Abstract
Clinical practice guidelines are important for guiding the management of specific diseases by medical practitioners, trainees, and nurses. In some cases, the guidelines are utilized as a reference for health policymakers in controlling diseases with a large public impact. With this in mind, practice guidelines for the management of chronic hepatitis B (CHB) have been developed in the United States, Europe, and Asian-Pacific regions to suggest the best-fit recommendations for each social and medical circumstance. Recently, the Korean Association for the Study of the Liver published a revised version of its clinical practice guidelines for the management of CHB. The guidelines included updated information based on newly available antiviral agents, the most recent opinion on the initiation and cessation of treatment, and updates for the management of drug resistance, partial virological response, and side effects. Additionally, CHB management in specific situations was comprehensively revised. This review compares the similarities and differences among the various practice guidelines to identify unmet needs and improve future recommendations.
Keywords: Hepatitis B, Chronic; Hepatitis B virus; Clinical practice guidelines
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a global health problem [1]. Although several therapeutic agents have been approved and used for patients with HBV infection, the disease remains difficult to cure, and the eradication of chronic infections remains challenging [2-5]. Current clinical practice guidelines or guidance by the American Association for the Study of Liver Diseases (AASLD) [6], European Association for the Study of the Liver (EASL) [7], and Asian-Pacific Association for the Study of the Liver (APASL) provides general recommendations for the management of chronic hepatitis B (CHB) (Table 1) [8]. Recently, in 2019, the Korean Association for the Study of the Liver (KASL) published new clinical practice guidelines for the management of CHB in this journal and described comprehensive management strategies including prevention, monitoring, treatment, and special considerations [9]. Here, we compare the Korean guidelines with other international guidelines regarding when to start, when to change, and when to stop antiviral treatment for CHB.
Table 1.
Comparison of current clinical practice guidelines for chronic hepatitis B management
| KASL | AASLD | EASL | APASL | |
|---|---|---|---|---|
| Published year | Jun-19 | Apr-18 | Aug-17 | Jan-16 |
| Journal | Clinical Molecular Hepatology | Hepatology | Journal of Hepatology | Hepatology International |
| Type | Clinical practice guidelines | Guidance incorporated with guidelines | Clinical practice guidelines | Clinical practice guidelines |
| Listed author(s) | KASL | An expert panel of the AASLD | EASL | A panel of Asian experts chosen by the APASL |
| Recommendation | GRADE | Guidance developed by the consensus of an expert panel, GRADE (2016) | GRADE | GRADE |
| Interval since the previous update | 3 years | 2 years | 5 years | 4 years |
| Target population | Korean | American | European | Asian |
| Suggested normal ALT (IU/L) | ||||
| Male | <34 | <35 | <40 | <40 |
| Female | <30 | <25 | <40 | <40 |
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; GRADE, Grading of Recommendations Assessment, Development and Evaluation system; ALT, alanine aminotransferase.
NATURAL HISTORY
CHB is a life-long disease that can start at the beginning of life by perinatal transmission, especially in Asian countries [3]. Five characteristic phases of CHB have been identified according to immunological features, virology, biochemistry, and histology (Table 2) [3].
Table 2.
Comparison of terminology and characteristics associated with the natural history of chronic hepatitis B
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | ||
|---|---|---|---|---|---|---|
| Terminology | ||||||
| KASL | Immune tolerant CHB (CHB, immune tolerant phase) | Immune active HBeAg-positive CHB (HBeAg-positive CHB, immune active phase) | Immune inactive CHB (CHB, Immune inactive phase) | Immune active HBeAg-negative CHB (HBeAg-negative CHB, immune active phase) | Resolved CHB, (HBsAg loss phase) | |
| AASLD | Immune tolerant CHB | Immune active HBeAg-positive CHB | Inactive CHB | Immune active HBeAg-negative CHB | Resolved CHB (functional cure state) | |
| EASL | HBeAg-positive chronic HBV infection | HBeAg-positive CHB | HBeAg-negative chronic HBV infection | HBeAg-negative CHB | Resolved HBV infection | |
| APASL | Immune tolerant chronic HBV infection (immune tolerant phase) | HBeAg-positive CHB (immune reactive phase) | Low replicative chronic HBV infection (low replicative phase) | HBeAg-negative CHB (reactivation phase) | Resolved hepatitis B infection | |
| Characteristics | ||||||
| HBsAg HBeAg | High Positive | High/intermediate Positive | Low Negative | Intermediate Negative | Negative Negative | |
| HBV DNA level | >106–107* IU/mL | >2×104 IU/mL (104–107 IU/mL)‡ | <2,000 IU/mL | >2,000 IU/mL | Undetectable | |
| ALT level | Persistently normal | Elevated | Persistently normal | Elevated | Normal | |
| Histological activity† | None/minimal | Moderate/severe | Minimal | Moderate/severe | None | |
KASL, Korean Association for the Study of the Liver; CHB, chronic hepatitis B; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; HBV, hepatitis B virus; ALT, alanine aminotransferase.
HBV DNA >106 IU/mL by the AASLD, HBV DNA >107 IU/mL by the KASL and EASL, and no clear cut-off by the APASL although ranges from >2×106–107 IU/mL are favored.
Activity depends on necroinflammation, and fibrosis stage can vary according to the degree of liver injury accumulation.
EASL criteria for HBeAg-positive chronic hepatitis B.
The first phase is the CHB immune tolerant phase (immune tolerant CHB). It is characterized by very high levels of HBV replication, persistently normal alanine aminotransferase (ALT) levels, and minimal or no necroinflammatory activity. During this phase, patients are typically positive and show high titers of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg). Inflammatory activity is assumed to be absent, which prompted the EASL to revise the name of the first phase to HBeAg-positive chronic HBV infection [7]. However, normal ALT levels do not exclude the presence of necroinflammation and fibrosis, particularly as determined by conventional laboratory criteria [10,11]. Therefore, the KASL adjusted the cut-off of normal ALT to 34 IU/L for males and 30 IU/mL for females on the basis of a recent study involving 12,486 Korean CHB patients [9,12]. Experts from the AASLD recommended the use of similar cut-offs of 35 IU/L for males and 25 IU/mL for females, whereas the EASL and APASL insist on using traditional laboratory reference values of 40 IU/mL for both sexes (Table 1) [6]. Another issue differentiating the immune tolerant phase from other phases is the cut-off of the very high HBV DNA levels. The HBV DNA criterion (≥107 IU/mL) for the immune tolerant phase as defined by the KASL and EASL is somewhat different from that of the AASLD (>106 IU/mL) and the APASL (>2×106 IU/mL) [6-9]. However, given that a decreasing HBV DNA titer suggests immunological interactions between the host and virus, a higher cut-off would be suitable to exclude patients in transition phases who may need antiviral treatment [13]. Indeed, the definition of the immune tolerant phase is still under debate [13,14], causing investigators to continue evaluating the appropriate cut-offs of the ALT and HBV DNA levels for the accurate prediction of long-term prognosis and recommendation of suitable treatment.
The second phase is the HBeAg-positive immune active phase. This is also termed the immune reactive or immune clearance phase [3]. The level of HBV DNA remains high (104 –107 IU/mL according to the EASL) but may fluctuate [3]. Elevated ALT suggests the presence of intrahepatic necroinflammation and can be associated with liver damage [15]. The outcome of this phase varies according to the degree of liver injury; thus, prompt antiviral therapy is recommended [15].
The third phase is the immune inactive phase, previously known as the immune controlled phase [3]. Other terminologies for this phase include HBeAg-negative chronic HBV infection, as proposed by the EASL, and low replicative chronic HBV infection or low replicative phase, as proposed by the APASL [7,8], emphasizing the minimal intrahepatic inflammation and low viral replication. The level of HBV DNA is typically as low as <2,000 IU/mL, and the ALT level is within the upper limit of the normal (ULN) range. If patients remain in this phase, prognosis is favorable, and the HBsAg levels may decrease at 1–3% per year. However, low level persistent viremia can be associated with live disease progression, and a number of patients transit to the HBeAg-negative immune-active phase of CHB annually [16].
The fourth phase is the HBeAg-negative CHB immune active phase, which was previously known as the immune escape phase or reactivation phase [3,17]. The moderate to high levels of HBV replication (>2,000 IU/mL) and negative HBeAg status in this phase are caused by mutations on the pre-core or core promoter regions of HBV DNA, blocking HBeAg production [18,19]. The prolonged viral replication and intrahepatic necroinflammation observed during this phase are associated with progression to liver cirrhosis or the development of hepatocellular carcinoma (HCC) [3,20].
The last phase is the HBsAg loss phase, in which HBsAg is spontaneously cleared [21]. Although the incidence of HBsAg loss is very low (<0.5% per year), the risk of disease progression substantially decreases [21,22]. However, HCC surveillance should be continued if HBsAg loss occurs after age ≥50 years as recommended by the KASL. The AASLD suggests continued HCC surveillance in patients with HBsAg loss after ages >40 years for males and >50 years for females [6,9].
TREATMENT GOALS AND AIMS
The treatment goals and aims were updated in the KASL guidelines [9]. The goals of treatment are to decrease mortality due to liver disease and improve survival by preventing the progression of liver fibrosis to cirrhosis and preventing HCC, which are consistent with other guidelines [7,8]. The aims of anti-HBV treatment suggested by the KASL include ALT normalization, undetectable serum HBV DNA, serum HBeAg loss or seroconversion, and serum HBsAg loss or seroconversion [9]. Specifically, serum HBsAg loss or seroconversion is proposed as an ideal endpoint for CHB treatment [9]. The EASL also suggests HBsAg loss with or without anti-HBs seroconversion as an optimal endpoint, whereas the APASL considers HBsAg loss an ideal, although not realistic, endpoint [7,8]. Hence, the APASL suggests a sustained off-therapy virological response in both HBeAg-positive (with HBeAg to anti-HBe seroconversion) and HBeAg-negative patients as a satisfactory endpoint [8].
WHEN TO START: COMPARISON OF TREATMENT INDICATORS
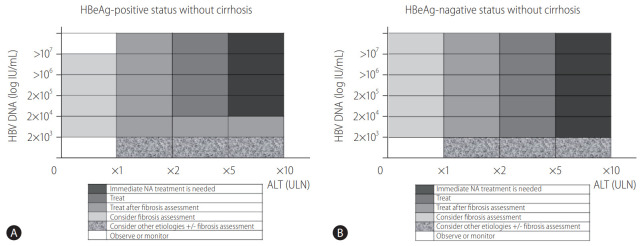
High level HBV replication is associated with an increased risk of liver damage and liver-related complications [23,24]. Antiviral therapy using interferons or nucleos(t)ide analogs (NAs) efficiently reduces these risks by suppressing HBV replication [25-29]. Current guidelines recommend treatment initiation with antiviral agents before the accumulation of liver injury or progression of fibrosis. However, intrahepatic covalently closed circular DNA cannot be eradicated, even with long-term treatment [6-9]. To determine when to initiate antiviral treatment, the level of HBV replication by HBV DNA measurement, the degree of liver injury measured by ALT or liver biopsy, and the stage of liver fibrosis assessed by noninvasive methods or liver biopsy should be considered (Fig. 1) [9,30]. Additionally, information regarding age, co-morbidity, and family history of HCC or liver cirrhosis may be helpful in determining when to start treatment.
Figure 1.
Nomogram of treatment indicators for chronic hepatitis B without liver cirrhosis for (A) HBeAg-positive patients and (B) HBeAg-negative patients. Gradation toward dark gray suggests immediate treatment, and gradation toward white suggests observation and monitoring. Intermediate gradation suggests the need for fibrosis assessment before determining treatment according to the international guidelines and the Korean Association for the Study of Liver Guidelines. The initiation of antiviral therapy is indicated by a noninvasive fibrosis test suggesting evidence of significant fibrosis or a liver biopsy showing significant necroinflammation or fibrosis (≥A2 or ≥F2). HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; ALT, alanine aminotransferase; ULN, upper limit of normal; NA, nucleos(t)ide analog.
CHB, immune tolerant phase
Previously, most practice guidelines did not recommend antiviral therapy for CHB patients in the immune tolerant phase (Table 3) [17,31]. Recent data regarding the treatment of CHB patients in the immune tolerant phase suggest that the risk of progression to liver cirrhosis and HCC development could be reduced by antiviral therapy [13,32]. Therefore, patients needing treatment should be differentiated from truly immune tolerant CHB patients who do not require antiviral therapy. However, initiation of antiviral therapy for patients in the immune tolerant phase remains very controversial [6-8]; further studies are needed to appropriately define the immune tolerant phase, as discussed above.
Table 3.
Comparison of treatment indicators for chronic hepatitis B
| KASL | AASLD | EASL | APASL | |
|---|---|---|---|---|
| Immune tolerant CHB | 1) Monitor patients with very high HBV DNA (≥107 IU/mL) and normal ALT (male <34 IU/mL, female <30 IU/mL) | 1) Monitor patients with high HBV DNA (≥106 IU/mL) and normal ALT (male <35 IU/mL, female <25 IU/mL) | 1) Monitor patients with high HBV DNA (≥107 IU/mL) and normal ALT (<40 IU/L) if there are no signs of chronic hepatitis | 1) Monitor patients with high HBV DNA (e.g., >2×106–107 IU/mL) and normal ALT (<40 IU/L) if age <30 years |
| 1) Monitor | 2) Liver biopsy to determine treatment if there are risk factors (age ≥30–40 years, HBV DNA levels <107 IU/mL, noninvasive fibrosis tests suggesting significant hepatic fibrosis, or ALT is approaching the ULN) | 2) Antiviral therapy is suggested in selected patients (age >40 years with normal ALT and elevated HBV DNA [1,000,000 IU/mL], liver biopsy showing significant necroinflammation or fibrosis) | 2) Antiviral therapy may be indicated for patients >30 years of age, regardless of the severity of liver histological lesions | 2) Liver biopsy if indicated (age is >35 years or there is a family history of HCC or cirrhosis, noninvasive tests suggest evidence of significant fibrosis, persistently elevated ALT) Treat if ≥A2 or ≥F2 |
| 2) Consider | Patients with a family history of HCC or cirrhosis and extrahepatic manifestations can be treated | |||
| Immune active CHB | 1) Treat if HBV DNA ≥20,000 (for HBeAg-positive CHB) or ≥2,000 (for HBeAg-negative CHB) IU/mL and serum ALT level ≥2× ULN | 1) Treat if elevated HBV DNA (≥20,000 IU/mL for HBeAg-positive or ≥2,000 IU/mL for HBeAg-negative CHB) and ALT ≥2× ULN or there is evidence of significant histological disease | 1) Treat if HBV DNA >20,000 IU/mL and ALT >2× ULN, regardless of the degree of fibrosis | 1) Treat if HBV DNA >20,000 IU/mL for HBeAg-positive or >2,000 IU/mL for HBeAg-negative CHB and ALT levels are elevated >2× ULN |
| 1) Treat | 2) Consider liver biopsy if ALT is 1–2× ULN and treat if there is moderate to severe necroinflammation (≥A2) or significant fibrosis (≥F2) | 2) Consider the severity of liver disease to determine treatment for patients with ALT >1–2× ULN | 2) Treat all patients with HBeAg-positive or -negative CHB, defined by HBV DNA >2,000 IU/mL, ALT>ULN (40 IU/L), and/or at least moderate liver necroinflammation or fibrosis by biopsy | 2) Patients with high HBV DNA levels (>20,000 IU/mL for HBeAg-positive and >2,000 IU/mL for HBeAg-negative CHB) but ALT <2× ULN should depict a noninvasive fibrosis assessment |
| 2), 3) Consider | 3) In HBeAg-negative patients with HBV DNA ≥2,000 IU/mL and normal ALT levels, follow-up or liver biopsy/noninvasive fibrosis tests can be considered | Biopsy should be considered if indicated* | ||
| Antiviral therapy is recommended if ≥A2 or ≥F2 | ||||
| Immune inactive CHB | 1) Monitor | 1) Monitor | 1) Monitor | 1) Monitor |
| 1) Monitor | 2) Patients with HBeAg-negative chronic HBV infection, family history of HCC or cirrhosis, and extrahepatic manifestations can be treated even if typical treatment indications are not present | 2) HBeAg-negative patients with HBV DNA <2,000 IU/mL, should be evaluated for other causes if ALT is elevated and obtain a noninvasive fibrosis assessment | ||
| 2) Consider | Biopsy should be considered if indicated* | |||
| Antiviral therapy is recommended if ≥A2 or ≥F2 | ||||
| First-line agents | Entecavir, tenofovir DF, tenofovir AF, besifovir, peg-interferon | Entecavir, tenofovir DF, tenofovir AF, peg-interferon | Entecavir, tenofovir DF, tenofovir AF, peg-interferon | Entecavir, tenofovir DF, peg-interferon |
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; CHB, chronic hepatitis B; HBV, hepatitis B virus; ALT, alanine aminotransferase; ULN, upper limit of normal; HCC, hepatocellular carcinoma; ≥A2, moderate to severe inflammation; ≥F2, significant fibrosis or more; HBeAg, hepatitis B e antigen; tenofovir DF, tenofovir disoproxil fumarate; tenofovir AF, tenofovir alafenamide fumarate; peg-interferon, pegylated interferon.
APASL recommends the consideration of liver biopsy if noninvasive tests suggest evidence of significant fibrosis, ALT becomes persistently elevated, age is >35 years, or there is a family history of HCC or cirrhosis.
The KASL guidelines suggest liver biopsy if the patient is ≥30–40 years of age, the serum HBV DNA levels are <107 IU/mL, a noninvasive fibrosis test shows a range of significant hepatic fibrosis, or ALT is at the borderline of the ULN [9]. Biopsy findings of moderate to severe inflammation (≥A2) or significant fibrosis (≥F2) are treatment indicators. Age cut-offs for the consideration of liver biopsy or treatment vary among the guidelines and are >40 years according to the AASLD, >30 years according to the EASL, and >35 years according to the APASL [6-8]. The EASL specifically emphasizes the age; it recommends starting treatment regardless of the severity of histological liver lesions if a patient is >30 years of age [7]. However, this recommendation requires further validation. In other cases, histological criteria should be used to determine when to initiate treatment; these values are the same among the guidelines (≥A2 or ≥F2) [6-9].
CHB, immune active phase
Antiviral treatment during the immune active phase decreases the risk of liver cirrhosis, hepatic decompensation, and HCC [25-29]. Therefore, antiviral therapy is recommended for patients in this phase. The criteria for treatment differ slightly among the guidelines (Table 3). Regarding HBV DNA levels, the KASL, AASLD, and APASL suggest levels of ≥20,000 IU/mL for HBeAg-positive and ≥2,000 IU/mL for HBeAg-negative CHB as treatment indicators if ALT is elevated >2× ULN [6-9]. If the ALT levels are 1–2× ULN, liver biopsy or noninvasive fibrosis tests are necessary to determine whether treatment should be initiated [6-9]. The EASL recommends the treatment of patients with HBV DNA >20,000 IU/mL and ALT >2× ULN, regardless of the degree of fibrosis, whereas all patients with HBV DNA >2,000 IU/mL and ALT >1× ULN require fibrosis assessment before treatment (≥A2 or F2) [7]. Previously, a 3–6 months monitoring period was recommended for HBeAg-positive immune active CHB patients expecting spontaneous HBeAg seroconversion [8,17]. However, this is currently not recommended by the KASL and international guidelines, with the exception of the APASL, owing to an increased risk of liver failure during the follow-up period [6-9,33]. Additionally, most guidelines recommend immediate antiviral therapy for patients with acute exacerbation, such as elevation of ALT to ≥5–10× ULN or signs of liver failure [6-9,34].
On the contrary, for HBeAg-negative patients with elevated HBV DNA levels (≥2,000 IU/mL) and normal ALT levels, treatment may be delayed or considered after liver biopsy, because these patients are considered to be in a gray area or transitional zone [6-9]. The KASL, EASL, and APASL also recommend noninvasive fibrosis tests to assess these patients [6,7,9].
CHB, immune inactive phase
The KASL suggested that the immune inactive phase, which features low HBV DNA levels (<2,000 IU/mL) and normal ALT, is not an indicator for antiviral therapy [9]. However, the EASL and APASL recommend considering treatment if there is a family history of HCC or liver cirrhosis or significant histological findings upon liver biopsy [7,8]. Considering that HBsAg loss is an ideal endpoint for therapy, treatment of CHB in this phase may facilitate HBsAg clearance [35]; thus, the treatment strategy could be changed in the future.
Compensated liver cirrhosis
Most guidelines recommend treating compensated liver cirrhosis if the HBV DNA level is ≥2,000 IU/mL, regardless of the ALT level [6-9]. Furthermore, even patients with detectable but low HBV DNA (<2,000 IU/mL) should be considered for treatment (Table 4) [6-9]. This approach is supported by recent data regarding the decrease in liver-related events induced by NA therapy in cirrhotic patients with low level viremia [36].
Table 4.
Comparison of treatment indicators for patients with liver cirrhosis
| KASL | AASLD | EASL | APASL | |
|---|---|---|---|---|
| Compensated cirrhosis | 1) Treat if HBV DNA level is ≥2,000 IU/mL, regardless of the ALT level | 1) Treat if HBV DNA is >2,000 IU/mL, regardless of the ALT level | 1) Treat for any detectable HBV DNA, regardless of the ALT levels, in patients with compensated or decompensated cirrhosis | 1) Treat if HBV DNA is >2,000 IU/mL, even if the ALT levels are normal |
| 1) Treat | ||||
| 2) Consider | 2) Treatment can be considered if HBV DNA is detectable but low (<2,000 IU/mL), regardless of the ALT level | 2) Treat patients with low level viremia (HBV DNA <2,000 IU/mL), regardless of the ALT level | 2) Treatment can be considered irrespective of HBV DNA and ALT levels | |
| Decompensated cirrhosis | 1) Treat with a NA if serum HBV DNA is detected, regardless of the ALT level | 1) Treat with antiviral therapy indefinitely, regardless of the HBV DNA level, HBeAg, or ALT level | 1) Immediately treat with a NA with high barrier to resistance, irrespective of the HBV replication level | 1) Immediately treat with a NA for patients with detectable HBV DNA |
| 1) Treat | 2) Consider liver transplantation | 2) Consider liver transplantation | 2) Assess for the possibility of liver transplantation | 2) Consider treatment for all patients with hepatic decompensation, irrespective of HBV DNA levels |
| 2), 3) Consider | 3) Consider liver transplantation | |||
| First-line agents* | Entecavir, tenofovir DF, tenofovir AF,† besifovir† | Entecavir, tenofovir DF, tenofovir AF† | Entecavir, tenofovir DF, tenofovir AF† | Entecavir, tenofovir DF |
KASL, Korean Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; HBV, hepatitis B virus; ALT, alanine aminotransferase; NA, nucleos(t)ide analog; HBeAg, hepatitis B e antigen; tenofovir DF, tenofovir disoproxil fumarate; tenofovir AF, tenofovir alafenamide fumarate.
Peg-interferon can only be used, with caution, for compensated cirrhosis, but may not be preferred owing to safety concerns.
Insufficient data for decompensated cirrhosis.
Decompensated liver cirrhosis
Antiviral therapy should be initiated regardless of the ALT level if serum HBV DNA is detected in patients with decompensated liver cirrhosis [6-9]. Preferably, all HBsAg-positive decompensated cirrhosis patients should receive antiviral therapy, even if HBV DNA is not detected. However, the benefits of NAs for HBV DNA-undetected patients require further studies. Liver transplantation should also be considered (Table 4) [8].
WHAT TO CHOOSE: SELECTION OF ANTIVIRAL AGENTS
Pegylated interferon
Interferons and oral NAs represent the currently available antiviral agents [5-9,37]. Among interferons, pegylated interferon (peg-interferon) replaced conventional interferon owing to its onceweekly dosing and improved efficacy [37,38]. However, various adverse events and the inconvenience associated with the injection of peg-interferon have limited its use despite its unique immune-modulatory actions [37-39]. Nonetheless, peg-interferon should be considered for a finite duration of treatment achievable by the immune-mediated control of HBV, leading to sustained off-treatment responses [37-39]. Peg-interferon is not preferred in patients with liver cirrhosis due to safety concerns and is contraindicated for decompensated cirrhosis patients by all international guidelines [6-9].
NAs
Contrary to interferons, NAs are directly acting antiviral agents that inhibit HBV replication and have no fixed treatment duration [6-9]. NAs are now widely used for CHB treatment owing to their low incidence of adverse effects as well as convenience. Antiviral resistance was a major drawback of first-generation NAs (lamivudine and adefovir). Resistance was very rare in second-generation NAs (entecavir and tenofovir disoproxil fumarate [tenofovir DF]) [40-42]. Additionally, newer generation drugs (besifovir dipivoxil maleate [besifovir] and tenofovir alafenamide fumarate [tenofovir AF]) have alleviated the safety concerns associated with tenofovir DF (renal and bone toxicity), while maintaining a high genetic barrier to resistance [43-45]. Therefore, the KASL recommends NAs with a high genetic barrier to resistance, including entecavir, tenofovir DF, tenofovir AF, and besifovir, rather than those with a low genetic barrier to resistance (lamivudine, telbivudine, clevudine, and adefovir) as first-line agents for CHB treatment [9].
Lamivudine and adefovir have been used for extended periods but are no longer recommended given their low potency and high incidence of resistance [42,46]. Telbivudine and clevudine are comparable to entecavir in their antiviral potency but are currently not recommended owing to the frequent development of antiviral resistance and serious muscle-related problems [42,46-49].
Entecavir and tenofovir DF have been the preferred antiviral agents for more than a decade since their approval for CHB treatment. Recently, these drugs were compared in terms of long-term treatment outcomes, especially for the prevention of HCC [50-53]. The initial report using data from the Korean National Health Insurance Service database suggested that tenofovir DF was associated with a significantly lower risk of HCC compared to entecavir [50]. However, subsequent reports using multicenter academic teaching hospital data were contradictory and found no difference between the two therapies regarding the incidence of HCC, all-cause mortality, and liver transplantation, even after a thorough adjustment of baseline characteristics [51,52]. The issue remains contentious and requires further longer term and larger scale studies with the appropriate adjustment of possible biases to reach a consensus [53].
Currently, generic and less expensive forms of entecavir and tenofovir (tenofovir disoproxil or tenofovir fumarate aspartate) are available in Korea and other countries, which can improve the cost-effectiveness of antiviral treatment [54,55]. Unfortunately, there is limited clinical data on the antiviral efficacy of generic antiviral drugs for CHB [55].
Tenofovir AF is a nucleotide reverse transcriptase inhibitor and a novel prodrug of tenofovir. It has greater plasma stability than tenofovir DF and efficiently delivers the active form of tenofovir to hepatocytes at a lower dose [44,45]. In phase 3 clinical trials, tenofovir AF was found to be as effective as tenofovir DF and induced significantly smaller decreases in the estimated glomerular filtration rate and spine/hip bone density than tenofovir DF after up to 96 weeks of treatment [44,45].
Besifovir is an acyclic nucleotide phosphonate developed in Korea that was approved by the Ministry of Food and Drug Safety in 2017 [43]. However, it is still not available outside Korea. The KASL guidelines are the first to include besifovir as one of the initial choices for CHB treatment [9]. The advantage of besifovir over tenofovir DF has been well described [43]. Briefly, in phase 3 randomized controlled trials, besifovir was comparable to tenofovir DF in terms of antiviral efficacy after 48 weeks of treatment. Additionally, the renal and bone safety profiles of besifovir were superior to those of tenofovir DF. The estimated glomerular filtration rate and hip/spine bone mineral density were significantly higher in the besifovir group than in the tenofovir DF group [43]. After 48 weeks, all patients were rolled over into an open-label extensional study where everyone received besifovir. In patients who switched from tenofovir DF to besifovir, the estimated glomerular filtration rate and hip/spine bone mineral density improved to baseline levels at 96 weeks [43].
Currently, there is limited data regarding the use of besifovir or tenofovir AF in patients with decompensated liver cirrhosis or HCC. However, there seems to be no reason not to use these drugs. In the future, more data regarding besifovir and tenofovir AF will be available for CHB patients in various situations. The AASLD and EASL recommend entecavir, tenofovir DF, and tenofovir AF monotherapy as the preferred regimens for the treatment of CHB and liver cirrhosis patients. The APASL only recommends entecavir and tenofovir DF, likely owing to the limited data available at the time of its publication in 2016 [6-8].
No guidelines recommend a combination of peg-interferon and NA or a combination of NAs as initial therapy due to their limited benefits [6-9].
WHEN TO CHANGE: TREATMENT MODIFICATIONS
Partial virological responses (PVR)
Although the antiviral efficacy of drugs has remarkably improved, patients with very high HBV DNA levels may show PVR featuring a decreased but still detectable level of HBV DNA after at least 48 weeks of continued treatment with high genetic barrier drugs. Other causes of PVR include decreased susceptibility owing to previous drug exposure, decreased medication compliance, and altered drug metabolism. The clinical significance of low level viremia due to PVR is unclear although an increased risk of liver-related complications was found in patients with advanced liver diseases [36,56].
There are slightly different definitions of PVR. They are summarized in Table 5. Briefly, the KASL defines PVR at different time points based on the genetic barrier of the drugs, at 48 weeks of therapy for high genetic barrier drugs and at 24 weeks for low genetic barrier drugs [9]. The APASL defines PVR as detectable HBV DNA at 24 weeks of therapy, whereas the EASL and AASLD define PVR or persistent viremia at 48 weeks and 96 weeks of entecavir or tenofovir treatment, respectively [6-8].
Table 5.
Comparison of partial virological response management during chronic hepatitis B treatment
| KASL | AASLD | EASL | APASL | |
|---|---|---|---|---|
| Definition | A decreased but detectable level of HBV DNA after at least 48 weeks of therapy using high genetic barrier drugs (24 weeks for low genetic barrier drugs) | Persistent viremia is defined as a plateau in the decline of HBV DNA and/or failure to achieve an undetectable HBV DNA level after 96 weeks of therapy | A decrease in HBV DNA level of more than 1 log10 IU/mL but HBV DNA remains detectable after at least 12 months of therapy | Reduction of serum HBV DNA level >1 log IU/mL but still detectable at 24 weeks of therapy |
| PVR to low genetic barrier (e.g., lamivudine or telbivudine) | 1) Switch: switching to NAs with high genetic barriers and no cross-resistance is recommended | – | – | 1) Switch: treatment can be modified |
| 1) Switch | ||||
| PVR to high genetic barrier (e.g., entecavir or tenofovir) | 1) Continue: treatment can be continued | 1) Continue: patients with persistent low level viremia (HBV DNA <2,000 IU/mL) on entecavir or tenofovir may continue treatment | 1) Continue: patients with declining serum HBV DNA levels may continue treatment with the same agent | 1) Continue: for patients with detectable HBV DNA after 24 weeks, continuation of the same treatment is recommended |
| 1) Continue | 2) Switch: switching to tenofovir is recommended in the case of partial virological response to entecavir | 2) Switch or add: in cases where HBV DNA levels plateau, a switch to another drug or a combination of entecavir/tenofovir can be considered | ||
| 2) Switch/add |
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; HBV, hepatitis B virus; PVR, partial virological responses; NA, nucleos(t)ide analog.
Modification of therapy may be considered for PVR, especially when using low genetic barrier drugs (Table 5). However, the recommendations for managing PVR during entecavir or tenofovir DF therapy are not consistent across the guidelines (Table 5). All guidelines indicate that high genetic barrier drugs can be continued, with the possibility of switching to another high genetic barrier drug (KASL, EASL) or add-on (EASL), especially in patients with advanced liver diseases [6-9]. The AASLD argues that there is insufficient comparative evidence to advocate the addition of a second drug or switching to another drug. However, a recent randomized controlled trial compared switching to tenofovir DF with continuation of entecavir for the treatment of CHB with PVR and observed a better virological outcome upon switching to tenofovir DF [57]. Hence, the KASL recommends switching to tenofovir DF for patients with entecavir PVR [9].
Antiviral resistance
Although antiviral resistance is uncommon in previously treatment-naïve patients receiving high genetic barrier drugs, entecavir resistance was found in 1–3% of patients, and variants resistant to tenofovir DF have been identified [40,42,58,59]. Entecavir resistance rates increase up to 50% in the fifth year in treatment-experienced or refractory patients [40,42]. Adefovir monotherapy also has a high risk of resistance in patients with lamivudine resistance with up to 25% resistance at two years of treatment, leading to multidrug resistance [60,61]. The collective findings highlight the importance of paying attention to patients’ adherence to medication; antiviral resistance testing should be performed in case of virological breakthrough [42].
After antiviral resistance is confirmed, it is assumed that one of two strategies can be applied: switching to another class of antiviral monotherapy with a high genetic barrier to resistance or adding a second antiviral drug that lacks cross-resistance (Table 6). The recent KASL guidelines recommend that patients with resistance to L-nucleoside analogs (lamivudine and telbivudine) be switched to tenofovir DF/AF [9]. For adefovir or entecavir resistance, switching to tenofovir DF/AF monotherapy or a combination of tenofovir DF/AF and entecavir is recommended. For multidrug resistance, switching to a combination of tenofovir DF/AF and entecavir or tenofovir DF/AF monotherapy is recommended [9]. Similar recommendations were provided by the AASLD, EASL, and APASL favoring tenofovir DF/AF monotherapy, except in cases of multidrug resistance [6-8]. However, considering recent reports of the long-term efficacy of tenofovir DF for multidrug resistance, tenofovir DF/AF monotherapy could also be a safe option [62,63].
Table 6.
Comparison of antiviral resistance management during chronic hepatitis B treatment
| KASL* | AASLD* | EASL | APASL | |
|---|---|---|---|---|
| Lamivudine/telbivudine resistance | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to TDF |
| 1) Switch | ||||
| Entecavir resistance | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to TDF |
| 1) Switch | 2) Combine with tenofovir (TDF or TAF) | |||
| 2) Combine | ||||
| Adefovir resistance | 1) Switch to tenofovir (TDF or TAF) | 1) Switch to ETV or tenofovir (TDF or TAF) | 1) Switch to ETV or tenofovir (TDF or TAF) (LAM-naïve) | 1) Switch to either ETV or TDF (no LAM-resistance) |
| 1) Switch | 2) Combine ETV plus tenofovir (TDF or TAF) | 2) Switch to tenofovir (TDF or TAF) (LAM-resistance) | 2) Switch to TDF monotherapy (LAM-resistance) | |
| 2) Switch/combine | If HBV DNA plateaus: combine ETV or switch to ETV | |||
| Tenofovir resistance | 1) Combine with ETV | 1) Switch to ETV | 1) Switch to ETV (LAM-naïve) | – |
| 1) Combine/switch | 2) Combine with ETV (LAM-resistance) | |||
| 2) Combine | ||||
| Multidrug resistance | 1) Combine ETV and tenofovir (TDF or TAF) | 1) Combine ETV and tenofovir (TDF or TAF) | 1) Combine ETV and tenofovir (TDF or TAF) | 1) Combine ETV and TDF |
| 1) Combine | 2) Switch to tenofovir (TDF or TAF) | |||
| 2) Switch |
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide fumarate; LAM, lamivudine; HBV, hepatitis B virus; ETV, entecavir.
Preferred treatment was suggested.
There are insufficient data regarding tenofovir resistance, although guidelines recommend adding or switching to entecavir. In these cases, it is likely that new antiviral agents other than NAs, such as capsid assembly inhibitors, may be needed [58].
Adverse effects
NAs are relatively safe drugs, even with long-term use. However, all drugs may have side effects. Clinically significant adverse effects associated with NA therapy include lactic acidosis (entecavir, tenofovir DF, adefovir, lamivudine, and telbivudine), nephropathy, osteomalacia, Fanconi syndrome (tenofovir DF and adefovir), increasing low-density lipoprotein cholesterol (tenofovir AF), carnitine depletion (besifovir), pancreatitis (lamivudine), peripheral neuropathy, creatinine kinase elevation, and myopathy (telbivudine and clevudine) [9]. When NA-related adverse effects are suspected, it is essential to confirm the diagnosis. In cases of serious complications, immediate cessation of the drug or switching to another drug should be considered. For example, switching to entecavir or tenofovir DF is a reasonable option for clevudine- or telbivudine-associated myopathy [48,49,64].
Among the high genetic barrier drugs preferred as first-line agents for CHB treatment, tenofovir DF has an increased risk of renal and bone toxicity [65]. The KASL recommends substituting tenofovir DF with entecavir, tenofovir AF, or besifovir in such patients based on previous treatment history [9]. The AASLD and EASL also recommend that patients using tenofovir DF who are at risk of developing and/or have underlying renal dysfunction or metabolic bone disease consider switching to entecavir or tenofovir AF, depending on their previous lamivudine exposure [6,7]. For lamivudine or other NA-experienced or refractory patients, tenofovir AF is preferred to entecavir.
WHEN TO STOP: TREATMENT CESSATION
The standard duration of peg-interferon therapy is 48 weeks for HBeAg-positive or -negative CHB [38,66]. Thus, it would be acceptable to stop treatment after the planned schedule is completed. Extending the treatment duration may be more effective for HBeAg-negative CHB [67,68] but cannot be routinely recommended (Table 7).
Table 7.
Comparison of cessation criteria for chronic hepatitis B treatment
| KASL | AASLD | EASL | APASL | ||
|---|---|---|---|---|---|
| NAs | |||||
| HBeAg-postive CHB | 1) HBsAg loss | 1) HBeAg seroconversion with 12 months consolidation plus undetectable HBV DNA | 1) HBsAg loss with/without anti-HB seroconversion | 1) HBeAg seroconversion with 12 months consolidation (preferably 3 years) | |
| 2) HBeAg loss/seroconversion with 12 months consolidation plus undetectable HBV DNA | 2) Alternatively, treat until HBsAg is lost | 2) HBeAg seroconversion with 12 months consolidation plus undetectable HBV DNA | 2) HBsAg loss or seroconversion | ||
| HBeAg-negative CHB | 1) HBsAg loss | 1) Indefinite | 1) HBsAg loss with/without seroconversion | 1) HBsAg loss or seroconversion | |
| 2) May be considered after HBsAg loss | 2) May be considered after long-term (≥3 years) virological suppression after NA therapy | 2) Undetectable HBV DNA for at least 2 years on 3 separate occasions each 6 months apart | |||
| Liver cirrhosis | 1) Long-term treatment for compensated cirrhosis | 1) Indefinite (may be considered after HBsAg loss) | 1) Indefinite | 1) NA therapy should be continued for life in compensated and decompensated cirrhotic patients | |
| 2) Indefinite for decompensated cirrhosis | |||||
| Peg-Interferon | |||||
| HBeAg (+) | 48 weeks | 48 weeks | 48 weeks | 48 weeks | |
| HBeAg (-) | 48 weeks | 48 weeks | 1) 48 weeks | 48 weeks | |
| 2) Extending treatment beyond 48 weeks may be beneficial | |||||
1) Preferred, 2) alternative.
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; NA, nucleos(t)ide analog; HBeAg, hepatitis B e antigen; CHB, chronic hepatitis B; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; peg-interferon, pegylated interferon.
There is no predefined duration for NA therapy. As such, stopping NAs before achieving the ultimate goal of antiviral therapy, which is the improvement of survival by the prevention of liver disease progression and HCC development [7-9], may lead to the loss of treatment benefits and risk clinical exacerbations. Therefore, appropriate biomarkers are needed for proper decision making regarding cessation of NA therapy.
HBeAg-positive CHB
Previously, HBeAg loss or seroconversion was considered an indication to stop therapy in HBeAg-positive CHB [17,69]. Cessation of antiviral therapy was recommended after 12 months of consolidation therapy. However, most patients experienced virological or clinical relapse. On the contrary, HBsAg loss or seroconversion is rare during antiviral therapy, but the prognosis of patients who cleared HBsAg is much improved; relapse of viral replication is very rare, and liver transplant-free survival or HCC-free survival is significantly better than for those who did not clear HBsAg [22]. Based on this information, the KASL and EASL guidelines suggest that HBsAg loss is the ideal endpoint of therapy and should be the primary goal [7,9]. Still, the AASLD and APASL consider HBeAg seroconversion as a satisfactory endpoint, probably owing to the rarity of HBsAg loss (Table 7) [6,8].
HBeAg-negative CHB
Relapse is common after stopping antiviral therapy in HBeAg-negative CHB patients owing to the presence of immune escape mutants [70]. Therefore, the AASLD suggests that treatment may be continued indefinitely or until HBsAg is lost [6]. The KASL and EASL also suggest the cessation of NAs after HBsAg loss (Table 7) [7,9]. However, the EASL also proposed that NA discontinuation may be considered in select patients with long-term (≥3 years) virological suppression under NAs [7]. Similarly, the APASL suggested that treatment be withdrawn after HBsAg loss following either anti-HB seroconversion or at least 12 months of consolidation therapy. Otherwise, at least ≥2 years of consolidation therapy confirming undetectable HBV DNA levels on three separate occasions can justify the end of treatment (Table 7) [8]. However, undetectable HBV DNA levels cannot likely be the sole factor in determining treatment cessation because most patients experience virological relapse after stopping therapy in the presence of HBsAg [70,71]. Recently, a low HBsAg titer (<2 log IU/mL) was proposed as an indicator of safe cessation of therapy before HBsAg loss, although further clinical experience may be necessary [72,73].
Liver cirrhosis
For patients with compensated or decompensated cirrhosis, indefinite therapy is recommended by most guidelines (Table 7) [6-9]. However, NA therapy may be discontinued after HBsAg loss or seroconversion in cases of compensated cirrhosis.
CONSIDERATIONS FOR SPECIAL POPULATIONS
CHB patients may face situations requiring special consideration. HCC may develop, accompanying renal or bone abnormalities may be detected, and anticancer chemotherapy or immunosuppressive therapy may be needed. Table 8 summarizes representative special conditions and compares international guidelines.
Table 8.
Comparison of chronic hepatitis B management for special populations
| KASL | AASLD | EASL | APASL | |
|---|---|---|---|---|
| Renal or bone disease | 1) Entecavir, TAF, and besifovir are preferred | 1) No preference between entecavir or TDF regarding the potential long-term risk of renal and bone complications TAF is associated with fewer bone and renal abnormalities than TDF | 1) Entecavir or TAF are preferred over TDF for patients with increasing age (>60 years), bone diseases, or renal alterations | 1) Entecavir or telbivudine are the first-line treatment options for chronic HBV-infected patients with any level of renal dysfunction and renal replacement therapy |
| 1) Selection | ||||
| 2) Switch | ||||
| 2) Treatment can be switched to TAF, besifovir, or entecavir in high risk patients | 2) TDF should be substituted with TAF or entecavir for TDF-associated renal dysfunction and/or bone disease | 2) Switching to entecavir or TAF should be considered for patients on TDF at risk of development and/or with underlying renal or bone disease | 2) Renal function and bone profiles should be monitored at least every 3 months if TDF or ADV is used | |
| HCC | 1) Antiviral therapy should be initiated in patients with HBV-related HCC if serum HBV DNA is detected | – | – | 1) NA treatment should be given to patients with HBV-related HCC (at least 1–2 weeks before, during, and after chemotherapy, locoregional therapies, resection, or liver transplantation), if there is detectable serum HBV DNA |
| 1) Treat | ||||
| 2) Prophylaxis | ||||
| 2) Prophylactic antiviral therapy should be considered in patients undergoing anticancer treatment, regardless of serum HBV DNA levels | ||||
| Immunosuppression or chemotherapy | 1) If either HBsAg is positive or HBV DNA is detected, prophylactic antiviral therapy should be initiated before immune-suppression or chemotherapy | 1) Anti-HBV prophylaxis should be initiated in HBsAg-positive anti-HBc-positive patients before starting immunosuppressive or cytotoxic therapy | 1) All HBsAg-positive patients should receive ETV, TDF, or TAF as treatment or prophylaxis | 1) Prophylactic antiviral therapy should be administered in anti-HBc-positive patients with either HBsAg-positive or detectable serum HBV DNA |
| 1) Prophylaxis (HBsAg-positive) | ||||
| 2) Monitor/treat/prophylaxis (HBsAg-negative) | ||||
| 2) HBsAg-negative anti-HBc-positive patients with undetectable HBV DNA should be monitored for serum HBsAg and HBV DNA during immunosuppression/chemotherapy and NAs should be initiated if HBV reactivation occurs | 2) HBsAg-negative anti-HBc-positive patients should be carefully monitored for ALT, HBV DNA, and HBsAg to provide therapy as needed | 2) HBsAg-negative anti-HBc-positive patients should receive anti-HBV prophylaxis if they are at high risk of HBV reactivation | 2) HBsAg-negative anti-HBc-positive patients with undetectable serum HBV DNA levels who receive chemotherapy and/or immunosuppression should be carefully monitored and be treated with NAs upon HBV reactivation | |
| If rituximab is included, initiate antiviral therapy at the start of immunosuppression or chemotherapy | For patients receiving anti-CD20 antibody therapy (e.g., rituximab) or undergoing stem cell transplantation, anti-HBV prophylaxis is recommended | |||
| Pregnant women | 1) Initiate or switch to TDF if treatment is needed | 1) TDF is preferred | 1) TDF should be continued, whereas ETV or other NAs should be switched to TDF | 1) TDF is the drug of choice for mothers requiring antiviral treatment |
| 1) Treat/switch | ||||
| 2) Prevention | ||||
| 3) Lactation | 2) For pregnant women with serum HBV DNA levels >200,000 IU/mL, TDF administration is recommended to prevent MTCT, beginning at 24–32 weeks of gestation and stopping 2–12 weeks after delivery | 2) If HBV DNA >200,000 IU/mL at 28–32 weeks of gestation, antiviral therapy is recommended to reduce the risk of perinatal transmission | 2) In all pregnant women with high HBV DNA levels (>200,000 IU/mL) or HBsAg levels >4 log10 IU/mL, antiviral prophylaxis with TDF should begin at 24–28 weeks of gestation and continue for up to 12 weeks after delivery | 2) For pregnant women with HBV DNA >6–7 log10 IU/mL, short-term maternal NA therapy with tenofovir or telbivudine is recommended beginning at 28–32 weeks of gestation |
| 3) TDF, which is relatively safe for the fetus and pregnant women, is not contraindicated during breastfeeding | 3) Breastfeeding is not contraindicated, but there are insufficient long-term safety data in infants born to mothers who received antiviral agents during pregnancy and while breastfeeding | 3) Breast feeding is not contraindicated in HBsAgpositive untreated women or on TDF-based treatment or prophylaxis | 3) Breast feeding is discouraged during maternal NA treatment | |
| Acute hepatitis B | 1) In patients with severe acute hepatitis B (e.g., coagulopathy, severe jaundice, liver failure), NA therapy can be initiated | 1) Indicators for antiviral therapy are total bilirubin >3 mg/dL, international normalized ratio >1.5, encephalopathy, or ascites | 1) Patients with severe acute hepatitis B, characterized by coagulopathy or protracted course, should be treated with NAs and considered for liver transplantation | 1) Treatment is only indicated for patients with fulminant hepatitis B or for those with severe or protracted acute hepatitis B |
| 1) Treat |
KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; APASL, Asian-Pacific Association for the Study of the Liver; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate; HBV, hepatitis B virus; ADV, adefovir; HCC, hepatocellular carcinoma; NA, nucleos(t)ide analog; HBeAg, hepatitis B e antigen; anti-HBc, antibody to hepatitis B core antigen; ALT, alanine aminotransferase; ETV, entecavir; MTCT, mother-to-child transmission.
Renal dysfunction or metabolic bone disease
Patients who develop renal dysfunction or decreased bone density during tenofovir DF or adefovir treatment may need to change medication, as described above concerning adverse effects [65]. If a treatment-naïve patient has pre-existing renal dysfunction (estimated glomerular filtration <60 min/mL/1.73 m2, dipstick proteinuria, urine albuminuria/creatinine >30 mg/g, or serum phosphate <2.5 mg/dL) or metabolic bone disease (chronic steroid use, taking medication that worsens bone density, or pre-existing osteoporosis/osteopenia) before starting the therapy, entecavir, tenofovir AF, and besifovir are preferred for treatment by the KASL. Although the AASLD previously suggested that there was no preference between entecavir or tenofovir DF regarding the potential long-term risk of renal and bone complications [74], their updated guidance recommends switching to tenofovir AF or entecavir if tenofovir DF-associated renal dysfunction or bone disease is suspected [6]. The EASL made detailed recommendations regarding renal or bone abnormalities in terms of when to consider entecavir or tenofovir AF over tenofovir DF when initiating antiviral therapy [7]. Similar criteria have been proposed by the KASL, as described above (Table 8). The EASL also included age >60 years, history of fragility fracture, and hemodialysis for similar candidates, but individualized approaches are needed [7]. These criteria must be clinically validated further [7]. The NA doses in all patients with renal dysfunction should be adjusted according to their creatinine clearance [9].
HCC
Only the KASL and APASL have recommendations for patients with HBV-related HCC (Table 8) [8,9]. Given that HCC is one of the most serious complications in CHB patients, appropriate measures should be taken. Importantly, antiviral therapy reduces both the incidence of de novo HCC and the recurrence of HCC in this population [25-29,75-77]. Furthermore, the reactivation of HBV can be effectively prevented by prophylactic antiviral therapy during anticancer treatment [78,79]. Therefore, the KASL recommends starting NAs before and during HCC treatment if HBV DNA is detected or prophylactically, even if HBV DNA is not detected [9]. All HBsAg-positive patients should receive NAs after HCC diagnosis, regardless of HBV DNA detection, but this requires confirmation in future studies.
Immunosuppression or chemotherapy
Patients with chronic HBV infection are at an increased risk of hematological and solid malignancies [80,81]. Therefore, the chance of receiving immunosuppression or anticancer chemotherapy is relatively high in CHB patients. If the immune system is suppressed by immunosuppression or chemotherapy, HBV can reactivate and lead to severe hepatic injury via the acute exacerbation of chronic HBV infection in HBsAg-positive patients or relapse of past HBV infection in HBsAg-negative/anti-HBc-positive patients [82-84]. HBV reactivation during anticancer chemotherapy has occurred in 41–53% of HBsAg-positive anti-HBc-positive patients and in 8–18% of HBsAg-negative anti-HBc-positive patients [6,85,86]. If reactivation occurs, dose reduction or discontinuation of anticancer therapy is necessary, which adversely affects the outcomes of cancer treatment. As such, HBsAg and anti-HBc testing should be performed in all patients before initiating any immunosuppressive or cytotoxic chemotherapy [6-9]. Additionally, particular caution should be exercised with high risk (>10% of reactivation) treatments using B cell-depleting agents (rituximab, ofatumumab, natalizumab, alemtuzumab, and ibritumomab), high-dose corticosteroids (prednisone ≥20 mg/day, ≥4 weeks), anthracyclines (doxorubicin and epirubicin), potent tumor necrosis factor-alpha inhibitors (infliximab, adalimumab, certolizumab, and golimumab), and local therapy for HCC (transcatheter arterial chemoembolization) [9,87]. High (>10%), moderate (1–10%), and low (<1%) risk of HBV reactivation in response to immunosuppressive or anticancer chemotherapy is well described in the KASL guidelines [9]. Currently, most guidelines, including the KASL, AASLD, ESAL, and APASL, recommend the initiation of NAs (entecavir or tenofovir DF/AF) before immunosuppression or chemotherapy in HBsAg-positive or HBV DNA-detected patients (Table 8) [6-9]. Not all patients who are exclusively anti-HBc-positive (HBsAg-negative and HBV DNA-undetectable) require routine administration of NAs before immunosuppression or chemotherapy. However, NA therapy should be initiated promptly if there is a high risk of reactivation (e.g., treated with a rituximab-containing regimen) or detectable HBV DNA and/or reversion of HBsAg during follow-ups. NAs should be continued during and at least 6 months (or 12 months for rituximab therapy) after the cessation of immunosuppressive therapy or chemotherapy [6-9].
Pregnant women
Immunological changes occur during pregnancy, and HBV may replicate more actively [88]. Immune responses are restored at the late phase of pregnancy or after delivery, leading to ALT flares. Hence, NA therapy may need to be initiated in patients during or after pregnancy [89,90]. Moreover, a high level of HBV DNA is related to an increased risk of mother-to-child viral transmission despite vaccine administration and hepatitis B immune globulin prophylaxis [88]. Currently, the NAs evaluated for safety and efficacy during pregnancy include lamivudine [91], telbivudine [92], and tenofovir DF [93-95]. Among these, tenofovir DF is the preferred NA owing to its excellent potency and high genetic barrier to resistance. Most guidelines recommend using tenofovir DF over other NAs for CHB treatment during pregnancy (Table 8). Additionally, the prophylactic use of tenofovir DF is recommended to prevent mother-to-child transmission beginning at 24–32 weeks of pregnancy (24–28 weeks by the EASL, 28–32 weeks by the AASLD and APASL, and 24–32 weeks by the KASL) and continuing until 2–12 weeks after delivery if pregnant women show serum HBV DNA levels >200,000 IU/mL [6-9].
The KASL, AASLD, and EASL agree that breastfeeding is generally not contraindicated, even if tenofovir DF is being administered to the mother, based on previous studies [6,7,9,96]. However, the AASLD is somewhat cautious about this issue, suggesting there are insufficient long-term safety data [6]. The APASL discourages breastfeeding during maternal NA treatment, which may need to be updated [8].
Acute hepatitis B
Although acute hepatitis B is generally a self-limiting disease, severe cases resulting in hepatic failure, liver transplantation, or even death have been reported, albeit uncommonly [97]. The use of antivirals in severely ill patients has been debated. While NA therapy might delay HBsAg sero-clearance, NA therapy can significantly reduce the mortality rate in patients with severe acute hepatitis B [98]. Considering this, the KASL guidelines recommend initiating NA therapy in patients with severe acute hepatitis B (e.g., coagulopathy, severe jaundice, or liver failure) in agreement with other guidelines [9].
The AASLD recommends using entecavir or tenofovir DF/AF, whereas the EASL refrains from recommending tenofovir AF owing to a lack of data [6,7]. The APASL includes lamivudine, telbivudine, or adefovir for severe acute hepatitis B considering their relatively short therapy duration [8]. They also recommend initiating NA treatment when it is difficult to distinguish between true severe acute hepatitis B and spontaneous reactivation of chronic HBV infection [8].
CONCLUSIONS
The KASL clinical practice guidelines for the management of CHB were recently revised, given the emergence of new NAs and continuously updated data regarding treatment initiation, modification, and cessation. Considering the 4- to 5-year interval of guideline revisions, other international guidelines are expected to be updated soon. Through a thorough and systematic approach for the management of CHB based on clinical practice guidelines, the cure of chronic HBV infection is expected to be a real treatment endpoint in the near future.
Acknowledgments
This work was funded in part by the Korean Association for the Study of the Liver and Korea University Research Grants.
Footnotes
Authors’ contribution
YHJ conceptualized and designed the study. YHJ, KJH, PJY, YEL, PH, KJH, SDH, LSH, LJ-H, and LHW performed a review of literature, revised the current KASL guidelines, and incorporated those revisions into the manuscript through interactive discussion. YHJ wrote the manuscript. YHJ, KJH, PJY, YEL, PH, KJH, SDH, LSH, LJH, and LHW reviewed and approved the final submission of the manuscript.
Conflicts of Interest: YHJ received research funds from Gilead Sciences Korea, Ildong Pharm, and Yuhan Corporation. LJ-H received honoraria from Gilead Science Korea, and Daewoong Pharm during the conduct of the study. KJH, PJY, YEL, PH, KJH, SDH, LSH, and LHW have nothing to disclose.
REFERENCES
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15 Suppl 6:S13–S24. doi: 10.3350/kjhep.2009.15.S6.S13. [DOI] [PubMed] [Google Scholar]
- 3.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2 Suppl 1):S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 4.Liang LY, Wong GL. Unmet need in chronic hepatitis B management. Clin Mol Hepatol. 2019;25:172–180. doi: 10.3350/cmh.2018.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93–159. doi: 10.3350/cmh.2019.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HN, Sinn DH, Gwak GY, Kim JE, Rhee SY, Eo SJ, et al. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int. 2012;32:937–944. doi: 10.1111/j.1478-3231.2011.02749.x. [DOI] [PubMed] [Google Scholar]
- 11.Park JY, Park YN, Kim DY, Paik YH, Lee KS, Moon BS, et al. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with genotype C and high serum HBV DNA levels. J Viral Hepat. 2008;15:615–621. doi: 10.1111/j.1365-2893.2008.00989.x. [DOI] [PubMed] [Google Scholar]
- 12.Shim JJ, Kim JW, Oh CH, Lee YR, Lee JS, Park SY, et al. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: a large national cohort study. Liver Int. 2018;38:1751–1759. doi: 10.1111/liv.13705. [DOI] [PubMed] [Google Scholar]
- 13.Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immunetolerant-phase chronic hepatitis B. Gut. 2018;67:945–952. doi: 10.1136/gutjnl-2017-314904. [DOI] [PubMed] [Google Scholar]
- 14.Wong GL. Management of chronic hepatitis B patients in immunetolerant phase: what latest guidelines recommend. Clin Mol Hepatol. 2018;24:108–113. doi: 10.3350/cmh.2017.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–1457. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 17.Korean Association for the Study of the Liver KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:18–75. doi: 10.3350/cmh.2016.22.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52–61. doi: 10.1046/j.1365-2893.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 19.Lok AS, Akarca U, Greene S. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc Natl Acad Sci U S A. 1994;91:4077–4081. doi: 10.1073/pnas.91.9.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croagh CM, Bell SJ, Slavin J, Kong YX, Chen RY, Locarnini S, et al. Increasing hepatitis B viral load is associated with risk of significant liver fibrosis in HBeAg-negative but not HBeAg-positive chronic hepatitis B. Liver Int. 2010;30:1115–1122. doi: 10.1111/j.1478-3231.2010.02267.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–1089. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 22.Kim GA, Lim YS, An J, Lee D, Shim JH, Kim KM, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325–1332. doi: 10.1136/gutjnl-2013-305517. [DOI] [PubMed] [Google Scholar]
- 23.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 26.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Ahn H, Lee DH, Lee JH, Jung YJ, Chang Y, et al. Entecavir and tenofovir reduce hepatitis B virus-related hepatocellular carcinoma recurrence more effectively than other antivirals. J Viral Hepat. 2018;25:707–717. doi: 10.1111/jvh.12855. [DOI] [PubMed] [Google Scholar]
- 28.Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631–3638. doi: 10.1002/cncr.29537. [DOI] [PubMed] [Google Scholar]
- 29.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–1859. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 31.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 32.Chang Y, Choe WH, Sinn DH, Lee JH, Ahn SH, Lee H, et al. Nucleos(t) ide analogue treatment for patients with hepatitis B virus (HBV) e antigen-positive chronic HBV genotype C infection: a nationwide, multicenter, retrospective study. J Infect Dis. 2017;216:1407–1414. doi: 10.1093/infdis/jix506. [DOI] [PubMed] [Google Scholar]
- 33.Song BC, Cho YK, Jwa H, Choi EK, Kim HU, Song HJ, et al. Is it necessary to delay antiviral therapy for 3-6 months to anticipate HBeAg seroconversion in patients with HBeAg-positive chronic hepatitis B in endemic areas of HBV genotype C? Clin Mol Hepatol. 2014;20:355–360. doi: 10.3350/cmh.2014.20.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH, Chan HY, et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol. 2011;54:236–242. doi: 10.1016/j.jhep.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Cao Z, Liu Y, Ma L, Lu J, Jin Y, Ren S, et al. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology. 2017;66:1058–1066. doi: 10.1002/hep.29213. [DOI] [PubMed] [Google Scholar]
- 36.Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62:694–701. doi: 10.1002/hep.27889. [DOI] [PubMed] [Google Scholar]
- 37.Li WC, Wang MR, Kong LB, Ren WG, Zhang YG, Nan YM. Peginterferon alpha-based therapy for chronic hepatitis B focusing on HBsAg clearance or seroconversion: a meta-analysis of controlled clinical trials. BMC Infect Dis. 2011;11:165. doi: 10.1186/1471-2334-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 39.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 40.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, et al. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg-patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68–74. doi: 10.1111/jvh.12613. [DOI] [PubMed] [Google Scholar]
- 42.Yim HJ, Hwang SG. Options for the management of antiviral resistance during hepatitis B therapy: reflections on battles over a decade. Clin Mol Hepatol. 2013;19:195–209. doi: 10.3350/cmh.2013.19.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, et al. Efficacy and safety of besifovir dipivoxil maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2019;17:1850–1859. doi: 10.1016/j.cgh.2018.11.001. e4. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 45.Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 46.Yoon EL, Yim HJ, Lee HJ, Lee YS, Kim JH, Jung ES, et al. Comparison of clevudine and entecavir for treatment-naive patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol. 2011;45:893–899. doi: 10.1097/MCG.0b013e31821f8bdf. [DOI] [PubMed] [Google Scholar]
- 47.Su QM, Ye XG. Effects of telbivudine and entecavir for HBeAg-positive chronic hepatitis B: a meta-analysis. World J Gastroenterol. 2012;18:6290–6301. doi: 10.3748/wjg.v18.i43.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tak WY, Park SY, Cho CM, Jung MK, Jeon SW, Kweon YO, et al. Clinical, biochemical, and pathological characteristics of clevudine-associated myopathy. J Hepatol. 2010;53:261–266. doi: 10.1016/j.jhep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Kim EH, Park H, Lee KH, Ahn SH, Kim SM, Han KH. Two cases of telbivudine-induced myopathy in siblings with chronic hepatitis B. Clin Mol Hepatol. 2013;19:82–86. doi: 10.3350/cmh.2013.19.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol. 2019;71:456–464. doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69:1301–1308. doi: 10.1136/gutjnl-2019-318947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158:215–225. doi: 10.1053/j.gastro.2019.09.025. e6. [DOI] [PubMed] [Google Scholar]
- 54.Toy M, Hutton DW, So SK. Cost-effectiveness and cost thresholds of generic and brand drugs in a national chronic hepatitis B treatment program in China. PLoS One. 2015;10:e0139876. doi: 10.1371/journal.pone.0139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DY, Kim JH, Tak WY, Yeon JE, Lee JH, Yoon JH, et al. Baracle® vs Baraclude® for 48 weeks in patients with treatment-naïve chronic hepatitis B: a comparison of efficacy and safety. Drug Des Devel Ther. 2017;11:3145–3152. doi: 10.2147/DDDT.S149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 57.Yim HJ, Kim IH, Suh SJ, Jung YK, Kim JH, Seo YS, et al. Switching to tenofovir vs continuing entecavir for hepatitis B virus with partial virologic response to entecavir: a randomized controlled trial. J Viral Hepat. 2018;25:1321–1330. doi: 10.1111/jvh.12934. [DOI] [PubMed] [Google Scholar]
- 58.Park ES, Lee AR, Kim DH, Lee JH, Yoo JJ, Ahn SH, et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol. 2019;70:1093–1102. doi: 10.1016/j.jhep.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Sheldon J, Camino N, Rodés B, Bartholomeusz A, Kuiper M, Tacke F, et al. Selection of hepatitis B virus polymerase mutations in HIVcoinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 60.Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488–1495. doi: 10.1136/gut.2005.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44:703–712. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 62.Lee S, Ahn SH, Jung KS, Kim DY, Kim BK, Kim SU, et al. Tenofovir versus tenofovir plus entecavir for chronic hepatitis B with lamivudine resistance and entecavir resistance. J Viral Hepat. 2017;24:141–147. doi: 10.1111/jvh.12623. [DOI] [PubMed] [Google Scholar]
- 63.Lee HW, Park JY, Lee JW, Yoon KT, Kim CW, Park H, et al. Long-term efficacy of tenofovir disoproxil fumarate monotherapy for multidrug-resistant chronic HBV infection. Clin Gastroenterol Hepatol. 2019;17:1348–1355. doi: 10.1016/j.cgh.2018.10.037. e2. [DOI] [PubMed] [Google Scholar]
- 64.Lee JW, Lee YJ, Lee JJ, Kim JH, Jung YK, Kwon OS, et al. Efficacy of entecavir switching therapy in chronic hepatitis B patients with clevudine-induced myopathy. Korean J Gastroenterol. 2013;61:30–36. doi: 10.4166/kjg.2013.61.1.30. [DOI] [PubMed] [Google Scholar]
- 65.Duarte-Rojo A, Heathcote EJ. Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2010;3:107–119. doi: 10.1177/1756283X09354562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Chen X, Chen W, Ma X, Huang J, Chen R. Extended peginterferon alfa-2a (Pegasys) therapy in Chinese patients with HBeAg-negative chronic hepatitis B. J Med Virol. 2014;86:1705–1713. doi: 10.1002/jmv.24013. [DOI] [PubMed] [Google Scholar]
- 68.Lampertico P, Viganò M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, et al. Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62:290–298. doi: 10.1136/gutjnl-2011-301430. [DOI] [PubMed] [Google Scholar]
- 69.Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51:415–421. doi: 10.1002/hep.23323. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Kim K, Hwang SH, Kim SS, Lee D, Cheong JY, et al. Durability after discontinuation of nucleos(t)ide therapy in chronic HBeAg negative hepatitis patients. Clin Mol Hepatol. 2013;19:300–304. doi: 10.3350/cmh.2013.19.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang SH, Kang K, Jong Eun Y, Lee YS, Kim TS, Yoo YJ, et al. Antiviral response is not sustained after cessation of lamivudine treatment in chronic hepatitis B patients: a 10-year follow-up study. J Med Virol. 2017;89:849–856. doi: 10.1002/jmv.24715. [DOI] [PubMed] [Google Scholar]
- 72.Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515–522. doi: 10.1016/j.jhep.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 73.Lee HA, Seo YS, Park SW, Park SJ, Kim TH, Suh SJ, et al. Hepatitis B surface antigen titer is a good indicator of durable viral response after entecavir off-treatment for chronic hepatitis B. Clin Mol Hepatol. 2016;22:382–389. doi: 10.3350/cmh.2016.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 76.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 77.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 78.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 79.Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004;99:2369–2375. doi: 10.1111/j.1572-0241.2004.40069.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from non-liver causes: results from the Haimen City cohort study. Int J Epidemiol. 2005;34:132–137. doi: 10.1093/ije/dyh339. [DOI] [PubMed] [Google Scholar]
- 81.Marcucci F, Spada E, Mele A, Caserta CA, Pulsoni A. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma - a review. Am J Blood Res. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59:2092–2100. doi: 10.1002/hep.26718. [DOI] [PubMed] [Google Scholar]
- 83.Liu WP, Wang XP, Zheng W, Ping LY, Zhang C, Wang GQ, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma. 2016;57:1355–1362. doi: 10.3109/10428194.2015.1116121. [DOI] [PubMed] [Google Scholar]
- 84.Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013;57:209–214. doi: 10.1016/j.jcv.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 87.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Söderström A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35:814–819. doi: 10.1080/00365540310016547. [DOI] [PubMed] [Google Scholar]
- 89.ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37–41. doi: 10.1111/j.1365-2893.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 90.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau D, et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am J Gastroenterol. 2016;111:1410–1415. doi: 10.1038/ajg.2016.296. [DOI] [PubMed] [Google Scholar]
- 91.van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–297. doi: 10.1046/j.1365-2893.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 92.Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 93.Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62:375–386. doi: 10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- 94.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 95.Hyun MH, Lee YS, Kim JH, Je JH, Yoo YJ, Yeon JE, et al. Systematic review with meta-analysis: the efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus. Aliment Pharmacol Ther. 2017;45:1493–1505. doi: 10.1111/apt.14068. [DOI] [PubMed] [Google Scholar]
- 96.Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA study, step 2. Antimicrob Agents Chemother. 2011;55:1315–1317. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang CY, Zhao P, Liu WW, Acute Liver Failure Study Team Acute liver failure caused by severe acute hepatitis B: a case series from a multi-center investigation. Ann Clin Microbiol Antimicrob. 2014;13:23. doi: 10.1186/1476-0711-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyake Y, Iwasaki Y, Takaki A, Fujioka S, Takaguchi K, Ikeda H, et al. Lamivudine treatment improves the prognosis of fulminant hepatitis B. Intern Med. 2008;47:1293–1299. doi: 10.2169/internalmedicine.47.1061. [DOI] [PubMed] [Google Scholar]