Abstract
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a source of significant morbidity and death worldwide, and effective treatments are urgently needed. Clinical trials have focused largely on direct antiviral therapies or on immunomodulation in patients with severe manifestations of COVID-19. One therapeutic approach that remains to be clinically investigated is disruption of the host-virus relationship through amino acid restriction, a strategy used successfully in the setting of cancer treatment. Arginine is an amino acid that has been shown in nonclinical studies to be essential in the life cycle of many viruses. Therefore, arginine depletion may be an effective therapeutic approach against SARS-CoV-2. Several arginine-metabolizing enzymes in clinical development may be a viable approach to induce a low arginine environment to treat COVID-19 and other viral diseases. Herein, we explore the rationale for arginine depletion as a therapeutic approach for COVID-19.
Keywords: SARS-CoV-2, COVID-19, Arginine depletion
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a tremendous burden on healthcare systems and a risk to both lives and livelihoods around the globe. Many clinical trials are under way, with current strategies focusing on directly disrupting viral machinery, such as with the RNA polymerase inhibitor remdesivir, or modulating the intensity of the immune response, such as with the anti-cytokine antibodies tocilizumab and sarilumab. Manipulation of the host-virus relationship to disrupt the viral lifecycle is a novel therapeutic strategy. Like other viruses, SARS-CoV-2 is obligately reliant on host machinery and nutrients for the synthesis of viral macromolecules. The deprivation of key nutrients—an approach used in the oncology field to treat tumors—may therefore interfere with viral replication. Although this metabolic starvation approach has yet to be clinically applied to virus control, preclinical studies support this concept. Arginine is a key nutrient shown to be essential in vitro in the lifecycle of many DNA and RNA viruses, and therapeutic depletion of arginine may therefore inhibit SARS-CoV-2 replication.
Several arginine-depleting enzymes are already in clinical development (Supplemental Table 1). Pegzilarginase is an engineered, pegylated enzyme that degrades arginine to produce ornithine and urea and is currently in a phase 3 clinical study in patients with arginase 1 (ARG1) deficiency. Pegylated recombinant human ARG1 (BCT-100) is currently in development to treat malignancies. Pegylated bacterial arginine deiminase (ADI-PEG 20) uses a different enzymatic mechanism, generating citrulline and ammonia from arginine. In a recent cancer-focused clinical trial, ADI-PEG 20 was shown to have antiviral activity in patients with hepatitis C virus (HCV) infection with hepatocellular carcinoma. These arginine-degrading therapeutics may therefore be readily accessible treatments for COVID-19. Herein, we review the evidence for arginine depletion as a strategy to treat SARS-CoV-2 infection.
Arginine metabolism
Arginine is a semiessential amino acid that can be obtained from the diet or produced in certain cells via the complete or partial urea cycle. In addition to its important role in the make-up of essential proteins, arginine is a substrate for the various isoforms of nitric oxide synthase (NOS), which converts arginine into nitric oxide (NO) and citrulline. Both citrulline and ornithine, as direct products of arginine metabolism, also undergo further modifications into other bioactive compounds (Morris, 2016).
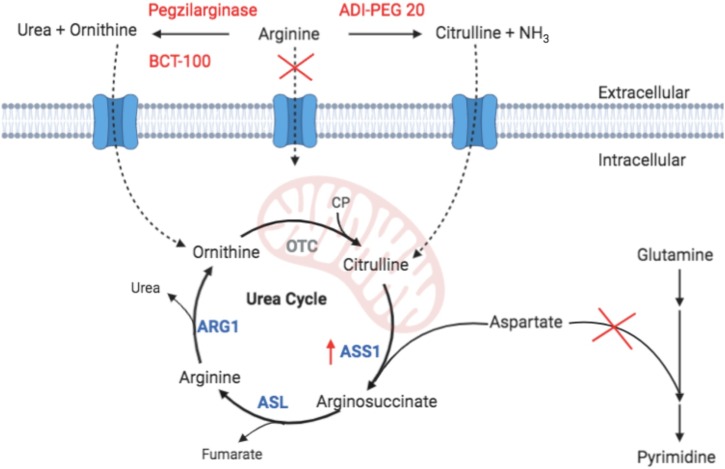
In the urea cycle, arginine is converted by ARG1 into ornithine and urea, thereby allowing excretion of excess nitrogen produced by protein catabolism. Ornithine is then recycled to remake arginine in the urea cycle through use of the enzymes ornithine transcarbamylase (OTC), argininosuccinate synthetase 1 (ASS1), and arginosuccinate lyase (ASL) (Figure 1 ). Of these urea cycle enzymes, only the liver and parts of the small intestine express all four, as most other cells in the body do not express OTC. The importance of this expression pattern and of the expression level of ASS1 as pertaining to arginine deprivation as an antiviral therapeutic approach are discussed herein.
Figure 1.
Extracellular metabolism of arginine by BCT-100, pegzilarginase, and ADI-PEG 20 (pegylated bacterial arginine deiminase) reduces cellular uptake of free arginine, resulting in upregulation of argininosuccinate synthetase 1 (ASS1). Ornithine generated by BCT-100 and pegzilarginase cannot be converted into citrulline outside the liver and intestines as ornithine transcarbamylase (OTC) is not expressed (gray); therefore, arginine cannot be synthesized and utilized for viral replication. In contrast, citrulline produced by ADI-PEG 20 is more easily converted to arginine outside the liver since ASS1 and arginosuccinate lyase (ASL) are expressed in most tissues; therefore, viral replication may not be impeded as effectively. A consequence of ASS1 upregulation is that any available citrulline will be rapidly conjugated to aspartate, preventing its utilization in pyrimidine ring synthesis, thereby restricting viral replication of another key building block. ARG1, arginase 1; CP, carbamoyl phosphate.
In an arginine-deprived environment, arginine is generated endogenously from either ornithine (for those cells that express OTC) or citrulline (for cells that express ASS1 and ASL but not OTC). Therefore, in the context of treatment with the ornithine-generating enzyme pegzilarginase or BCT-100, most cells in the body will not be able to endogenously synthesize arginine (Figure 1). To compensate for the low-arginine environment, cells upregulate ASS1 and convert circulating citrulline into arginine, consuming aspartic acid in the process (Feun et al., 2008). The importance of aspartic acid availability for viral replication was highlighted by Grady et al. (2013) as they showed that a low ASS1 expression status, which would allow continued use of aspartic acid as a substrate for pyrimidine synthesis rather than for arginine production, favored replication of herpes simplex viruses (HSVs). This same concept has also been proposed as a rationale for the relatively faster proliferation rates of ASS1-deficient tumor cells (Rabinovich et al., 2015). On the basis of this link between arginine synthesis via the urea cycle and pyrimidine production, it is interesting to contemplate the potential additive or synergistic effects of combining an arginine-depleting approach with nucleotide analog therapies such as remdesivir.
Arginine depletion as an antiviral strategy
Arginine depletion has long been investigated in vitro as a potential antiviral strategy, with most studies performed on the families Herpesviridae and Adenoviridae. A number of other RNA and DNA viruses have also been investigated (Supplemental Table 2), but to date there have been no studies of arginine depletion in the coronavirus family.
Human Herpesviridae family
Early studies showed that HSV yield increases with increasing arginine levels in the medium of HSV-1-infected cells and that complete arginine deprivation inhibits viral replication (Inglis, 1968, Becker et al., 1967). Viral DNA synthesis was not affected by acute arginine depletion, which suggests that either viral RNA synthesis or protein production impacts viral replication (Becker et al., 1967). Of note, reintroduction of arginine after deprivation in HSV-1-infected cells resulted in resumption of normal virus production, thereby highlighting the importance of arginine (Inglis, 1968). Given these early findings, it was postulated that arginase activity may be a key antiviral tool. A supporting experiment found that release of arginase by macrophages inhibits replication of HSV-1, implying that control of local arginine levels is an antiviral mechanism (Wildy et al., 1982).
More recently, arginine depletion was studied in HSV-1-infected cells treated with a pegylated form of native ARG1 (peg-ARG1). Peg-ARG1 treatment inhibits viral replication, halts production of viral progeny with reduction in cell-to-cell transmission, and blocks the classic cytopathic effects of HSV-1. Furthermore, peg-ARG1 exhibited more antiviral activity compared with acyclovir (Sanchez et al., 2016).
Cytomegalovirus (CMV) has also been shown to replicate in a dose-dependent manner as arginine is introduced to cell culture medium (Garnett, 1975). In contrast to findings in the HSV studies, the antiviral effects of arginine deprivation impacted DNA production. Another study, in a murine CMV model, noted that arginine deprivation decreased viral DNA, RNA, and protein production, but could not conclude that synthesis of these macromolecules was completely inhibited (Aono and Minamishima, 1984). Both of these studies demonstrated that reintroduction of arginine stimulates viral production up to 8 days later.
Adenovirus
Early studies of amino acid deprivation in adenovirus identified arginine as an essential nutrient in viral replication (Rouse and Schlesinger, 1967). These studies found that viral DNA and protein accumulate even in the absence of arginine (Everitt et al., 1971, Raška et al., 1972). However, on reintroduction of arginine, these viral components cannot be packaged into mature virions, and the viral lifecycle depends on synthesis of new components (Rouse and Schlesinger, 1972). This suggests that viral proteins made in the absence of arginine are defective or inaccessible to virion packaging. One study also notes that packaging of viral DNA into new capsids may require an arginine-dependent process (Raška et al., 1972).
Experience with other viruses
Other viruses have been studied in the context of arginine deprivation (Supplemental Table 2). The RNA influenza virus has conserved arginine residues critical for viral replication, which is in line with studies showing decreased influenza virus yield in cultures depleted of arginine (Schierhorn et al., 2017, Becht, 1969). The vaccinia virus is a DNA virus shown to be dependent on arginine for both early (DNA production) and late (virion packaging) viral stages (Archard and Williamson, 1971). Arginine depletion studies in the polyomavirus simian virus 40 show that DNA and viral proteins can be synthesized but not packaged into mature virions, similar to findings in adenovirus (Goldblum et al., 1968, Tan, 1977). Measles morbillivirus, the RNA virus responsible for measles, is not essentially dependent on arginine, but low arginine levels reduce viral progeny (Romano and Scarlata, 1973). The DNA alphaherpesvirus responsible for Marek’s disease in poultry is reliant on arginine for viral protein formation, but not for viral entry or DNA synthesis (Mikami et al., 1974).
A retrospective analysis of a clinical study of hepatocellular carcinoma patients treated with ADI-PEG 20 supports the concept and safety of therapeutic arginine depletion as an antiviral approach (Izzo et al., 2007). This study demonstrated that five of ten HCV serotype 1B patients had a greater than 90% reduction in viral load. Decreases of 47–82% were demonstrated in three other patients. These data are striking given the potential rescue effect associated with the generation of citrulline, as well as the high incidence of anti-drug antibody (ADA) formation (Yang et al., 2010). It is possible that the latter may have contributed to the variability in the HCV titer response.
Arginine deprivation for the treatment of COVID-19
Conceptually, and distinct from traditional antiviral agents, arginine depletion may possess a twofold mechanism of action: limitation of the amino acid and pyrimidine nucleotide pools may impede translation of viral proteins and inhibit replication of the viral genome, respectively. A number of steps in the viral lifecycle of SARS-CoV-2 have been shown to depend on key arginine residues. For example, a low-arginine environment could impact production of the nucleocapsid (N) protein (contains 6.9% arginine content), which plays an important role in interacting with the negatively charged RNA strands, thus helping to bind and wrap them to aid in efficient virion packing (McBride et al., 2014).
Additionally, arginine residues on the spike (S) protein may be crucial to stabilize viral interaction with the host cell receptor angiotensin-converting enzyme 2 (ACE2) to facilitate viral entry. Saha et al. (2020) found that a substitution of isoleucine for arginine at position 407 of the S protein in a SARS-CoV-2 isolate predicts a destabilized interaction of the S protein with ACE2. It is worth noting that another group found that this same substitution may actually stabilize the structure of the S protein, although it is unclear whether this increased stability is favorable for interaction with ACE2 (Khan et al., 2020). Importantly, an arginine motif at the S1/S2 site of the S protein has been shown to be a critical cleavage site for host machinery after viral binding. Appropriate cleavage at this site is necessary for successful fusion of the viral membrane with the host cell membrane (Hoffmann et al., 2020).
Of note, our group has unpublished in vitro data supporting the above hypothesis, as the arginine-depleting enzyme pegzilarginase inhibits SARS-CoV-2 replication in Vero cells. Mechanistic studies are ongoing, and these findings require further internal and external validation.
In addition to the putative direct antiviral activity with regard to SARS-COV-2, arginine depletion may also attenuate the pulmonary inflammation seen in COVID-19. Severe cases of COVID-19 are characterized by a hyperinflammatory lung pathology and local tissue damage that contribute to poor patient outcome (Xu et al., 2020). Arginine is the substrate for NOS-mediated production of NO, a signaling molecule that is a key mediator of the innate inflammatory immune response imparted by viral infections (Ricciardolo et al., 2006). In experimental models of severe influenza virus infection, which shares in common with COVID-19 a state of pulmonary hyperinflammation, induced NO overproduction directly contributed to animal morbidity and death (Perrone et al., 2013). Clinically, reduction of NO has been demonstrated following prolonged dosing with ADI-PEG 20 in patients with HCV infection with hepatocellular carcinoma (Izzo et al., 2007). By limiting the availability of arginine for production of NO, especially production of NO mediated by inducible NOS, it may be possible to limit the extent of the hyperinflammatory response in COVID-19.
Clinical data for arginine-depleting enzymes
Pegzilarginase has been studied in a number of clinical trials in patients with solid malignant tumors (NCT02561234) and hematologic diseases (NCT02732184), and is currently in phase 3 clinical development for the treatment of patients with ARG1 deficiency (NCT03921541). Pegzilarginase is well tolerated and shows sustained reduction of serum arginine concentration to 10% or less of normal-range baseline levels for 48 h or more. Although low-titer anti-pegzilarginase antibodies have been reported in a small number of patients, these antibodies were undetectable after the treatment period and had minimal impact on the arginine-lowering effectiveness with continued dosing (Rasco et al., 2018).
ADI-PEG 20 has been studied as a single agent and in combination with a number of other antineoplastic agents in solid tumors and hematologic diseases, and also has a favorable safety profile (Patil et al., 2016). Serum arginine concentrations are completely depleted in most patients treated with ADI-PEG 20 by day 8 following the first dose. As most patients treated with the drug develop anti-ADI-PEG 20 antibodies, arginine depletion is not sustained at lower doses following long-term exposure (Yang et al., 2010).
BCT-100 has been studied as an antineoplastic agent primarily as a single agent (NCT01092091) and in combination with other therapies (NCT02089633). BCT-100 is generally well tolerated and demonstrates sustained reduction in serum arginine levels in weekly repeated dosing trials. Anti-BCT-100 antibodies have not been detected in patients (Yau et al., 2015).
All clinical trials involving arginine-depleting enzymes can be found in Supplemental Table 3.
Given the above clinical profiles, a pegylated form of human ARG1 may be more suited for the treatment of SARS-CoV-2 infection in patients, as the development of ADAs to ADI-PEG 20 could reduce clinical efficacy. The clinical implications of frequent ADA development following treatment with ADI-PEG 20 will likely depend on the length of treatment and the required duration of arginine suppression. ADA development may also complicate considerations for repeated administration of ADI-PEG 20 in the event of reinfection with SARS-CoV-2.
Given the potential favorable impact on viral replication, treatment earlier in the disease course may have advantages. Insights regarding the selection of patients and the timing of use of remdesivir and other antiviral approaches will likely continue to inform these considerations. The additional immunomodulatory potential of arginine-depleting approaches could offer advantages over other antiviral approaches as the disease pathogenesis evolves to a more dominant immune-driven component. However, as is the case with the use of other immune-modifying approaches in infectious diseases, there is a risk that any beneficial effects may be offset by harmful unanticipated effects on the quality and effectiveness of inflammatory and immune defenses. Given the potential that immune suppression before the development of more severe symptoms may be harmful, as suggested by the trend in RECOVERY trial patients who received dexamethasone before requiring oxygen support, this dynamic between antiviral and potential immune-modifying effects will be important in considerations on the optimal dosing window for an arginine-depleting enzyme in the clinical course of COVID-19 (Horby et al., 2020).
Conclusion
Although arginine depletion has long been proposed as a potential antiviral mechanism, this strategy has yet to be applied in the clinic. Given the preclinical evidence summarized in this article, arginine appears to be a key metabolite important for successful viral replication, and there are clear steps in the SARS-CoV-2 lifecycle that rely on conserved arginine residues. Furthermore, arginine is also a key substrate in the host inflammatory response, and reduction of serum plasma arginine levels could plausibly attenuate the severe inflammatory response in SARS-CoV-2 infection. As multiple arginine-depleting enzymes have been demonstrated to be safe and effective in reducing systemic arginine levels, the field is well positioned to further develop preclinical understanding of the antiviral effectiveness of arginine deprivation and advance clinical trials of these approaches in patients with COVID-19. If successful against SARS-CoV-2, the use of arginine-depleting enzymes could more broadly be applied as an antiviral approach, thus enabling a therapeutic preparedness option for future viral disease pandemics.
Conflict of interest
Joseph M. Grimes, Shaheer Khan, and Richard D. Carvajal have no competing interests to declare. Mark Badeaux, Ravi M. Rao, and Scott W. Rowlinson are employees of Aeglea Biotherapeutics Inc. and each have equity interest in the company.
Funding source
No funding was required for this work.
Ethical approval
This review did not involve the use of animals or human participants, so ethical approval was not required.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.100.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aono J., Minamishima Y. Effect of arginine deprivation on murine cytomegalovirus replication. Microbiol Immunol. 1984;28:129–133. doi: 10.1111/j.1348-0421.1984.tb02954.x. [DOI] [PubMed] [Google Scholar]
- Archard L.C., Williamson J.D. The effect of arginine deprivation on the replication of vaccinia virus. J Gen Virol. 1971;12:249–258. doi: 10.1099/0022-1317-12-3-249. [DOI] [PubMed] [Google Scholar]
- Becht H. Induction of an arginine-rich component during infection with influenza virus. J Gen Virol. 1969;4:215–220. doi: 10.1099/0022-1317-4-2-215. [DOI] [PubMed] [Google Scholar]
- Becker Y., Olshevsky U., Levitt J. The role of arginine in the replication of herpes simplex virus. J Gen Virol. 1967;1:471–478. doi: 10.1099/0022-1317-1-4-471. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971;8:742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun L., You M., Wu C.J. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett H.M. The effect of arginine deprivation on the cytopathogenic effect and replication of human cytomegalovirus. Arch Virol. 1975;48:131–145. doi: 10.1007/BF01318146. [DOI] [PubMed] [Google Scholar]
- Goldblum N., Ravid Z., Becker Y. Effect of withdrawal of arginine and other amino acids on the synthesis of tumour and viral antigens of SV 40 virus. J Gen Virol. 1968;3:143–146. doi: 10.1099/0022-1317-3-1-143. [DOI] [PubMed] [Google Scholar]
- Grady S.L., Purdy J.G., Rabinowitz J.D., Shenk T. Argininosuccinate synthetase 1 depletion produces a metabolic state conducive to herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 2013;110:E5006–15. doi: 10.1073/pnas.1321305110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A Multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78 doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis V.B.M. Requirement of arginine for the replication of herpes virus. J Gen Virol. 1968;3:9–17. doi: 10.1099/0022-1317-3-1-9. [DOI] [PubMed] [Google Scholar]
- Izzo F., Montella M., Orlando A.P. Pegylated arginine deiminase lowers hepatitis C viral titers and inhibits nitric oxide synthesis. J Gastroenterol Hepatol. 2007;22:86–91. doi: 10.1111/j.1440-1746.2006.04463.x. [DOI] [PubMed] [Google Scholar]
- Khan M.I., Khan Z.A., Baig M.H. Comparative genome analysis of novel coronavirus (SARS-CoV-2) from different geographical locations and the effect of mutations on major target proteins: an in silico insight. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T., Onuma M., Hayashi T.T.A. Requirement of arginine for the replication of Marek’s disease herpes virus. J Gen Virol. 1974;22:115–128. doi: 10.1099/0022-1317-22-1-115. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Jr. Arginine metabolism revisited. J Nutr. 2016;146 doi: 10.3945/jn.115.226621. 2579s–86s. [DOI] [PubMed] [Google Scholar]
- Patil M.D., Bhaumik J., Babykutty S., Banerjee U.C., Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35:4957–4972. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone L.A., Belser J.A., Wadford D.A., Katz J.M., Tumpey T.M. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013;207:1576–1584. doi: 10.1093/infdis/jit062. [DOI] [PubMed] [Google Scholar]
- Rabinovich S., Adler L., Yizhak K. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527:379–383. doi: 10.1038/nature15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasco D.W., Eckhardt S.G., Davar D. Abstract CT030: phase I dose escalation trial of pegzilarginase in patients with advanced solid tumors. Cancer Res. 2018;78 CT030-CT. [Google Scholar]
- Raška K., Prage L., Schlesinger R.W. Effects of arginine starvation on macromolecular synthesis in infection with type 2 adenovirus: II. Synthesis of virus-specific RNA and DNA. Virology. 1972;48:472–484. doi: 10.1016/0042-6822(72)90058-x. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F.L., Di Stefano A., Sabatini F., Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533:240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Romano N., Scarlata G. Amino acids requirements of measles virus in hela cells. Arch Virusforsch. 1973;43:359–366. doi: 10.1007/BF01556153. [DOI] [PubMed] [Google Scholar]
- Rouse H.C., Schlesinger R.W. An arginine-dependent step in the maturation of type 2 adenovirus. Virology. 1967;33:513–522. doi: 10.1016/0042-6822(67)90128-6. [DOI] [PubMed] [Google Scholar]
- Rouse H.C., Schlesinger R.W. The effects of arginine starvation on macromolecular synthesis in infection with type 2 adenovirus: I. Synthesis and utilization of structural proteins. Virology. 1972;48:463–471. doi: 10.1016/0042-6822(72)90057-8. [DOI] [PubMed] [Google Scholar]
- Saha P., Banerjee A.K., Tripathi P.P., Srivastava A.K., Ray U. A virus that has gone viral: amino acid mutation in S protein of Indian isolate of coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci Rep. 2020;40 doi: 10.1042/BSR20201312. BSR20201312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.D., Ochoa A.C., Foster T.P. Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways. Antiviral Res. 2016;132:13–25. doi: 10.1016/j.antiviral.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierhorn K.L., Jolmes F., Bespalowa J. Influenza A virus virulence depends on two amino acids in the N-terminal domain of its NS1 protein to facilitate inhibition of the RNA-dependent protein kinase PKR. Journal of Virology. 2017;91:e00198–17. doi: 10.1128/JVI.00198-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K.B. The effect of arginine deprivation on DNA, thymidine kinase and RNA polymerase synthesis in simian virus 40-infected monkey kidney cells. Arch Virol. 1977;53:133–138. doi: 10.1007/BF01314854. [DOI] [PubMed] [Google Scholar]
- Wildy P., Gell P.G., Rhodes J., Newton A. Inhibition of herpes simplex virus multiplication by activated macrophages: a role for arginase? Infect Immun. 1982;37:40–45. doi: 10.1128/iai.37.1.40-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.S., Lu S.N., Chao Y. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer. 2010;103:954–960. doi: 10.1038/sj.bjc.6605856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T., Cheng P.N., Chan P. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:496–504. doi: 10.1007/s10637-014-0200-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.