Abstract
COVID-19 is a disease caused by a coronavirus named as SARS-CoV-2. It has become pandemic due to its contagious nature. Majority of the patients are asymptomatic or having mild flu like symptoms. Few need hospitalisation due to severe acute respiratory infection (SARI). Co-morbidity like diabetes, hypertension, renal failure etc. are associated with severe COVID-19 that often causes death. There have been only two published case reports of monoclonal gammopathy of unknown significance (MGUS) in patients with COVID-19 disease. Cytokine storm is often observed in severe COVID-19 and various cytokines including IL-6 that activates plasma cells are increased in blood in this condition. Here we present a case of severe COVID-19 patient with bioclonal gammopathy. He was known diabetic and hypertensive on treatment. He developed SARI, cytokines storm and septicaemia, treated with antibiotics, enoxaparin, hydroxychloroquine, insulin, anti-hypertensives, put on ventilator, subsequently developed septicaemia, multi-organ failure and died. Two M-bands on serum capillary electrophoresis with presence IgG-κ on both the M-bands indicates a biclonal gammopathy of unknown significance in this patient. We conclude that like MGUS, early stage biclonal gammopathy, although rare, gets manifested with M-bands on plasma protein electrophoresis. It is probably due to high level of IL-6 associated with cytokine storm in severe COVID-19 that stimulate early stage dyscratic plasma cells. Such biclonal gammopathy might be a risk factor for severe COVID-19 and associated mortality.
1. Introduction
SARS-CoV-2, a new corona virus was detected in humans in December 2019 in Wuhan, China. The disease was named as COVID-19 and as it was highly contagious, it soon became a global health emergency. It was declared a pandemic by WHO in March 2020 [1]. Majority of SARS- CoV2 infection in humans remain asymptomatic or have mild flu like symptoms with fever, cough, diarrhoea, anosmia and ageusia. Only few develop severe acute respiratory syndrome (SARS), need hospitalisation and oxygen therapy to maintain adequate saturation. A few of them may require ICU care and ventilatory support. The diagnosis is by RT -PCR or by detection of antigen in the swabs collected from the nasopharynx and oro-pharynx. Asymptomatic patients need no treatment. Mild cases are treated with antipyretics and other symptomatic medications. Role of hydroxychloroquine (HCQ) remains controversial in covid-19 treatment [2]. Moderate to severe cases are administered antiviral therapy e.g., Remdesivir, Favipiravir etc with varying success [3]. Beside severe acute respiratory infection (SARI), increased level of D-dimer and consequent clot embolism is a major cause of death [4]. Few patients develop cytokine storm that aggravates SARI. These patients are treated with steroids and anti-IL-6 receptor antibodies, like Tocilizumab [5]. Mortality rate is approximately 3.4% [1]. Old age, diabetes mellitus, hypertension, obesity, COPD, renal disease etc. are known risk factors for disease severity and mortality.
In capillary electrophoresis (CE), plasma proteins are resolved into few bands and are often used to detect M-band for the diagnosis of multiple myeloma and other gammopathies. A premalignant form of clonal plasma cell dyscrasia, known as monoclonal gammopathy of unknown significance (MGUS) often remain asymptomatic and may progress to multiple myeloma [6]. MGUS is associated with immune suppression and deep vein thrombosis [7], [8]. The rise in antibody titre following any viral infection is polyclonal in nature and increases the height and area under curve of gamma band, but does not usually produce M-band. Only two studies of two and seven cases of monoclonal gammopathy each have been reported so far in COVID-19 patients by Vazzana et al. [9] and Gonzalez-Lugo et al. [10] Biclonal gammopathy, per se, is not as common as monoclonal gammopathy [11].
2. Case
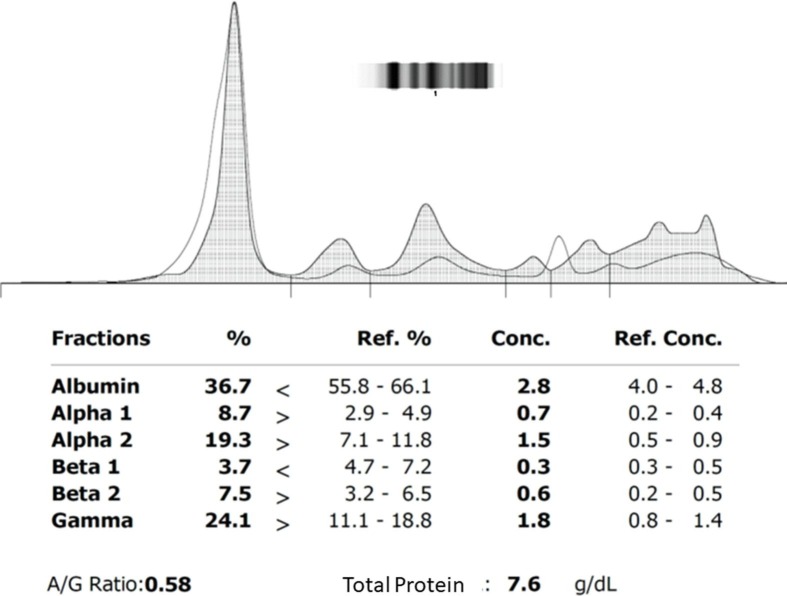
A 60 year old male subject who was on regular treatment for Type2 Diabetes Mellitus (T2DM) and hypertension, presented in the emergency department with cough and sore throat for 5 days and shortness of breath for 3 days. He was tested for COVID-19 by RT-PCR which was found to be positive. At the time of admission, he had tachypnea and SpO2 level was 84% on room air. He was shifted to ICU and received oxygen support, antibiotics, HCQ, anti-hypertensives, insulin, thromboprophylaxis with low molecular heparin along with other supportive treatments. On day 1, 2 and 4 of hospital admission, his blood reports were as shown in Table 1 . Blood gas analysis report on day 3 was suggestive of respiratory acidosis. CE showed two M-bands in gamma region (Fig. 1 a) and on immuno fixation (Fig. 1b), it was IgG-kappa in both the M-bands. After 2 days, patient developed SARI and was eventually put on ventilator. His kidney function and liver function got deranged, leading to multi-organ dysfunction. Later he developed septic shock. Vasopressors were administered to maintain adequate mean arterial pressure. He had a cardiorespiratory arrest on day 4 of admission and could not be revived.
Table 1.
Investigation reports of the patient on day 1, 2 and day 4.
| Parameters | Result Day 1 22-06-2020 |
Result Day 2 23-06-2020 |
Result Day 4 25/24-06-2020 |
Reference range |
|---|---|---|---|---|
| Hb% | 12.1 | 10.6 | 12–15.5 mg/dL | |
| TLC | 9320 | 20,370 | 5–10 × 103/mL | |
| Polymorphs % | 90 | 94 | 60–75% | |
| Lymphocytes% | 8 | 5 | 20–40% | |
| Monocytes | 1 | 1 | 2–6% | |
| Eosinophil | 1 | 0 | 1–3% | |
| APTT | 26.7 s | 30–40 s | ||
| PT | 16.1 s | 11–13.5 s | ||
| INR | 1.41 | 0.8–1.1 | ||
| Fibrinogen | 252 mg/dl | 200–400 mg/dL | ||
| D-dimer | 4375 | <500 ng/mL | ||
| Random Blood glucose | 338 mg/dl | 303 mg/dl | 229 mg/dl 280 mg/dl |
80–140 mg/dL |
| SpO2 | 70% | 94% | 94–100% | |
| ABG | pH:7.347, PO2:93.3, PCO2:23.8, cHCO3: 12.8, SO2: 96.4% |
pH:7.18 PO2:91, PCO2:68.5, cHCO3:25.4, SO2:94 |
pH:6.9 PO2: 113% PCO2: 66.9 cHCO3: 18 SO2: 63.8 |
pH: 7.3–7.45 PO2: 75–100 mmHg PCO2: 35–45 mmHg cHCO3: 22–26 meq/L SO2: 95–100% |
| hsCRP | 128 | <3 | ||
| Ferritin | 552 ng/ml | 12–300 ng/mL | ||
| LDH | 655 | 120–246 U/L | ||
| IL-6 | 93.7 | 0–16.4 pg/mL | ||
| Procalcitonin | 0.45 | 2.1 | 0.1–0.49 ng/mL | |
| BNP | 9909 | <100 pg | ||
| Urea | 89 | 170 | 18–40 mg/dL | |
| Creatinine | 1.6 | 4.9 | 0.7–1.3 mg/dL | |
| Sodium | 145 | 143 | 137–145 mmol/L | |
| Potassium | 6.2 | 8.2 | 3.5–5.1 mmol/dL | |
| Total Bilirubin | 0.9 | 1.3 | 0.2–1.3 mg/dL | |
| Direct Bilirubin | 0.8 | 0.9 | 0.0–0.3 mg/dL | |
| AST | 106 | 118 | 17–59 U/L | |
| ALT | 27 | 51 | 0–50 U/L | |
| ALP | 100 | 146 | 38–126 U/L | |
| Total Protein | 8.9 | 7.6 | 6.3–8.2 g/dL | |
| Albumin | 3.8 | 2.9 | 3.5–5.5 g/dL | |
| IgG | >2700 | 600–1600 mg/dL | ||
| IgM | 144.39 | 40–250 mg/dL | ||
| IgA | 523.67 | 80–300 mg/dL | ||
| Anti-covid-19 antibody level (arbitraty unit) | 7.2 | <0.9 | ||
| LDL-C | 103.9 mg/dl | <100 mg/dL | ||
| α-Fetoprotein | 2.07 | 0.00–7.22 IU/mL |
Fig. 1.
(a) Capillary electrophoretogram and (b) immunofixation test of plasma protein of the patient.
3. Discussion
The present case had COVID-19 with co-morbidities of T2DM and hypertension, which was complicated by SARI, septic shock and multi-organ dysfunction, fast progression and ultimately died despite best possible treatment available. CE is not done routinely in COVID-19 cases. Detection of M-bands (biclonal gammopathy) was totally accidental when CE of serum protein of the patient was done with the left over sample sent for serum IL-6 assay for checking the Capillary Electrophoresis equipment (Sebia, model: minicap flex piercing, France) performance after its preventive maintenance services. After getting two M-bands, immunofixation test was done that showed the presence of IgG-κ on both the M-bands (Fig. 2 a & b). Then the treating physician was informed about the accidental finding. There was no history suggestive of any plasma cell disorder in that patient. So it is suggestive of a Biclonal Gammopathy of Unknown Significance (BGUS) probably induced by immunological response to the virus. There have been two published reports of MGUS in COVID-19 cases [9], [10]. One of the study reported two cases, where both the patients recovered and M−band also disappeared after recovery [9]. The second study reported a series of 7 cases of COVID-19 with MGUS [10], where 01 of the 07 patients died. Although BGUS is not uncommon [11], till date BGUS in COVID-19 has not been reported. This is the first such a case to the best of our knowledge. Since the patient was very sick we were unable to do a bone marrow biopsy. In biclonal gammopathy, the electrophoretic mobility of immunoglobulins present in two M-bands differs mostly because of their isotypic difference in heavy chains or light chains or both. Uniqueness in this case is that both the M-bands are having the same isotype of immunoglobulins i.e., IgG with kappa light chains. Hence, we surmised that variations in electrophoretic mobility of immunoglobulins present in two M-bands is not due to isotypic variations but due to difference in amino acid sequence of either heavy chains or light chains or both [12]. So origin of these two peaks are from two different clones of dyscratic plasma cells producing two different clones of immunoglobulin molecules varying idiotypically. Hence, our diagnosis in this case was BGUS.
Fig. 2.
Immunofixation of serum protein of the patient (a) without antibody (ELP) (b) with anti-IgG (IgG) (c) with anti-IgA (IgA), (d) with anti-IgM (IgM), (e) with anti-κ (κ) and with anti-λ(L).
Blood picture showed no abnormal cells, the rise in TLC with predominantly polymorphs (90%) and high serum PCT was suggestive of SARI with septicaemia. Such gammopathies of unknown significance are known to cause immune suppression and proneness towards thrombus formation [8]. A prothrombotic state with or without thromboembolic complication is commonly seen in patients who have COVID-19 disease. INR in this case was 1.4 and D-dimer levels were 4375 ng/ml. So in this case, BGUS might have acted as an additional risk factor along with T2DM and hypertension which might have contributed to SARI, septicaemia, multi-organ failure and subsequent death. Appearance of M-band during acute phase of COVID-19 followed by its disappearance after recovery as shown by Vazzana et al. [9], might suggest a cytokine (IL-6)- induced proliferation of latent/ early stage plasma cell dyscrasia in these patients. Hence probably on recovery, loss of stimulus with decrease in cytokines might be responsible for M-band disappearance. In this case also, IL-6 was very high indicating the presence of a cytokine storm. Therefore, the rise of IL-6, an activator of plasma cells associated with cytokine storm in COVID-19 may be responsible for appearance of M-band(s) in MGUS (BGUS). The death of our patient along with one reported by Gonzalez-lugo et al might indicate probable effect of MGUS or BGUS on COVID-19 prognosis [10]. It is worth seeing if COVID-19, per se, accelerates the progression of early stage plasma cell dyscrasia to full blown multiple myeloma. Age of all the COVID-19 cases with M-band(s) including the present case was above 59 and none was reported from younger patients with COVID-19. MGUS or BGUS is commonly observed at old age. So probably the existence of an early stage MGUS or BGUS is more likely the cause of appearance of M-band(s) rather than appearance of an aberrant new malignant B cell clone due to cytokine stimulus and depletion of T-reg cells in COVID-19 as suggested by Vazzana et al. [9].
Besides M-bands, capillary electrophoretogram of this patient showed decrease in area under curve (AUC) of albumin band (constituted of albumin, retinol binding protein and transthyretin) & β1 band (constituted of transferrin and complement 4) and increase in AUC of α1 (that is constituted of α1 anti-trypsin, α-acid glycoprotein and α- fetoprotein), β2 band (constituted of β2-microglobulin, Complement 3 and LDL) & γ-band (constituted of immunoglobulins). The rise in serum CRP and ferritin levels that indicates viral infection induced acute phase reactants (APR) in this case. These changes in albumin, α and β bands are reflection of this acute phase response as albumin, transferrin, transthyretin & retinol-binding protein are known negative APR and many proteins in α1, α2 and β2 bands act as positive APR. The rise in γ-band reflects humoral immune response as evidenced by rise in anti-SAR-CoV antibody levels and rise in IgG, IgM and IgA levels in this case (Table 1). The diagnostic utility of these changes in management of COVID-19 is worth investigating.
References
- 1.C. Sohrabi, Z. Alsafi, N. O’Neill, M. Khan, A. Kerwan, A. Al-Jabir, C. Iosifidis, R. Agha, World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int. J. Surg. 76 (2020) 71–76. [DOI] [PMC free article] [PubMed]
- 2.Li Zou L., Dai X., Zhang Z., Zhang Z.Z. Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch. Pharm. Res. 2020:1–8. doi: 10.1007/s12272-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S. Crisafulli, V. Isgrò, L. La Corte, F. Atzeni, G. Trifirò, Potential Role of Anti-interleukin (IL)-6 Drugs in the Treatment of COVID-19: Rationale, Clinical Evidence and Risks., BioDrugs. (2020) 1–8. [DOI] [PMC free article] [PubMed]
- 6.Bladé J., Rosiñol L., Cibeira M.T., de Larrea C.F. Pathogenesis and progression of monoclonal gammopathy of undetermined significance. Leukemia. 2008;22:1651–1657. doi: 10.1038/leu.2008.203. [DOI] [PubMed] [Google Scholar]
- 7.Kristinsson S.Y., Tang M., Pfeiffer R.M., Bjorkholm M., Goldin L.R., Blimark C., Mellqvist U.-H., Wahlin A., Turesson I., Landgren O. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97:854–858. doi: 10.3324/haematol.2011.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristinsson S.Y., Fears T.R., Gridley G., Turesson I., Mellqvist U.-H., Björkholm M., Landgren O. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112:3582–3586. doi: 10.1182/blood-2008-04-151076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.N. Vazzana, S. Ognibene, F. Dipaola, “Acute” monoclonal gammopathy in severe COVID-19, Hematol. Transfus. Cell Ther., 2020. [DOI] [PMC free article] [PubMed]
- 10.Gonzalez-Lugo J.D., Bachier-Rodriguez L., Goldfinger M., Shastri A., Sica R.A., Gritsman K., Mehta V., Kabarriti R., Goel S., Verma A., Braunschweig I., Kornblum N., Mantzaris I. A case series of Monoclonal Gammopathy of Undetermined Significance and COVID-19. Br. J. Haematol. 2020;190 doi: 10.1111/bjh.16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahto M., Balakrishnan P., Koner B.C., Lali P., Mishra T.K., Saxena A. Rare case of biclonal gammopathy. Int. J. Case Reports Images. 2011;2:11. [Google Scholar]
- 12.Khalid A., Shahbaz I. Double Gammopathy of Undetermined Significance-A First Case Report from Pakistan. J. Hematol. Thrombo. Dis. 2019;6:297. [Google Scholar]