Abstract
Donation after circulatory death (DCD) is an increasing source of liver grafts for transplantation, yet outcomes have been inferior compared to donation after brain death liver transplantation. These worse outcomes are mainly due to the severe graft injury resulting from mandatory warm ischemia during DCD organ recovery. New evidence, however, indicates that improved donor selection and surgical techniques can decrease the risk of graft failure and ischemic cholangiopathy (IC). Under current best practices, DCD organs are retrieved with the super-rapid technique, optimizing timing and protecting the liver graft from detrimental warm ischemia. Graft viability is influenced by both the quantity and quality of warm ischemia, which is unique to each donor and causes various degrees of pathophysiologic consequences. Evidence also shows that the choice of preservation solution and premortem heparin administration influences graft viability. Additionally, although the precise mechanism of IC remains unknown, stasis of blood during donor warm ischemia may cause the formation of microthrombi in the peribiliary vascular plexus and ischemia of the bile duct. Importantly, thrombolytic protocols show a possible preventive modality for IC. Finally, while ex vivo machine perfusion technology has gained an interest in DCD liver transplantation, further studies are necessary to evaluate the effectiveness of this evolving field to improve graft quality and transplant outcomes.
Keywords: Tissue and organ procurement, Liver transplantation, Reperfusion injury, Graft survival, Warm ischemia
INTRODUCTION
Before the introduction of the brain death law, transplant organs were taken from non-heart beating donors, now commonly referred to as donation after circulatory death (DCD) donors. Due to better outcomes, however, the majority of liver transplantations have been performed using donation after brain death (DBD) donors. With an increasing demand for donor organs, a living donor option was added to mitigate waitlist mortality. To further expand the donor pool, transplant centers were forced to accept donors with increased risks. In the 1990s, DCD donors started regaining attention as a valuable source of liver grafts. Despite growing experiences of DCD in liver transplantation, increased risks of graft failure and ischemic biliary complications remain critical concerns.
The definition of DCD is organ donation from patients who have suffered a catastrophic brain injury but do not meet the criteria for brain death [1]. In 1995, an international workshop to examine issues surrounding non-heart beating donors was held in Maastricht, Netherlands. The workshop resulted in the classification of DCD donors into four categories: category I (dead on arrival), category II (unsuccessful resuscitation), category III (awaiting cardiac arrest), and category IV (cardiac arrest in brain dead donor) [2]. Currently, the majority of DCD liver grafts are recovered from category III donors under a controlled environment (controlled DCD). While uncontrolled DCD donors (category II) have been used in some European countries with acceptable outcomes, many transplant centers are reluctant to use them due to unknown warm ischemic time [3].
Organs recovered from DCD donors inevitably experience warm ischemic injury, which is the primary element distinguishing DCD from DBD (Fig. 1) [1]. A progressive drop in blood pressure and oxygen saturation during an extended withdrawal period is detrimental to graft viability. Since the liver graft is vulnerable to warm ischemic injury, DCD liver transplantation is associated with an increased risk of graft failure and surgical complications [4,5]. Nonetheless, with the need for liver transplantation is increasing worldwide, DCD donors will continue to be an essential source of lifesaving liver grafts. For the best use of DCD donors, it is crucial to understand the mechanisms of tissue injury of DCD liver grafts and how to maintain graft viability (Table 1). This article reviews the current practice of DCD organ recovery as well as DCD donor selection, which is essential to achieve successful outcomes in DCD liver transplantation. Further, critical advancement in this field will be discussed regarding preventive modalities for ischemic biliary complications and graft failure.
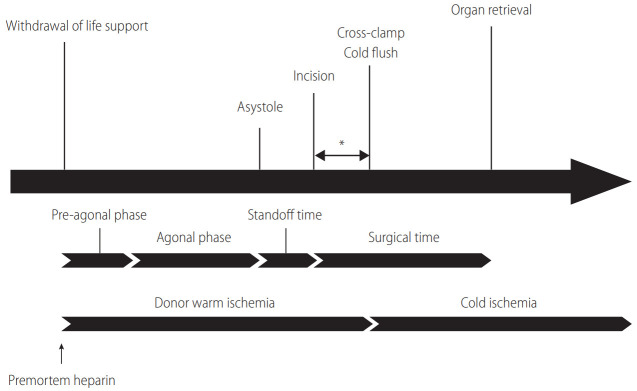
Figure 1.
Timeline of DCD organ recovery. The definition of the agonal phase varies based on each program’s policy regarding its cutoffs of MAP and SpO2. Generally, the agonal phase begins when blood pressure or SpO2 drops to certain levels (70–80 mmHg in systolic pressure, 50–60 mmHg in MAP, or 60–80% in SpO2). The standoff (no touch) time lasts at least 2 minutes, but not more than 5 minutes, to confirm the irreversibility of circulatory death. Time from incision to cross-clamp (*) is the only period that is surgically modifiable to shorten donor warm ischemia. DCD, donation after circulatory death; MAP, mean arterial pressure; SpO2, peripheral oxygen saturation.
Table 1.
Factors influencing DCD graft viability
| Factor | Reference |
|---|---|
| Donor selection | |
| Donor age | 6-10 |
| BMI (graft steatosis) | 10 |
| Cold ischemia time | 611 |
| Organ recovery | |
| Location of withdrawing life support | 12 |
| Premortem heparin | 16 |
| Preservation solution | 17-20 |
| Warm ischemia time | |
| Extubation to cross clamp | 6,22-26 |
| Agonal phase | 2,728 |
| Asystole to cross clamp | 29 |
| Thrombolytic agents | 33,39-42 |
| Ex vivo machine perfusion | 43-49 |
DCD, donation after circulatory death; BMI, body mass index.
DCD DONOR SELECTION
Donor age is one of the most potent indicators of graft survival in DCD liver transplantation. According to the Scientific Registry of Transplant Recipients data from September 2001 to April 2009 (n=1,576), donor age of 50–60 years increased the risk of graft failure by 39% when compared to younger donors. Moreover, the risk jumped to 88% for donors older than 60 years [6]. Similarly, based on UK national data on risk factors for graft failure (n=1,153) from January 2000 to December 2015, donor age has been included as an essential element of the DCD donor risk score [7].
On the other hand, conflicting results have been reported by single-center studies from high volume programs. For instance, a retrospective study by Mayo Clinic showed that recipients who received liver grafts from DCD donors ≥50 years of age achieved comparable graft survival to those who received liver grafts from younger DCD donors [8]. Our experience at Cleveland Clinic also demonstrated no significant impact of donor age on graft survival with an age cutoff of 45 years [9]. However, it should be noted that such favorable outcomes could have partly been achieved due to stringent donor and recipient selection criteria. Since the use of DCD donors per se is an independent risk factor, the coexistence of multiple risk factors should be avoided, such as high donor body mass index (BMI), graft steatosis, and high Model for End-Stage Liver Disease (MELD) score in recipients. In the Birmingham group’s single-center study, an age cutoff of 60 years failed to distinguish recipients with poorer graft and patient survival [10]. However, when donor age was combined with donor BMI ≥25 kg/m2, graft survival was significantly compromised [10]. Since high donor BMI is often associated with macrosteatosis in the liver graft, a graft biopsy may be necessary during organ recovery to further evaluate the degree of steatosis. Further, cold ischemia has a significant impact on outcomes in DCD liver transplantation [6,11]. Indeed, each hour in cold ischemia time increases the risk of graft failure by 6% [6]. Accordingly, when accepting aged DCD livers, careful risk assessment is essential to avoid not only donor- and recipient-related risks, but also long warm and cold ischemic time. It should also be noted that the use of younger DCD donors does not guarantee a promising outcome, particularly when other unfavorable factors exist [9].
DCD ORGAN RECOVERY
End of life care and withdrawal of life support
After an imminent death is identified, the Organ Procurement Organization (OPO) is notified to initiate an evaluation process to determine whether a patient is suitable to be an organ donor. If the patient does not fulfill the criteria of brain death despite a catastrophic brain injury, a discussion takes place with the family as to whether they wish to proceed with the withdrawal of life support. If the family agrees to proceed, a matched recipient(s) is identified, and an organ recovery team(s) arrives at the donor hospital. Withdrawal of life-sustaining therapy, which includes termination of cardiopulmonary support and all essential medications, often occurs in the operating room or intensive care unit in the presence of the family, depending on the OPO or hospital policy. The family needs to have as much time as needed to say goodbye to their loved one. Generally, the operating room is a preferred location to withdraw life support because the time for transport can be shortened to minimize warm ischemia. Indeed, not only is graft and patient survival better, but the incidence of complications is mitigated when life support is withdrawn in the operating room [12]. With all comfort care measures in place, the donor is extubated, and life-sustaining treatments are terminated. For the pronouncement of death in DCD organ recovery, the hospital staff must use objective and auditable criteria that are standardized by the law. If the patient does not expire within the time that makes organ donation possible, the process of organ recovery is aborted, and the patient is transferred back to intensive care unit. The donor surgical team needs to wait for at least two minutes, and not more than five minutes, of “standoff time” before starting organ recovery, depending on local policy after donor death is declared (Fig. 1) [1]. This standoff time is vital to confirm the irreversibility of circulatory death. It should be noted that operating room personnel who will be involved in organ recovery or transplantation must not be present when withdrawal of life support takes place.
Organ recovery by super-rapid technique
After the required standoff time, the surgical team begins organ recovery with a midline incision from the suprasternal notch to the symphysis pubis [13]. After placing a Balfour retractor, an assistant holds the small intestine upward to expose the sacral promontory. At approximately 5 cm superior to the sacral promontory, which may vary based on age, gender, and race, the bifurcation of the aorta can be identified in the retroperitoneum [14]. It is crucial to stay at the midline to avoid inadvertent injury of the ureters. The distal aorta is incised to insert a cannula to commence cold perfusion (Fig. 2). Then, the inferior vena cava next to the aorta is incised to vent blood. A pool suction tip is inserted into the vena cava to assist venting. Next, the sternum is split, and the thoracic aorta is cross-clamped. Finally, the pericardium is opened, and the inferior vena cava is incised below the right atrium to decompress the transplant organs further. The abdominal cavity is then filled with ice slush. If a thoracic team is present, the supraceliac aorta, instead of the thoracic aorta, is cross-clamped between the esophagus and the caudate lobe of the liver. When the lesser sac is opened to gain access to the aorta, special attention should be paid to avoid inadvertent injury to the left accessory hepatic artery. While the inferior mesenteric vein can be cannulated to perfuse the portal system, there is no evidence that this improves graft quality in DCD. Once core cooling is achieved, the liver is recovered using the same technique for DBD organ recovery. With an experienced surgical team, the process from skin incision to cross-clamp can be done within a few minutes.
Figure 2.
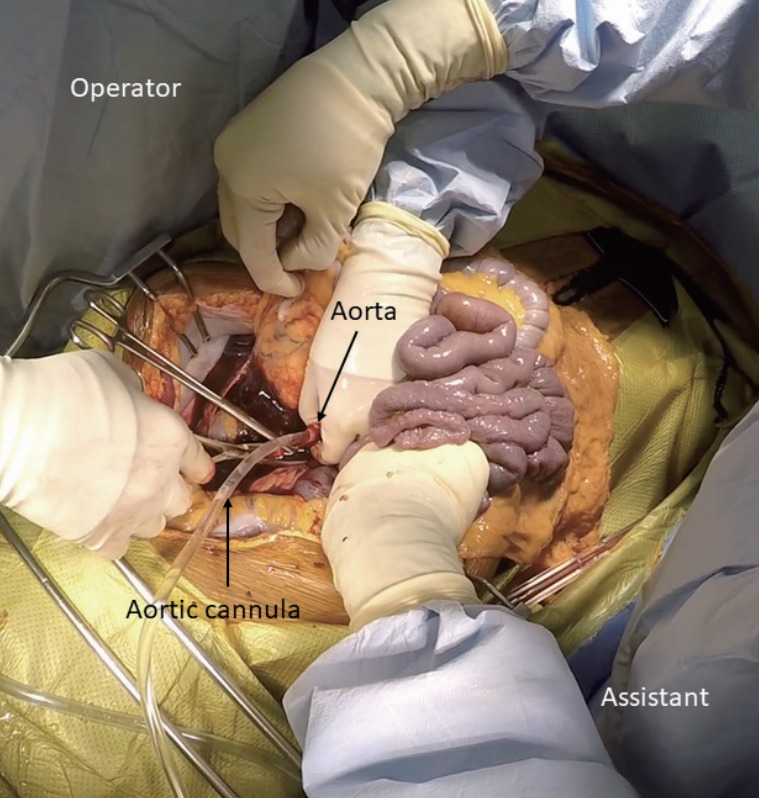
DCD organ recovery with the super-rapid technique. Aortic cannulation is done to commence cold perfusion while the assistant holds the small intestine upward to expose the retroperitoneum. DCD, donation after circulatory death.
Premortem heparin administration
Systemic administration of heparin is universally adopted in DBD organ recovery to prevent thrombus formation in transplanted organs. In DCD donors, however, premortem heparin administration (30,000 units) is ethically controversial because systemic heparinization may hasten the death of donors due to theoretical concerns of intracranial hemorrhage or worsening of bleeding [15]. According to the Scientific Registry of Transplant Recipients data, premortem heparin is currently used in >90% of DCD donors as a critical component of the DCD protocol [16]. When premortem heparin is prohibited due to a local policy, heparin can be mixed in an initial bag of the cold preservation solution. Without premortem heparin, the risks of graft failure and primary non-function increase by 18% and 81%, respectively [16]. Premortem heparin does not improve the discard rate of DCD livers [16].
Preservation solution
The selection of preservation solutions may be critical to maintaining the viability of DCD livers. Conflicting data exists regarding the comparison between University of Wisconsin solution versus histidine‐tryptophan‐ketoglutarate (HTK) solution in DCD liver transplantation [17-19]. Early studies failed to demonstrate significant differences in graft survival when comparing these solutions, possibly due to the small sample size. According to data from the United Network for Organ Sharing database from July 2004 to February 2008, however, the use of HTK solution was associated with an increased risk of graft failure by 44% in DCD grafts compared to University of Wisconsin solution (P=0.025), especially when cold ischemia time was >8 hours [20]. Despite the data, some transplant centers prefer HTK solution due to lower viscosity, which might be beneficial to prevent biliary complications [17].
QUANTITY AND QUALITY OF DONOR WARM ISCHEMIA TIME
In early experience, Feng et al. [21] reported that the use of DCD donors was one of the most substantial risk factors for liver graft failure. Compared to DBD, the inferiority of DCD mainly stems from a poorer quality of liver grafts. Donor warm ischemia time (dWIT) is generally defined as the time from extubation to cross-clamp (Fig. 1), the length of which has been considered a potent indicator of poor outcomes [22-24]. Since early experience has demonstrated that prolonged dWIT is associated with a lower graft survival and an increased risk of biliary complications, many transplant centers have adopted 30 minutes of dWIT as a cutoff to accept DCD livers. Indeed, the Scientific Registry of Transplant Recipients data demonstrated that the risk of graft loss increased by 84% when dWIT was ≥35 minutes [6]. However, many studies have shown an equivocal association between dWIT and transplant outcomes [25-28]. Such conflicting findings are mainly attributable to the heterogeneity of dWIT. Namely, from the withdrawal of life support to circulatory death, a wide range of hemodynamic perturbations is seen among donors. Changes in tissue perfusion and oxygenation during the agonal phase are not equal in each donor. Therefore, the trajectory of donor hemodynamics should be considered as an essential parameter to assess DCD liver graft quality.
During DCD organ recovery, the donor surgical team receives a minute-by-minute update of donor hemodynamics, including blood pressure, oxygen saturation, and heart rate. Peripheral arterial pressure and peripheral oxygen saturation (SpO2) are closely related to graft quality, and are particularly important. It is essential to understand that the time from withdrawal of life support to asystole consists of the pre-agonal and agonal phases (Fig. 1). Generally, the agonal phase begins when blood pressure or SpO2 drops to certain levels (70–80 mmHg in systolic pressure, 50–60 mmHg in mean arterial pressure [MAP], or 60–80% in SpO2) in DCD organ recovery. During the agonal phase, donors become progressively hypotensive and hypoxic. However, no donor has an identical change of these hemodynamic parameters to others, and the exact timing of the onset of liver graft injury is unknown (Fig. 3).
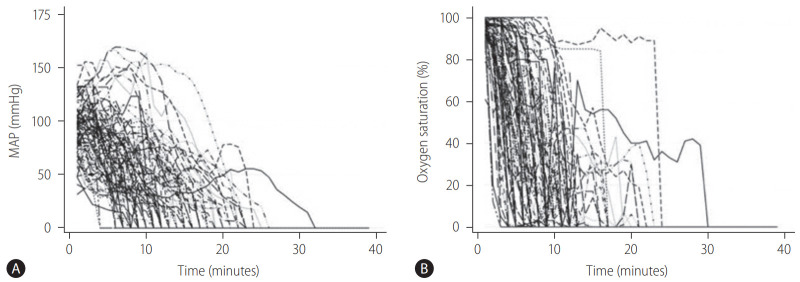
Figure 3.
Hemodynamic trajectories in DCD donors (n=87) from the time of withdrawal of life support to asystole by (A) MAP and (B) SpO2. Adapted from Firl et al. [28] with permission. DCD, donation after circulatory death; MAP, mean arterial pressure; SpO2, peripheral oxygen saturation.
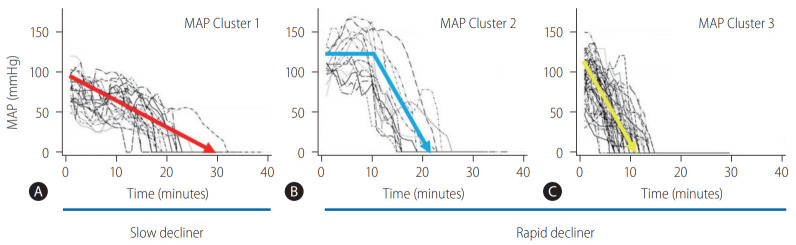
Based on the clinical data of 87 DCD donors at Cleveland Clinic, trajectories of MAP were categorized into three clinically meaningful phenotypes (Fig. 4) [28]. The patient group who had the worst graft survival (1-year graft survival, 68.9%) was the group of patients who received liver grafts from Cluster 1 donors. With minimal or short pre-agonal phase, MAP declined gradually to asystole with a prolonged agonal phase (≥15 minutes, slow decliner) (Figs. 4, 5). The median dWIT of Cluster 1 was 27 minutes (interquartile range [IQR], 24–30 minutes). While dWIT was comparable (median, 29 minutes; IQR, 25–32 minutes), Cluster 2 donors maintained stable MAP during the pre-agonal phase over 10 minutes, which was followed by a rapid decline with a short agonal phase (<15 minutes) (Fig. 4). Finally, Cluster 3 donors had the shortest dWIT (median, 19 minutes; IQR, 16–22 minutes; P<0.001 vs. Cluster 1 and 2) with a rapid decline of MAP from withdrawal to asystole (agonal phase <15 minutes). Interestingly and despite the longer dWIT, recipients who received Cluster 2 liver grafts had comparable graft survival to recipients with Cluster 3 donors (1-year graft survival, 90.9% in Cluster 2 and 91.3% in Cluster 3). This favorable outcome in Cluster 2 is probably due to the pre-agonal phase of dWIT, which appears to help maintain adequate perfusion and oxygenation of the liver graft. With a similar phenotype in the agonal phase, Cluster 2 and 3 donors can be categorized as the rapid decliner, who has better graft survival than the slow decliner (Figs. 4, 5). The difference between these phenotypes indicates that both the quantity (length) and quality of dWIT play a crucial role in determining graft survival. Similar findings were seen with SpO2 trajectories [28].
Figure 4.
Individual donor MAP trajectories shown by (A) Cluster 1 (n=24), (B) Cluster 2 (n=14), and (C) Cluster 3 (n=49). Cluster 1 donors are defined as the slow decliner due to a prolonged agonal phase (≥15 minutes) with a gradual MAP decline. On the other hand, Cluster 2 and Cluster 3 are defined as the rapid decliner because their agonal phases last less than 15 minutes. Cluster 2 donors maintain stable MAP over 10 minutes during the pre-agonal phase after withdrawal of life support. Adapted from Firl et al. [28] with permission. MAP, mean arterial pressure.
Figure 5.
Graft survival of the slow decliner (Cluster 1) and rapid decliner (Cluster 2 and 3) with respect to (A) MAP (P=0.038) and (B) SpO2 (P=0.039). The rapid decliner had significantly better graft survival than the slow decliner. Adapted from Firl et al. [28] with permission. MAP, mean arterial pressure; SpO2, peripheral oxygen saturation.
Taner et al. [29] reported that time from asystole to cross-clamp was important to determine the viability of DCD liver grafts. This asystole-cross clamp time consists of two critical periods, including the first portion as standoff time (2–5 minutes) to confirm the irreversibility of circulatory death, and the second portion which is the surgical time from incision to cross-clamp (Fig. 1). Both periods are critical because the liver graft receives no perfusion or oxygenation in the donor body due to stasis of blood. To shorten this asystole-cross clamp period, mandatory standoff time can be minimized to 2 minutes as recommended by the American Society of Transplant Surgeons [1]. However, it is also imperative to maintain public confidence in the irreversibility of death determination. Since the second portion is the only time that is surgically modifiable, it is essential for the surgical team to commence cold perfusion and cross-clamp as quickly as possible (Fig. 1).
ISCHEMIC CHOLANGIOPATHY (IC)
Increased risk of biliary complications is the Achilles’ heel of DCD liver transplantation. IC causes diffuse or focal intrahepatic biliary stricture, liver abscess, and necrosis, resulting in graft failure and retransplantation. The incidence of IC in DCD liver transplantation has been reported to be as high as 30–50%, and is one of the reasons transplant centers hesitate to accept DCD donors [17,22,25,30,31]. Patients who develop IC have significantly inferior graft survival compared to those who do not, because there is no effective therapeutic modality to salvage liver grafts from IC [5]. Therefore, it is vital to prevent the development of IC.
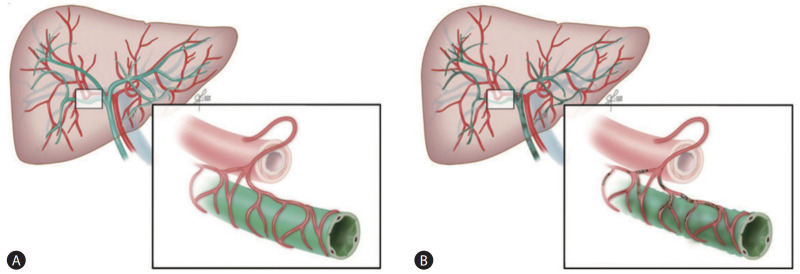
The precise mechanism for the development of IC remains unknown. Interestingly, clinical and radiological presentations of IC are similar to those of hepatic artery thrombosis. The peribiliary vascular plexus of the human liver receives blood supply only via the hepatic artery [32,33]. Therefore, a similar ischemic etiology may play a role in the development of IC. If microthrombi are formed in the peribiliary vascular plexus due to stasis of blood during warm ischemia, microcirculation of the intrahepatic bile duct can be disturbed. If this is the case, the formation of microthrombi causes ischemic biliary necrosis (Fig. 6) [33].
Figure 6.
The postulated mechanism of the development of IC in DCD liver transplantation. (A) The blood supply to the human biliary system depends solely on the hepatic artery via the peribiliary vascular plexus. (B) Formation of microthrombi in the peribiliary vascular plexus during donor warm ischemia may contribute to biliary ischemia. Adapted from Hashimoto et al. [33] with permission. DCD, donation after circulatory death.
Study results offer conflicting evidence regarding microthrombi formation in DCD livers. An animal model demonstrated an experimental evidence of microthrombi formation in the peribiliary vascular plexus [34]. A human study also demonstrated that peribiliary vascular injury with microthrombi is more prominent in DCD compared with DBD liver grafts, which correlates with the incidence of IC [35]. On the other hand, Verhoeven et al. [36] reported no evidence of increased microthrombi formation in human DCD liver grafts.
An early study from the Pittsburgh group demonstrated the usefulness of thrombolytic therapy with surgical revascularization to rescue liver grafts from hepatic artery thrombosis [37]. When such urgent intervention is conducted before irreversible tissue damage occurs, thrombolytic therapy can help prevent biliary necrosis [38]. According to these clinical findings, the use of thrombolytic agents may be of benefit to prevent the development of IC.
In August 2005, Cleveland Clinic implemented a new DCD protocol to use tissue plasminogen activator (tPA) to prevent IC. The protocol was used in 68 patients until December 2013; 22 patients in an initial pilot study [33] and 46 patients in a following prospective study [39]. Assuming the formation of microthrombi is the mechanism of IC, tPA (Activase® 1 mg/mL, 0.5 mg per 100 g liver weight; Genentech Inc., South San Francisco, CA, USA) was injected into the donor hepatic artery on the back table. After portal reperfusion, the hepatic artery was kept clamped for 10–15 minutes. To minimize the systemic introduction of tPA, the hepatic artery was unclamped before arterial anastomosis to allow excess tPA to back-bleed and discard the effluent. In the initial pilot study, the protocol dose of tPA was used in 12 grafts. Reduced doses (0.2–0.4 mg per 100 g liver weight) were used in 10 grafts based on the presence of various risk factors for bleeding such as donor age, dWIT, cold ischemia time, MELD score, and previous history of major laparotomy in recipients. Among the 22 patients in the pilot study, only two recipients (9%) developed IC, including one with diffuse necrosis requiring retransplantation and another with focal stricture of the intrahepatic bile duct with a functioning graft [33]. However, the randomized prospective study failed to demonstrate a preventive effect of tPA for IC (7% [3/46] with tPA vs. 3% [1/33] without tPA, P=0.6). Although there was a trend toward lower incidence of early allograft dysfunction with tPA (17% vs. 34%, P=0.07), there was no difference in the incidence of primary non-function, hepatic artery thrombosis, and retransplantation [39]. Since 2014, Cleveland Clinic abandoned the thrombolytic protocol based on the results of the prospective study, as well as the low incidence of IC even without the tPA treatment.
University of Toronto and Ochsner Clinic also adopted a new thrombolytic protocol [40,41]. With the Ochsner Clinic protocol, 2 mg of tPA was injected after 5 mg of verapamil into the donor hepatic artery after portal vein anastomosis. The University of Toronto protocol injected tPA (100 μg/kg donor body weight) into the donor hepatic artery before portal vein anastomosis. While 21.2% of patients at Ochsner Clinic and the University of Toronto experienced IC without tPA (n=33), IC occurred in less than 3.5% of patients with the tPA protocols (n=56 at Ochsner Clinic, n=29 at University of Toronto; P<0.005) [40]. A subsequent study by Ochsner Clinic with 100 DCD liver transplantations demonstrated that the tPA protocol achieved comparable graft and patient survival compared with DBD liver transplantation, with an IC incidence of only 3% [41]. Indiana University developed a different thrombolytic protocol along with a systematic approach, including shorter donor and recipient ischemia time, as well as shorter operation time [42]. In their protocol, 100 mg of tPA was mixed with 1 L of room temperature normal saline, which was administered via the donor aorta as an initial flush solution in organ recovery. This protocol achieved a zero incidence of IC in 30 patients who received DCD liver grafts.
Due to the risk of excessive bleeding, transplant centers are generally reluctant to adopt the use of thrombolytic agents. Yet results from the Centers that used these existing thrombolytic protocols do not support this concern [33,40-42]. Still, since coagulopathy, fibrinolysis, and thrombocytopenia are not uncommon in DCD liver transplantation, the risk of bleeding should not be underestimated even with the small dose of thrombolytic agents [33].
EX VIVO MACHINE PERFUSION
Ex vivo machine perfusion technology has recently gained the increased interest in DCD liver transplantation. Several single-center studies in the hypothermic setting have demonstrated less graft tissue damage, better graft survival, and lower incidence of IC, compared with static cold preservation [43-45]. On the other hand, normothermic machine perfusion, which creates more physiologic conditions to preserve, assess, and potentially repair liver grafts, is expected to improve outcomes for DCD liver transplantation even further. To date, however, clinical data are not available to support normothermic machine perfusion as a preventive and therapeutic modality [46-48]. While a randomized clinical trial of normothermic machine perfusion achieved a 50% reduction of graft injury, the study demonstrated no significant difference in survival or IC rates compared to static cold preservation [49]. It should be noted, however, that normothermic machine perfusion helped decrease the organ discard rate, providing an opportunity to increase the donor pool [49]. Future studies are necessary to further explore the potential of this evolving technology in terms of graft viability and ex vivo organ repair.
CONCLUSION
In real practice, the DCD liver graft increases access to lifesaving organs for patients awaiting liver transplantation. While the increased risks of graft failure and IC are of concern, outcomes after DCD liver transplantation have improved over the last two decades with a better understanding of donor selection and organ recovery techniques. It is vital to understand the importance of not only the quantity but also the quality of donor warm ischemia to determine DCD graft viability because each donor has different nature of warm ischemia, which induces various degrees of pathophysiologic consequences. Potential interventions, using thrombolytic agents and ex vivo machine perfusion technology, are evolving with encouraging reports from major transplant centers. Continued research is necessary to optimize existing protocols as well as develop new therapeutic approaches.
Acknowledgments
The author would like to acknowledge Ms. Sally Garrett Karyo for her editorial assistance.
Abbreviations
- BMI
body mass index
- DBD
donation after brain death
- DCD
donation after circulatory death
- dWIT
donor warm ischemia time
- HTK
histidine-tryptophanketoglutarate
- IC
ischemic cholangiopathy
- IQR
interquartile range
- MAP
mean arterial pressure
- MELD
Model for End-Stage Liver Disease
- OPO
Organ Procurement Organization
- SpO2
peripheral oxygen saturation
- tPA
tissue plasminogen activator
Footnotes
Conflicts of Interest: The author has no conflicts to disclose.
REFERENCES
- 1.Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D’Alessandro A, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–2011. doi: 10.1111/j.1600-6143.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 2.Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893–2894. [PubMed] [Google Scholar]
- 3.Fondevila C, Hessheimer AJ, Flores E, Ruiz A, Mestres N, Calatayud D, et al. Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am J Transplant. 2012;12:162–170. doi: 10.1111/j.1600-6143.2011.03834.x. [DOI] [PubMed] [Google Scholar]
- 4.Laing RW, Scalera I, Isaac J, Mergental H, Mirza DF, Hodson J, et al. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center. Am J Transplant. 2016;16:1795–1804. doi: 10.1111/ajt.13699. [DOI] [PubMed] [Google Scholar]
- 5.Croome KP, Lee DD, Perry DK, Burns JM, Nguyen JH, Keaveny AP, et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transpl. 2017;23:342–351. doi: 10.1002/lt.24713. [DOI] [PubMed] [Google Scholar]
- 6.Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512–2519. doi: 10.1111/j.1600-6143.2010.03293.x. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, et al. The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68:456–464. doi: 10.1016/j.jhep.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Croome KP, Mathur AK, Lee DD, Moss AA, Rosen CB, Heimbach JK, et al. Outcomes of donation after circulatory death liver grafts from donors 50 years or older: a multicenter analysis. Transplantation. 2018;102:1108–1114. doi: 10.1097/TP.0000000000002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firl DJ, Hashimoto K, O’Rourke C, Diago-Uso T, Fujiki M, Aucejo FN, et al. Impact of donor age in liver transplantation from donation after circulatory death donors: a decade of experience at Cleveland Clinic. Liver Transpl. 2015;21:1494–1503. doi: 10.1002/lt.24316. [DOI] [PubMed] [Google Scholar]
- 10.Schlegel A, Scalera I, Perera MTPR, Kalisvaart M, Mergental H, Mirza DF, et al. Impact of donor age in donation after circulatory death liver transplantation: is the cutoff “60” still of relevance? Liver Transpl. 2018;24:352–362. doi: 10.1002/lt.24865. [DOI] [PubMed] [Google Scholar]
- 11.Lee KW, Simpkins CE, Montgomery RA, Locke JE, Segev DL, Maley WR. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683–1688. doi: 10.1097/01.tp.0000250936.73034.98. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Shahrestani S, Chew HC, Crawford M, Macdonald PS, Laurence J, et al. Donation after circulatory death for liver transplantation: a meta-analysis on the location of life support withdrawal affecting outcomes. Transplantation. 2016;100:1513–1524. doi: 10.1097/TP.0000000000001175. [DOI] [PubMed] [Google Scholar]
- 13.Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Bronsther O, et al. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59:197–203. doi: 10.1097/00007890-199501000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal A, Abayazeed A, Francis SL, Tolentino J, Ostergard DR, Seow A, et al. Correlation of patient age with CT-measured aortasacral promontory distance. Int Urogynecol J. 2015;26:887–891. doi: 10.1007/s00192-014-2621-5. [DOI] [PubMed] [Google Scholar]
- 15.DuBois JM, Delmonico FL, D’Alessandro AM. When organ donors are still patients: is premortem use of heparin ethically acceptable? Am J Crit Care. 2007;16:396–400. [PubMed] [Google Scholar]
- 16.Narvaez JRF, Nie J, Noyes K, Kayler LK. Transplant outcomes of donation after circulatory death livers recovered with versus without premortem heparin administration. Liver Transpl. 2020;26:247–255. doi: 10.1002/lt.25685. [DOI] [PubMed] [Google Scholar]
- 17.Fung JJ, Eghtesad B, Patel-Tom K. Using livers from donation after cardiac death donors--a proposal to protect the true Achilles heel. Liver Transpl. 2007;13:1633–1636. doi: 10.1002/lt.21388. [DOI] [PubMed] [Google Scholar]
- 18.Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, et al. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14:365–373. doi: 10.1002/lt.21372. [DOI] [PubMed] [Google Scholar]
- 19.Gulsen MT, Girotra M, Cengiz-Seval G, Price J, Singh VK, Segev DL, et al. HTK preservative solution is associated with increased biliary complications among patients receiving DCD liver transplants: a single center experience. Ann Transplant. 2013;18:69–75. doi: 10.12659/AOT.883831. [DOI] [PubMed] [Google Scholar]
- 20.Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286–293. doi: 10.1111/j.1600-6143.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 22.Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. doi: 10.1097/01.TP.0000062574.18648.7C. [DOI] [PubMed] [Google Scholar]
- 23.Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791–796. doi: 10.1111/j.1600-6143.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 24.de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773–781. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 25.Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 26.Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abt PL, Praestgaard J, West S, Hasz R. Donor hemodynamic profile presages graft survival in donation after cardiac death liver transplantation. Liver Transpl. 2014;20:165–172. doi: 10.1002/lt.23777. [DOI] [PubMed] [Google Scholar]
- 28.Firl DJ, Hashimoto K, O’Rourke C, Diago-Uso T, Fujiki M, Aucejo FN, et al. Role of donor hemodynamic trajectory in determining graft survival in liver transplantation from donation after circulatory death donors. Liver Transpl. 2016;22:1469–1481. doi: 10.1002/lt.24633. [DOI] [PubMed] [Google Scholar]
- 29.Taner CB, Bulatao IG, Perry DK, Sibulesky L, Willingham DL, Kramer DJ, et al. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012;25:838–846. doi: 10.1111/j.1432-2277.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 30.Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645–1653. doi: 10.1002/lt.21212. [DOI] [PubMed] [Google Scholar]
- 31.Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543–552. doi: 10.1016/j.surg.2009.06.052. discussion 552-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto K, Sherman I, Phillips MJ, Fisher MM. Three-dimensional observations of the hepatic arterial terminations in rat, hamster and human liver by scanning electron microscopy of microvascular casts. Hepatology. 1985;5:452–456. doi: 10.1002/hep.1840050318. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto K, Eghtesad B, Gunasekaran G, Fujiki M, Uso TD, Quintini C, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665–2672. doi: 10.1111/j.1600-6143.2010.03337.x. [DOI] [PubMed] [Google Scholar]
- 34.Du Z, Dong S, Lin P, Chen S, Wu S, Zhang S, et al. Warm ischemia may damage peribiliary vascular plexus during DCD liver transplantation. Int J Clin Exp Med. 2015;8:758–763. [PMC free article] [PubMed] [Google Scholar]
- 35.op den Dries S, Westerkamp AC, Karimian N, Gouw AS, Bruinsma BG, Markmann JF, et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60:1172–1179. doi: 10.1016/j.jhep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Verhoeven CJ, Simon TC, de Jonge J, Doukas M, Biermann K, Metselaar HJ, et al. Liver grafts procured from donors after circulatory death have no increased risk of microthrombi formation. Liver Transpl. 2016;22:1676–1687. doi: 10.1002/lt.24608. [DOI] [PubMed] [Google Scholar]
- 37.Pinna AD, Smith CV, Furukawa H, Starzl TE, Fung JJ. Urgent revascularization of liver allografts after early hepatic artery thrombosis. Transplantation. 1996;62:1584–1587. doi: 10.1097/00007890-199612150-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiki M, Hashimoto K, Palaios E, Quintini C, Aucejo FN, Uso TD, et al. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery. 2017;162:1101–1111. doi: 10.1016/j.surg.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Eghtesad B, Hashimoto K, Watson M, Nazal M, Quintini C, Kelly D, et al. Use of tissue plasminogen activator (TPA) in liver transplantation from donation after cardiac death (DCD) donors: a controlled randomized trial. Am J Transplant. 2015;15:275. doi: 10.1111/j.1600-6143.2010.03337.x. [DOI] [PubMed] [Google Scholar]
- 40.Seal JB, Bohorquez H, Reichman T, Kressel A, Ghanekar A, Cohen A, et al. Thrombolytic protocol minimizes ischemic-type biliary complications in liver transplantation from donation after circulatory death donors. Liver Transpl. 2015;21:321–328. doi: 10.1002/lt.24071. [DOI] [PubMed] [Google Scholar]
- 41.Bohorquez H, Seal JB, Cohen AJ, Kressel A, Bugeaud E, Bruce DS, et al. Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy. Am J Transplant. 2017;17:2155–2164. doi: 10.1111/ajt.14261. [DOI] [PubMed] [Google Scholar]
- 42.Kubal C, Mangus R, Fridell J, Saxena R, Rush N, Wingler M, et al. Optimization of perioperative conditions to prevent ischemic cholangiopathy in donation after circulatory death donor liver transplantation. Transplantation. 2016;100:1699–1704. doi: 10.1097/TP.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 43.Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. 2015;262:764–770. doi: 10.1097/SLA.0000000000001473. discussion 770-771. [DOI] [PubMed] [Google Scholar]
- 44.van Rijn R, Karimian N, Matton APM, Burlage LC, Westerkamp AC, van den Berg AP, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907–917. doi: 10.1002/bjs.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera MTPR, Isaac JR, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70:50–57. doi: 10.1016/j.jhep.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 47.Selzner M, Goldaracena N, Echeverri J, Kaths JM, Linares I, Selzner N, et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: First North American results. Liver Transpl. 2016;22:1501–1508. doi: 10.1002/lt.24499. [DOI] [PubMed] [Google Scholar]
- 48.Bral M, Gala-Lopez B, Bigam D, Kneteman N, Malcolm A, Livingstone S, et al. Preliminary single-center Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant. 2017;17:1071–1080. doi: 10.1111/ajt.14049. [DOI] [PubMed] [Google Scholar]
- 49.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]