudotyped with SARS-CoV-2 spike (SARS-CoV-2 pp). Briefly, SARS-CoV-2 pp. were incubated in 1/100 dilution of sera at 37 °C for 1 h. The mix was added on reporter cells (VeroE6), spinoculated for 2 h (2.500 g, 25 °C). After 2 h of incubation, the inoculum was removed and replaced with fresh medium and cells were incubated for 72 h before FACS analysis. The level of infectivity was expressed as % of GFP positive cells and compared to cells infected with SARS-CoV-2 pp. incubated without serum. Prepandemic (including non SARS-CoV2 coronaviruses positive) sera from France were used as negative controls, and anti-SARS-CoV-2 RBD antibody was used as positive control. Seroneutralization specificity was 100% as already described.
2.4. Microsphere immunoassay
Dog and cat serum samples were tested using a multiplex Microsphere immunoassay (MIA). 10 μg of three recombinant SARS-CoV-2 antigens (nucleoprotein, spike subunit 1 and spike subunit 2 [The Native Antigen Company]) were used to capture specific serum antibodies, whereas a recombinant human protein (O6-methylguanine DNA methyltransferase) was used as a control antigen in the assay. Distinct MagPlex microsphere sets (Luminex Corp) were respectively coupled to viral or control antigens using the amine coupling kit (Bio-Rad Laboratories) according to manufacturers' instructions. This three microsphere immunoassays (MIA) were developed and provided by Institut Pasteur, Paris. The MIA procedure was performed by incubating the serum samples (50 μl), diluted 1:400 in assay buffer (PBS-1% BSA-0.05% Tween 20), with the mixture of antigen-coated bead sets (about 1250 beads of each type) protected from the light on an orbital shaker at 700 rpm for 30 min. After washing, 50 μl of biotinylated protein A and biotinylated protein G (Thermo Fisher Scientific) at a 4 μg/ml each in assay buffer were transferred to each well and incubated on an orbital shaker for 30 min at 700 rpm in the dark. After washing, the beads were incubated for 10 min at 700 rpm in the dark with 50 μl of Streptavidin-R-Phycoerythrin (Life technologies) diluted to 4 μg/ml in assay buffer. After washing, beads were resuspended in 100 μl of assay buffer. Measurements were performed using a Magpix instrument (Luminex), at least 100 events were read for each bead set and binding events were displayed as median fluorescence intensities (MFI). Relative Fluorescence Intensities (RFI) were calculated for each sample by dividing the MFI signal measured for the antigen-coated microsphere sets by the MFI signal obtained for the control microsphere set, to account for nonspecific binding of antibodies to beads. Specific seropositivity cut-off values for each antigen were set at three standard deviations above the mean RFI of the 37 dog and 14 cat samples from the control group sampled before 2019. Based on the prepandemic population, MIA specificity was set at 97,3% for dogs and 100% for cats.
2.5. Statistical analyses
Fisher's exact test was used to analyze differences in antibody detection between the COVID19+ household group and the unknown status household group, as well as tests comparing cats and dogs in COVID-19+ households.
3. Results
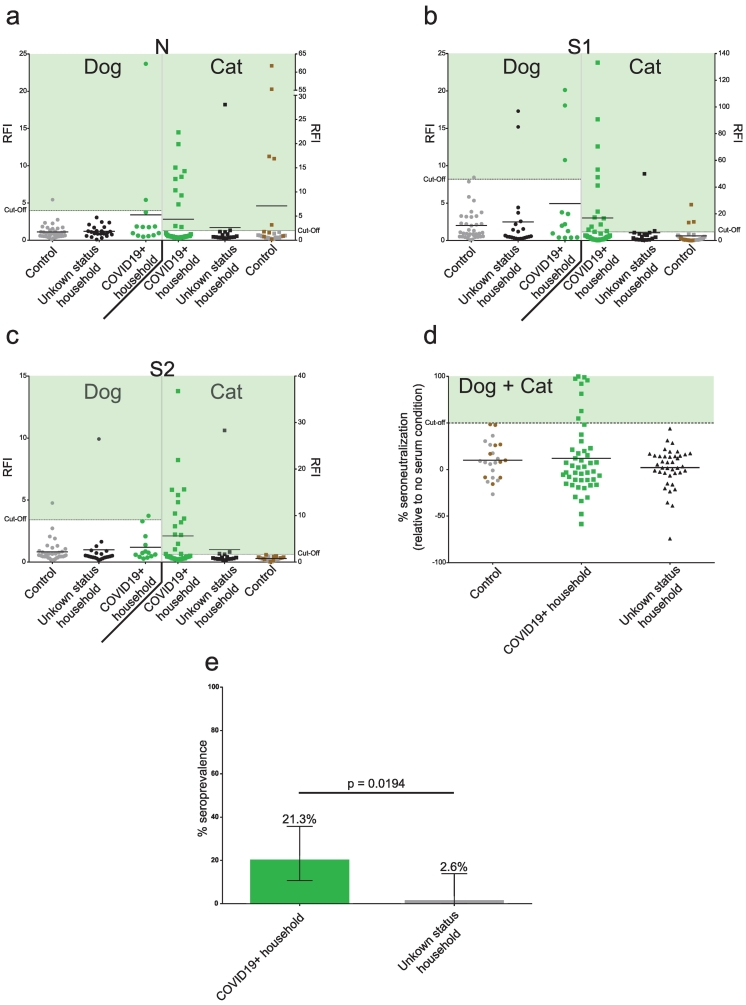
We combined four different tests based on two different techniques to ensure the greatest degree of specific-antibody detection. Three microsphere immunoassays (MIA) detected anti-SARS-CoV-2 IgGs produced in response to viral N, S1, or S2 proteins, and a retrovirus-based pseudoparticle assay detected SARS-CoV-2 neutralizing antibodies (Methods). Taking into account these two types of assays, animals were declared COVID-19 positive following a positive seroneutralization assay or if they were positive for all three MIA tests. This positivity criterion ensures 100% specificity, as none of the animals in the control group tested positive for the three MIAs or for seroneutralisation (Fig. 1a-d).
Fig. 1.
High prevalence of SARS-CoV-2 antibodies in COVID19+ household pets.
Serological evaluation of anti-SARS-CoV-2 antibodies in pets from unknown status and COVID19+ households. COVID19+ households had at least one COVID-19 laboratory-confirmed person (Green). Unknown status households were those with no confirmed SARS-CoV-2 infected person (Black). Control include pre-pandemic population (Grey) and FIPV infected cats (Brown). a: Anti-N antibody levels. b: Anti-S1 antibody levels. c: Anti-S2 antibody levels. SARS-CoV-2 specific antibody levels were assessed using MIAs and expressed as Relative Fluorescence Intensities (RFI) to control antigen. A pre-pandemic population was used to determine the cut-off (mean + 3*standard deviation). d: Percentage of neutralizing activity in pet sera. Neutralizing activity was assessed using a pseudoparticle assay and expressed as the percent neutralization relative to a no serum condition. For a,b,c,d mean line are presented. e: Prevalence based on positive anti-N, anti-S1, anti-S2, and seroneutralization tests in COVID19+ and unknown status households. 95% confidence interval are presented (± 95% confidence intervals). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A remarkably high 21.3% (10 of 47 animals tested) of pets in COVID-19+ households tested positive, including 23.5% of cats (8/34) and 15.4% of dogs (2/13), a non-significant difference (p = 0.70) (Fig. 1a-e, Supplementary tables 1–3). Of the 16 cats and 22 dogs tested from households of unknown status, only one animal (a cat) tested positive (Fig. 1a-e, Supplementary tables 1–3), representing a significantly lower seroprevalence than the COVID-19+ group (p = 0.0194). The risk of testing seropositive was eight times higher for pets sharing a home with a COVID-19+ person than for pets in homes of unknown status (relative risk of being seropositive = 8.1).
4. Discussion
Though we cannot definitively prove that all the ten positive animals were infected with SARS-CoV-2, the much greater seroprevalence in animals from COVID-19+ households provides strong evidence that pets have been infected with SARS-CoV-2.
The highly variable antibody responses to SARS-CoV-2 in human infections [13,14], calls into question our strict criteria for defining seropositive tests. If we consider an animal seropositive if any one test was positive, 53.2% in pets from COVID-19+ households show signs of having been infected (58.8% of cats (20/34) and 38.5% of dogs (5/13)) compared to 15.8% (6/38) of pets in homes of unknown status.
A recent Swiss study found that anti-N antibody assays substantially underestimate (i.e., by 30% to 45%) the proportion of SARS-CoV-2 exposed humans compared to anti-S antibody assays in population-based seroprevalence studies [15]. Assuming similar dynamics in pets, the actual seropositivity in COVID-19+ households is likely closer to 53% than 21%, indicating that infection risk in the pets of COVID-19 positive owners is much higher than previously described. Moreover, no factor (age, sex, vaccination status) was associated with the higher antibody prevalence observed among pets from COVID-19 households. However, the study's sample size may limit the ability to reach definitive conclusions.
Additionally, anti-SARS-COV-2 antibody prevalence did not appear associated with the number of pets in the households, suggesting that pet infections mainly occurred following transmission from their owners rather than from other animals. Given that cats and dogs may become infected, do they contribute to COVID-19 spread due to spillover back into humans? While viral shedding from pets does not appear sufficient for transmission to humans or other animals encountered during walks, for people in closer contact, precautionary measures should be considered as part of a ‘one health’ global control strategy.
Given the observation of potential transmission between cats following experimental infection [16,17], it is imperative to investigate the time course of the infection (especially viral load and duration of excretion) in natural conditions to better evaluate the risk of animal-to-animal and animal-to-human transmission. Moreover, a large-scale, longitudinal serological survey of SARS-CoV-2 infections in companion animals should be conducted to definitively determine the frequency of pet contamination, identify risk factors associated with pet infection, and characterize the impact of pet infection on COVID-19 epidemiology.
Animal ethics
Sampling of animals for this study was approved by VetAgro Sup ethical committee (approval number n°2031).
Author's contributions
Conceptualization: EML, VL; Data curation: MF, BR, EK, SD, BB; Formal analysis: all authors; Funding acquisition: EML, VL, CC; Investigation: MF, BR, VL, EML; Project administration: EML, VL, SGR, CC; Writing-original draft: MF, EML, VL; Writing-review & editing: all authors.
Funding
The study was funded by OIE through the European Union EBO-SURSY project, the French National Agency for Research EBOFAQ project (ANR-14-EBOL-003-01) and IDEXLYON project of Université de Lyon as part of the “Programme Investissements d'Avenir” (ANR-16-IDEX-0005). This work was supported by French state funds managed by the French National Agency for Research (ANR) within Programme Investissements d'Avenir, Institut Hospitalo-Universitaire FOReSIGHT (ANR-18-IAHU-0001) and Institut de Recherche pour le Développement (IRD).
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
We are grateful to the pet owners for giving us their permission to sample their pets. We thank all veterinarians that helped us with sampling, particularly Corentin Buisson, Julie Roggy and Maud Walliang for their important contribution to the sample collection and database. We thank Dr. Thierry Buronfosse for the kind gift of pre-pandemic sera. We also thank Kurt McKean for English editing of the manuscript (https://octopusediting.com/).
Contributor Information
Vincent Legros, Email: vincent.legros@vetagro-sup.fr.
Eric M. Leroy, Email: eric.leroy@ird.fr.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Le Poder S. Feline and canine coronaviruses: common genetic and pathobiological features. Adv. Virol. 2011;2011 doi: 10.1155/2011/609465. 609465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stavisky J., Pinchbeck G.L., German A.J., Dawson S., Gaskell R.M., Ryvar R., Radford A.D. Prevalence of canine enteric coronavirus in a cross-sectional survey of dogs presenting at veterinary practices. Vet. Microbiol. 2010;140:18–24. doi: 10.1016/j.vetmic.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell E.T., Toribio J.A., White J.D., Malik R., Norris J.M. Seroprevalence study of feline coronavirus in owned and feral cats in Sydney, Australia. Aust. Vet. J. 2006;84:74–81. doi: 10.1111/j.1751-0813.2006.tb12231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kummrow M., Meli M.L., Haessig M., Goenczi E., Poland A., Pedersen N.C., Hofmann-Lehmann R., Lutz H. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin. Diagn. Lab. Immunol. 2005;12:1209–1215. doi: 10.1128/CDLI.12.10.1209-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taharaguchi S., Soma T., Hara M. Prevalence of feline coronavirus antibodies in Japanese domestic cats during the past decade. J. Vet. Med. Sci. 2012;74:1355–1358. doi: 10.1292/jvms.11-0577. [DOI] [PubMed] [Google Scholar]
- 6.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020:100133. doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J. Virol. 2020;94 doi: 10.1128/jvi.00127-20. e00127-00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Huang C., Zhang Y., Zhang S., Jin M. Severe acute respiratory syndrome Coronavirus 2-specific antibodies in pets in Wuhan, China. J. infect. 2020 doi: 10.1016/j.jinf.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J., Jin Y., Liu Y., Sun J., Hao L., Bai J., Huang T., Lin D., Jin Y., Tian K. Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Sailleau C., Dumarest M., Vanhomwegen J., Delaplace M., Caro V., Kwasiborski A., Hourdel V., Chevaillier P., Barbarino A., Comtet L., Pourquier P., Klonjkowski B., Manuguerra J.C., Zientara S., Le Poder S. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., E. M. W. To, Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grzelak L., Temmam S., Planchais C., Demeret C., Tondeur L., Huon C., Guivel-Benhassine F., Staropoli I., Chazal M., Dufloo J., Planas D., Buchrieser J., Rajah M.M., Robinot R., Porrot F., Albert M., Chen K.Y., Crescenzo-Chaigne B., Donati F., Anna F., Souque P., Gransagne M., Bellalou J., Nowakowski M., Backovic M., Bouadma L., Le Fevre L., Le Hingrat Q., Descamps D., Pourbaix A., Laouenan C., Ghosn J., Yazdanpanah Y., Besombes C., Jolly N., Pellerin-Fernandes S., Cheny O., Ungeheuer M.N., Mellon G., Morel P., Rolland S., Rey F.A., Behillil S., Enouf V., Lemaitre A., Creach M.A., Petres S., Escriou N., Charneau P., Fontanet A., Hoen B., Bruel T., Eloit M., Mouquet H., Schwartz O., van der Werf S. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 2020 doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okba N.M.A., Müller M., Li W., Wang C., GeurtsvanKessel C., Corman V., Lamers M., Sikkema R., de Bruin E., Chandler F., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C.B.E.M., Bosch B.-J., Drosten C., Koopmans M.P.G., Haagmans B. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. J. 2020;26:1478. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenwick C., Croxatto A., Coste A.T., Pojer F., Andre C., Pellaton C., Farina A., Campos J., Hacker D., Lau K., Bosch B.J., Gonseth Nussle S., Bochud M., D’Acremont Genton V., Trono D., Greub G., Pantaleo G. Changes in SARS-CoV-2 Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. medRxiv. 2020 doi: 10.1101/2020.07.14.20153536. 2007.2014.20153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudreault N.N., Trujillo J.D., Carossino M., Meekins D.A., Morozov I., Madden D.W., Indran S.V., Bold D., Balaraman V., Kwon T., Artiaga B.L., Cool K., García-Sastre A., Ma W., Wilson W.C., Henningson J., Balasuriya U.B.R., Richt J.A. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020:1–36. doi: 10.1080/22221751.2020.1833687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halfmann P.J., Hatta M., Chiba S., Maemura T., Fan S., Takeda M., Kinoshita N., Hattori S.I., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Kawaoka Y. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]