Abstract
Background
The 2019 novel coronavirus disease (COVID-19) can complicate the perioperative course to increase postoperative mortality in operative patients, and also is a serious threat to medical staff. However, studies summarizing the impact of COVID-19 on the perioperative mortality of patients and on the safety of medical staff are lacking.
Methods
We searched PubMed, Cochrane Library, Embase and Chinese database National Knowledge Infrastructure (CNKI) with the search terms “COVID-19″ or “SARS-CoV-2″ and “Surgery” or “Operation” for all published articles on COVID-19 from December 1, 2019 to October 5, 2020.
Findings
A total of 269 patients from 47 studies were included in our meta-analysis. The mean age of operative patients with COVID-19 was 50.91 years, and 49% were female. A total of 28 patients were deceased, with the overall mortality of 6%. All deceased patients had postoperative complications associated with operation or COVID-19, including respiratory failure, acute respiratory distress syndrome (ARDS), short of breath, dyspnea, fever, cough, fatigue or myalgia, cardiopulmonary system, shock/infection, acute kidney injury and severe lymphopenia. Patients who presented any or more of the symptoms of respiratory failure, ARDS, short of breath and dyspnea after operation were associated with significantly higher mortality (r = 0.891, p < 0.001), while patients whose symptoms were presented as fever, cough, fatigue or myalgia only demonstrated marginally significant association with postoperative mortality (r = 0.675, p = 0.023). Twenty studies reported the information of medical staff infection, and a total of 38 medical staff were infected, and medical staff who used biosafety level 3 (BSL-3) protective equipment did not get infected.
Interpretation
COVID-19 patients, in particular those with severe respiratory complications, may have high postoperative mortality. Medical staff in close contact with infected patients is suggested to take high level personal protective equipment (PPE).
Funding
Heilongjiang postdoctoral scientific research developmental fund and the National Natural Science Foundation of China.
Keywords: Surgery, COVID-19, Mortality, Medical staff safety, Personal protective equipment
Research in context.
Evidence before this study
COVID-19 complicated the postoperative course to increase the mortality of operative patients, and brought serious threats to the safety of medical staff serving operative patients. We searched PubMed for all articles describing the clinical characteristics and outcomes of operative patients with COVID-19 up to October 5 2020, we found only some case reports, however, no studies performed a systematic review and meta-analysis on the perioperative mortality of operative patients with COVID-19 and no data related the risk factors for poor outcome.
Added value of this study
We searched PubMed, Cochrane Library, Embase and CNKI with the search terms “COVID-19″ or “SARS-CoV-2″ and “Surgery” or “Operation” for all published articles on COVID-19 from December 1, 2019 to October 5, 2020. A total of 269 patients from 47 studies were included in our meta-analysis. The mean age of operative patients with COVID-19 was 50.91 years, and 49% were female. A total of 28 patients were deceased, with the overall mortality of 6%. The operative patients who had respiratory complications or COVID-19 typical symptoms may have higher mortality. Twenty studies reported the information of medical staff infection, and a total of 38 medical staff were infected, and medical staff who used biosafety level 3 (BSL-3) protective equipment did not get infected.
Implications of all the available evidence
COVID-19 patients may have high postoperative mortality, and postoperative respiratory complications and COVID-19 typical symptoms may be the higher risk factors for poor outcome after operation. Medical staff serving operative patients is at high risks of cross-infection, and effective personal protective procedures can reduce the risk of COVID-19 infection of medical staff.
Alt-text: Unlabelled box
1. Introduction
The 2019 novel coronavirus disease (COVID-19) pandemic continues to infect a large number of patients, with fever, dry cough, fatigue, and shortness of breath, acute respiratory distress syndrome (ARDS) as major symptoms. These symptoms are also the risk factors for ventilator dependence [1]. As of October 5, 2020, over 36,600,000 cases and 1,000,000 deaths related to COVID-19 have been reported in at least 200 countries [2]. COVID-19 is caused by SARS-CoV-2, which belongs to the Betacoronavirus genus such as SARS-CoV, and MERS-CoV [3]. SARS-CoV-2 has a lower pathogenicity as compared with SARS-CoV, but has higher pandemic potential [4], [5], [6], [7]. Respiratory droplets, close contact transmission, and aerosol transmission in a relatively closed environment are the major routes of transmission [8]. Thus, surgical procedures may place clinicians at particularly high risk when caring for infected patients.
Surgical stress may impair cell-mediated immunity to reduce the resistance to viruses. Meanwhile, COVID-19 may complicate the postoperative course to increase the mortality of operative patients [9,10], while the major factors contributing to the increased postoperative mortality in patients with COVID-19 remain unelucidated. At present, little is known about the clinical characteristics and outcomes of operative patients with COVID-19 during the perioperative period.
COVID-19 brought serious threats to the safety of medical staff in addition to the general public [11]. Among medical staff, surgeons, anesthesiologists and operating nursing staff are at the highest risk of infection due to the exposure to respiratory droplets or aerosol from infected patients during airway manipulations and surgery [12]. An early report showed that fifteen hospital staff members in Wuhan Union Hospital (China) who had closed contact with infected patients, were confirmed as being infected with COVID-19 [13]. Thus, effective personal protective procedures and cautions should be taken to prevent medical staff from COVID-19 infection. Our knowledge of the protective measures of COVID-19 during the perioperative period is inadequate and limited.
Thus, the present analysis aimed to describe the clinical outcomes of operative patients with COVID-19, and the safety of medical staff during the perioperative period to take appropriate protective measures to avoid cross-infection. It is out hope that our findings of the COVID-19 associated postoperative mortality and reasonable advises will benefit the global community in the battle against COVID-19 infection.
2. Methods
This meta-analysis was accomplished in agreement with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [14].
2.1. Search strategy and study selection
We systematically searched PubMed, Cochrane Library, Embase and Chinese database National Knowledge Infrastructure (CNKI) with the search terms “COVID-19″ or “SARS-CoV-2″ and “Surgery” or “Operation” for all published articles on COVID-19 from December 1, 2019 to October 5, 2020. Only full articles involving humans were considered. Duplicate results were removed. The remaining articles were screened for relevance by its abstracts independently by two authors (Changshuai Wu and Kun Wang). The remaining investigators (Zhenglian Gao and Xiaowang Zhang) read full selected articles that met the requirements. In addition, closely relevant references to the current research topic were also manually searched. These articles were thoroughly read, and those that fulfilled our criteria were included in the study.
2.2. Inclusion/exclusion criteria
The inclusion criteria were as follows: (1) research types: randomised controlled trials (RCT), case report and case series; (2) research subjects: patients with COVID-19 underwent surgery and (3) data items: including clinical characteristics, outcomes, or medical staff safety. Exclusion criteria were as follows: (1) repeated research, and (2) lack of data.
2.3. Data extraction
Data extraction was performed independently by two authors (Changshuai Wu and Jian Xu), and we used standardized forms that include first author, publication date, country, number of patients, age, gender, comorbidities, surgery intervention, anesthetic method, surgical difficulty category, medical staff infection, study design, and clinical outcome, and so on. If there was any ambiguity in the search process, the decision was made by a third investigator (Zhengyuan Xia).
The primary outcome was the mortality rate of operative patients with COVID-19 and the secondary outcome was medical staff safety (i.e., the number of medical staff being infected with COVID-19 in the hospital).
2.4. Statistical analysis
Statistical analyses were performed using RStudio meta R package (version 3.6.2). Arcsine differences (ASD) were used as the measure of risk differences. The main advantages of using ASD are that the variance of the point estimate is determined solely by the sample size and that it handles occurrences of 0 counts, allowing for incorporation of trials with 0 events in both control and treatment groups into meta analyses [15].The combined prevalence and 95% confidence interval (CI) were calculated using a random effects model or fixed effects model. The selection of the model was determined according to Q statistics. When Q statistics (p < 0.10) indicated heterogeneity, the random effect model was utilized for meta-analysis. When Q statistics (p ≥ 0.10) indicated the lack of heterogeneity, then a fixed-effect model was utilized for meta-analysis. Spearman's rank correlation was used to analyze the correlations among preoperative comorbidities, age, postoperative complications and the mortality rate.
Sensitivity analysis by leave-one-out was performed to single out heterogeneity. Heterogeneity was assessed with the Q statistic test and the I2 test. The I2 statistic measured the percentage of total variation across the studies aroused from clinical or methodological heterogeneity rather than by chance. The Egger test was performed to assess publication bias in all literature works, and p < 0.05 was considered as the exist of publication bias, and the funnel plot showed the publication bias intuitively.
2.5. Role of the funding source
The funding agencies had no role in study design, data collection and analysis. The corresponding authors have full access to all data in the study and are fully responsible for the decision of submitting for publication.
3. Results
3.1. Study selection and demographical characteristics
Using the above selection criteria, we identified a total of 1426 records, and 574 papers remained after exclusion of irrelevant topics and duplicates. Of those, a total of 66 citations met the inclusion criteria and remained for title and abstract screening. Four of these 66 items did not have a full text. After assessing 62 full-text articles for eligibility, we further excluded 15 full-text articles due to the exist of one of the following reasons: 1) no operative patients (8 articles) or relevant data (5 articles), and 2) review articles (2 articles). Eventually, 47 studies were included in this meta-analysis, and the trial selection process was shown in Fig. 1 .
Fig. 1.
Diagram of documents retrieval.
3.2. Characteristics of studies
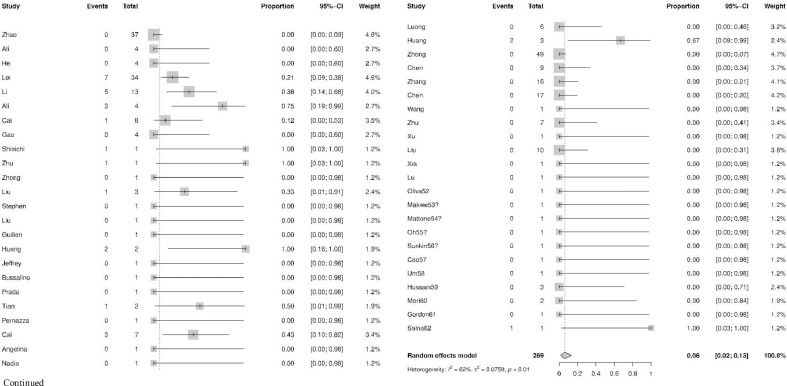
The characteristics of included trials were presented in Table 1 . A total of 269 patients from 47 studies [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62] were included in our meta-analysis. The mean age of operative patients with COVID-19 was 50.91 [95% CI, 42.49; 59.34], and 49% [95% CI, 0.33, 0.65] patients were female. Among operative patients with COVID-19, the number of discharged cases was 210, severe cases who needed prolonged in-hospital stay were 31, and the total number of the deceased cases were 28. And, the overall mortality rate was 6% [95% CI, 0.02; 0.13], as shown in Fig. 2 .
Table 1.
Characteristics of the included literature.
| First author (year) | Country | Sample (n) | Mean age | Gender (n) | Surgery Intervention | Anesthetic method (n) | Infected medical staff (n) | Literature type | Clinical outcome (Death, n) |
|---|---|---|---|---|---|---|---|---|---|
| Zhao16 (2020) | China | 37 | 41.0 | Female 23 Male 14 |
Neurosurgery, Cardiovascular, Abdominal, Orthopedic, Obstetric gynecological, Other | GA (n = 26) SA (n = 11) |
3 | A retrospective, multicenter study | 0 |
| Ali17 (2020) | Iran | 4 | 45.5 | Female 3 Male 1 |
Gastric bypass operation | GA | – | Case series | 0 |
| He18 (2020) | China | 4 | 55.8 | Female 1 Male 3 | Aortic dissection repair | GA | 0 | Case series | 0 |
| Lei19 (2020) | China | 34 | 55.0 | Female 20 Male 14 |
Cesarean section, Appendectomy, Lobectomy, Gastrectomy, Colectomy, Renal transplant | GA, SA, EA | 3 | A multicenter, retrospective study | 7 |
| Li20 (2020) | China | 13 | 60.0 | Female 3 Male 10 |
Lung/Esophagus operation | GA | 12 | A single-centred retrospective |
5 |
| Ali21 (2020) | Iran | 4 | 64.3 | Female 2 Male 2 |
Incisional henia repair, Cholecystectomy Gastric bypass |
GA | – | Case series | 3 |
| Cai22 (2020) | Chnia | 8 | 68.0 | Female 6 Male 2 |
Appendectomy, Gastrectom, Enterocolectomy, Cholecystostomy, Pancreaticojejunostomy, Gastric perforation repair |
GA | – | A single-centred, retrospective | 1 |
| Gao23 (2020) | Chnia | 4 | 56.8 | Female 1 Male 3 |
Partialenterectomy, Primary duodenal repair, Primary small bowel repair |
GA | – | Case series | 0 |
| Shinichi24 (2020) | USA | 1 | 52.0 | Male | Acute type A aortic dissection | GA | 0 | Case report | 1 |
| Zhu25 (2020) | China | 1 | 70.0 | Male | Endonasal Endoscopic Pituitary Adenoma Resection | GA | 14 | Case report | 1 |
| Zhong26 (2020) | China | 1 | 37.0 | Male | Liver transplanation | GA | – | Case report | 0 |
| Liu27 (2020) | China | 3 | 65.7 | Male | Heart or lung transplanation | GA | – | Case series | 1 |
| Stephen28 (2020) | America | 1 | 0.5 | Female | Liver transplanation | GA | – | Case report | 0 |
| Liu29 (2020) | China | 1 | 50 | Male | Liver transplantation | GA | 0 | Case report | 0 |
| Guillen30 (2020) | Spain | 1 | 50 | Male | Kidney transplantation | GA | – | Case report | 0 |
| Huang31 (2020) | China | 2 | 54.5 | Male | Bone marrow transplantation, Kidney transplantation |
GA | – | Case series | 2 |
| Jeffrey32 (2020) | USA | 1 | 39.0 | Male | Dual heart and kidney transplantation | GA | 0 | Case report | 0 |
| Bussalino33 (2020) | Italy | 1 | 32.0 | Male | Kidney transplantation | GA | – | Case report | 0 |
| Prada34 (2020) | Italy | 1 | 28.0 | Male | Tendon transfer surgery | GA | – | Case report | 0 |
| Tian35 (2020) | China | 2 | 78.5 | Female 1 Male 1 | Lung lobectomies for adenocarcinoma | GA | – | Case series | 1 |
| Pernazza36 (2020) | Italy | 1 | 61.0 | Male | Thoracoscopic lobectomy with lymph node dissection | GA | – | Case report | 0 |
| Cai37 (2020) | China | 7 | 60.3 | Female 2 Male 5 | Lung resection | GA | – | Case series | 3 |
| Luca38 (2020) | Italy | 1 | 64.0 | Female | Exploratory laparotomy | GA | – | Case report | 0 |
| Nadia39 (2020) | France | 1 | 56.0 | Female | Head and neck oncology surgery | GA | 3 | Case report | 0 |
| Luong40 (2020) | France | 6 | 55.7 | Male | Resection of colon cancer Gastrectomy, Pancreatactomy, Cholecystectomy, Gastroplasty, Resection of a rectal cancer |
GA | – | A non-interventional retrospective study | 0 |
| Huang41 (2020) | China | 3 | 70.7 | Female 2 Male 1 |
Thoracoscopic lobectomy |
GA | – | Case series | 2 |
| Zhong42 (2020) | China | 49 | 31.0 | Female 42 Male 7 |
Caesarean, Orthopedic, caesarean section, lower-limb surgery |
SA | 3 | A retrospective, single centre, observational cohort | 0 |
| Chen43 (2020) | China | 9 | 29.9 | Female | Caesarean | SA | – | A retrospective review | 0 |
| Zhang44 (2020) | China | 16 | 29.3 | Female | Caesarean | SA | – | A retrospective review | 0 |
| Chen45 (2020) | China | 17 | 29.1 | Female | Caesarean | EA | 0 | Case series | 0 |
| Wang46 (2020) | China | 1 | 28.0 | Female | Caesarean | EA | 0 | Case report | 0 |
| Zhu47 (2020) | China | 7 | 26.3 | Female | Caesarean | EA | – | Retrospectively analyzed | 0 |
| Xu48 (2020) | China | 1 | 30.0 | Female | Caesarean | EA | – | Case report | 0 |
| Liu49 (2020) | China | 10 | 32.0 | Female | Caesarean | EA | – | A Preliminary Analysis | 0 |
| Xia50 (2020) | China | 1 | 27.0 | Female | Caesarean | SA | 0 | Case report | 0 |
| Lu51 (2020) | China | 1 | 11.0 | Female | Caesarean | EA | – | Case report | 0 |
| Oliva52 (2020) |
USA |
1 | 35 | Female | Caesarean | SA | – | Case report | 0 |
| Makwe53 (2020) |
Nigeria |
1 | 37 | Female | Caesarean | SA | 0 | Case report | 0 |
| Mattone54 (2020) |
Italy | 1 | 68 | Female | Laparoscopic cholecystectomy |
GA | – | Case report | 0 |
| Oh55 (2020) |
Singapore |
1 | 66 | Male | Laparoscopic cholecystectomy | GA | 0 | Letter to the Editor |
0 |
| Sunkin56 (2020) |
USA | 1 | 64 | Male | Total knee arthroplasty |
GA | 0 | Case report | 0 |
| Cao57 (2020) |
China | 1 | 45 | Male | Pedicle screw internal fifixation |
GA | 0 | Case report | 0 |
| Um58 (2020) |
Korea |
1 | 86 | Male | Orthopedic surgery |
SA | 0 | Case report | 0 |
| Hussain59 (2020) |
UK |
3 | 57.3 | Male | Cardiopulmonary bypass |
GA | 0 | Case report | 0 |
| Mori60 (2020) |
USA | 2 | 68 | Female 1 Male 1 |
Cardiopulmonary bypass | GA | – | Case report | 0 |
| Gordon61 (2020) |
USA | 1 | 69 | Male | Otologic Surgery |
GA | 0 | Case report | 0 |
| Salna62 (2020) |
USA | 1 | 57 | Male | Cardiopulmonary bypass | GA | – | Case report | 1 |
Abbreviations: GA, General anesthesia; SA, Spinal anesthesia; EA, Epidural anesthesia; PTGD, Percutaneous transhepatic gallbladder drainage.
Fig. 2.
The mortality rate of operative patients with COVID-19 infection.
3.3. Characteristics of the deceased patients
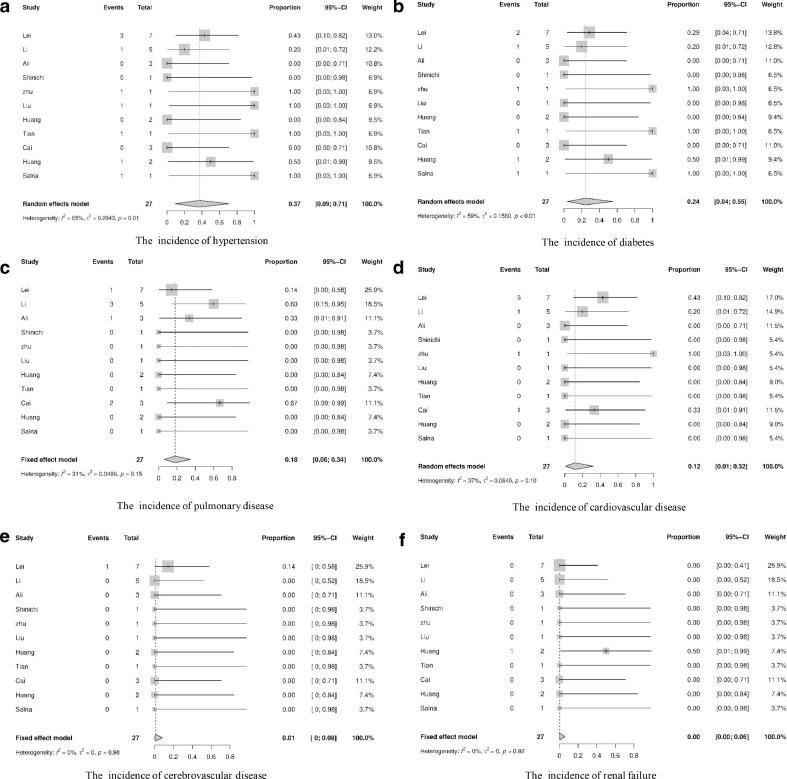
Among the 28 deceased patients, the mean age was 63.05 [95% CI, 58.47;67.63], and 43% [95% CI, 0.25, 0.61] patients were female. One death case in Cai et al.'s study [22] was not included in this analysis owing to the lack of related perioperative information. Twenty of the 27 deceased patients had comorbidities, which included 37% [95% CI, 0.09; 0.71] with hypertension, 24% [95% CI, 0.04; 0.55] with diabetes, 18% [95% CI, 0.06; 0.34] with pulmonary disease, 12% [95% CI, 0.01; 0.32] with cardiovascular disease, 1% [95% CI, 0.00; 0.08] with cerebrovascular disease, and 0.00% [95% CI, 0.00; 0.06] with renal injury (Table 2 and Fig. 3 ). In terms of the grade of surgical difficulty for the deceased patients, 1 case was surgical difficulty grade I, 2 cases were grade II, while 6 and 18 cases were respectively in grade III and IV. The majority of the deceased patients (24 in 27) underwent grade III and III surgeries, and all deceased patients received general anesthesia with endotracheal incubation.
Table 2.
Characteristics of the deceased patients.
| Patient number | Mean age | Gender | Comorbidities | Surgery type | Surgical difficulty category | Anesthetic method | Complications/Signs and symptoms Of COVID-19 |
|---|---|---|---|---|---|---|---|
| 1 [Lei 19] |
34 | Female | None | Pancreatoduo-denectomy | Level IV | GA | Respiratory failure, ARDS, Shock, Secondary infection, Acute kidney injury |
| 2 [Lei 19] |
55 | Male | Cardiovascular disease, Hypertension, COPD | Total esophagectomy |
Level IV | GA | Respiratory failure, ARDS, Shock, Arrhythmia, Acute cardiac injury, Secondary infection, Acute kidney injury |
| 3 [Lei 19] |
63 | Male | None | Thoracoscopic lobectomy | Level IV | GA | Respiratory failure, ARDS, Shock, Acute cardiac injury |
| 4 [Lei 19] |
48 | Female | Diabetes | Radical resection of rectal cancer | Level III | GA | Respiratory failure, ARDS, Shock, Arrhythmia, Acute cardiac injury |
| 5 [Lei 19] |
55 | Female | Cardiovascular disease | Thoracoscopic lobectomy | Level IV | GA | Respiratory failure, ARDS, Arrhythmia |
| 6 [Lei 19] |
83 | Male | Cardiovascular disease, Hypertension, Cerebrovascular disease |
Artificial femoral head replacement | Level IV | Intraspinal anesthesia | Respiratory failure, ARDS, Arrhythmia, Secondary infection |
| 7 [Lei 19] |
77 | Female | Cardiovascular disease, Hypertension |
Total hip replacement | Level IV | Intraspinal anesthesia | Respiratory failure, ARDS, Acute cardiac injury |
|
8–12 [Li 20] |
>51 | Female 4 Male 1 |
Hypertension, Diabetes, COPD, Coronary heart disease |
Lung/Esophagus operation | Level IV | GA | Fever, Cough, Fatigue or muscular soreness, Short of breath, Diarrhea, Lowed lymphocyte count, Renal function damage, Electrolyte disturbance |
| 13 [Ali 21] |
75 | Female | None | Incisional henia repair | Level I | GA | Fever, Cough, Dyspnea ARDS, MOF |
| 14 [Ali 21] |
81 | Male | None | Cholecystectomy | Level II | GA | Fever, Dyspnea, Diarrhea, ARDS, Sepsis, Acute cardiac injury |
| 15 [Ali 21] |
44 | Male | Severe respiratory distress | Gastric bypass | Level III | GA | Cardiopulmonary arrest |
| 16 [Shinichi 24] |
52 | Male | None |
Acute type A aortic dissection | Level IV | GA | Respiratory and renal failure |
| 17 [Zhu 25] |
70 | Male | Hypertension, Diabetes, Heart attack |
Endonasal endoscopic surgery | Level II | GA | Fever, Fatigue, Dry cough, Sputum production, Shortness of breath |
| 18 [Liu 27] |
66 | Male | Hypertension | Heart and lung transplantation | Level IV | GA | Ventricular fibrillation |
| 19 [Huang 31] |
51 | Male | None | Allogeneic bone marrow transplantation | Level IV | GA | Fever, Cough, Runny nose |
| 20 [Huang 31] |
58 | Male | Renal failure | Kidney transplantation | Level IV | GA | Fever, Dough, Shortness of breath |
| 21 [Tian 35] |
84 | Female | Hypertension, Diabetes | Lung lobectomies for adenocarcinoma | Level III | GA | Difficulty in breathing, Dry cough, Coma |
| 22 [Cai 37] |
63 | Male | Lung disease | Lung lobectomies for adenocarcinoma | Level III | GA | Short of breath, Productive cough, Myalgia |
| 23 [Cai 37] |
68 | Male | COPD | Lung lobectomies for adenocarcinoma | Level III | GA | Short of breath, Palpitation |
| 24 [Cai 37] |
56 | Female | Coronary atherosclerosis | Lung lobectomies for adenocarcinoma | Level III | GA | Dry cough, Diarrhea |
| 25 [Huang 41] |
84 | Female | Hypertension Diabetes |
Thoracoscopic lung surgery for adenocarcinoma |
Level IV | GA | Cough, Expectoration and dyspnea, Fatigue, Fever, Respiratory failure, Lymphocyte count decreased |
| 26 [Huang 41] |
55 | Female | None | Thoracoscopic lung surgery for adenocarcinoma |
Level IV | GA | Decreased lymphocyte count, Serious cough and fever, Severe dyspnea |
| 27 [Salna 62] |
57 | Male | Hypertension Diabetes |
Cardiopulmonary bypass | Level IV | GA | Fever, ARDS, Shock |
Abbreviations: COPD, Chronic obstructive pulmonary disease; ARDS, Acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome; GA, General anesthesia.
Fig. 3.
The incidence of preoperative comorbidities of the deceased patients.
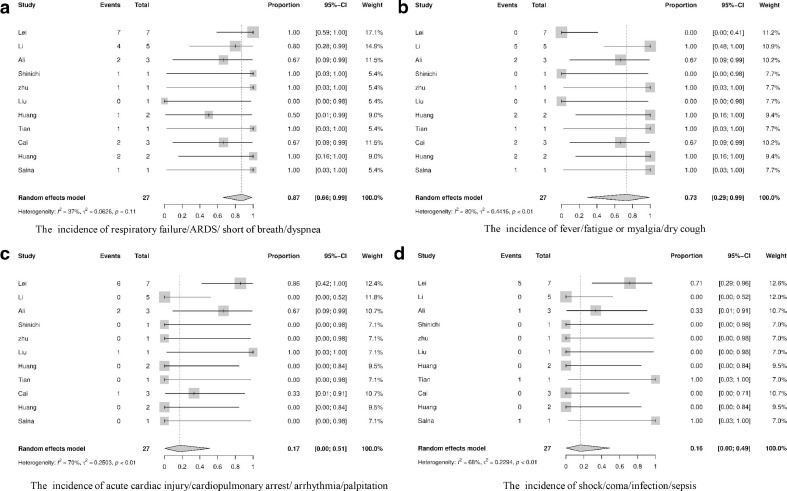
After surgery, all deceased patients had postoperative complications associated with operation or COVID-19 symptoms. The incidence of respiratory failure/ARDS/short of breath/dyspnea was 87% [95% CI, 0.66;0.99], that of fever/cough/ fatigue or myalgia was 73% [95% CI, 0.29;0.99], that of postoperative acute cardiac injury/cardiopulmonary arrest/arrhythmia/palpitation was 17% [95% CI, 0.00;0.51], that of shock/coma/secondary infection/sepsis was 16% [95% CI, 0.00;0.49], as shown in Fig. 4 , that of acute kidney injury was 9% [95% CI, 0.01;0.22], that of lowed lymphocyte count was 9% [95% CI, 0.00;0.41], that of diarrhea was 3% [95% CI, 0.00;0.12], that of electrolyte disturbance was 2% [95% CI, 0.00;0.20], and that of multiple organ failure (MOF) was 1% [95% CI, 0.00;0.07].
Fig. 4.
The incidence of postoperative complications comorbidities of the deceased patients.
Patients who presented any or more of the symptoms of respiratory failure, ARDS, short of breath and dyspnea after operation were associated with significantly higher mortality (r = 0.891, p < 0.001), while patients whose symptoms were presented as fever, cough, fatigue or myalgia only demonstrated marginally significant association with postoperative mortality (r = 0.675, p = 0.023). Preoperative comorbidities, the age of patients, and other postoperative complications were not significantly associated with increased risk of mortality. This suggests that postoperative respiratory complications and COVID-19 typical symptoms may be the major risk factors for poor outcome after operation.
3.4. Medical staff infection
Of the 47 studies included in the analysis, only 20 studies [16,[18], [19], [20],24,25, 29,32,39,42,45,46,50,53,[55], [56], [57], [58], [59],61] reported the information of medical staff infection which identified that a total of 38 medical staff were infected, and medical staff who used biosafety level 3 (BSL-3) protective equipment during the perioperative period did not get infected.
3.5. Publication bias
We carried out Egger's regression test and confirmed the absence of publication bias (Egger, p = 0.06) for the final articles included for analysis, and the funnel plot was symmetrical, which indicate that publication bias did not exist.
4. Discussion
The main focus of this study was to investigate the mortality rate of patients with COVID-19 undergoing surgery, and the related risk factors of the death during the perioperative period. We found that operative patients with COVID-19 infection had higher rate of mortality and the occurrence of postoperative complications. In particular, respiratory failure/ARDS/short of breath/dyspnea or fever/ cough/fatigue or myalgia were significantly associated with postoperative death in patients with COVID-19. Twenty-eight of the 269 operative patients died of operation or COVID-19 associated complications, the overall mortality rate was 6%, with a mortality rate much higher than the 1.8–4.5% postoperative mortality in ASA-III patients as reported [63]. Most of the deceased patients had complications associated with COVID-19 symptom and respiratory syndrome. The patient's immune function is a major determinant of the disease severity, and surgical stress may not only impair immune function [64], but also induce systemic inflammatory response [65]. The immune suppression after surgery should have exacerbated the progression and severity of COVID-19 infection. Most of those patients quickly present with typical symptoms such as fever, dry cough, fatigue or myalgia. COVID-19 can cause quick deterioration of lung function because the lung is the main target organ of the virus. In our study, the majority of patients rapidly developed respiratory failure/ARDS/short of breath/ dyspnea, which rendered them vulnerable to death. This is consistent with the findings of Chen et al.'s study who showed that 17% patients developed ARDS and, among them, 11% patients' condition worsened in a short period of time and died of MOF [66].
In addition to cause the progression to respiratory syndrome, COVID-19 disease also impairs other organ functions (e.g. heart, kidneys, liver) [67]. In our study, patients developed cardiac injury/cardiopulmonary arrest/arrhythmia/palpitation, acute kidney injury, diarrhea and even MOF. Furthermore, several patients rapidly progressed to shock/coma/secondary infection/sepsis that were concomitant with severe lymphopenia and electrolyte disturbance. This is consistent with the findings of Lei et al.'s study who showed that the most common complications of patients in non-survivors included shock, hyperleukocytemia, and lymphopenia [19]. Thus, operative patients with COVID-19 infection have higher perioperative mortality [68, 69].
Medical staff serving operative patients is at high risks of the cross-infection. The availability and especially proper utilization of valuable personal protective equipment (PPE) are of utmost importance. Clinicians have to balance a possible delay in cancer treatment against the risk for a potential COVID-19 exposure [70,71]. Alternative therapeutic approaches should be pursued, especially in very early - or very advanced-stage diseases. Turaga et al.'s study found that most cancer surgeries can be safely delayed beyond the current waiting time for at least 4 weeks without having a significant impact on patient survival or cancer progression [72]. Timely treatment of urgent cases with COVID-19 infection and the optimal of the protection of medical staff should both be taken into serious consideration. During a pandemic, it is essential to ensure emergency surgery care. If non-operative management failed and surgery is deemed necessary, appropriate PPE and precautions should be adopted, and surgery should not be delayed whilst waiting for the swab results [73,74]. The decision and plan to recognize whether surgery is required should be conducted by a senior clinician with the experienced surgeon, anaesthetist and infection control experts [75].
The protection level of the surgical gowns depends on the type of procedure [76]. An filtering face pieces (FFP) 2 mask filters 94% of all particles that are 0.3 mm in diameter or larger; while N95 masks block 95% and FFP3 masks block 99% [77]. A class 2 or 3 FFP face mask should be worn when working in close contact with patients with suspected or confirmed COVID-19, and only to use surgical face masks in a crisis scenario of shortage of FFP 2 and 3 respirators [78]. Airborne transmission risks are high during aerosol generating procedures such as laparoscopy, endoscopy and tracheal incubation to exposure patients' oropharynx and airway secretions with a high viral load [79]. We suggest that surgical team members, including anesthesiologists, surgeons and operating nursing staff should ware highly protective levels of PPE when treating patients known to have been infected with COVID-19 [77]. Most recent information from Italy reported that 12% of healthcare workers were infected at the beginning of COVID-19 pandemic [67], and this incidence was greatly reduced when PPE was used properly and infection control measures were followed [80]. In our analysis, 38 medical staff were infected as reported in 20 studies, while medical staff who used biosafety level 3 (BSL-3) protective equipment did not get infected. Thus, implementation of strict protections for medical staff is essential to decrease the cross-infection risks. Additionally, the choice between laparoscopy and laparotomy as a surgical approach needs to be cautious. Laparoscopy is an option but a potential risk of aerosol exposure must be considered for SARS-CoV-2 even though there is not current demonstration of SARS-CoV-2 RNA presence in the surgical smoke [81,82], but aerosolization of blood born viruses has been previously detected in surgical smoke during laparoscopy [83,84]. For critically ill patients with lung dysfunction, sepsis or shock, open surgery is advised [67]. Special care must be taken to reduce smoke formation (e.g., lowering electrocautery power settings, using bipolar electrocautery, using electrocautery or ultrasonic scalpels parsimoniously), and to limit smoke dispersal or spillage from trocars (e.g., lowering the pneumoperitoneum pressure) in the OR [85]. Pneumoperitoneum and surgical smoke should be evacuated only using a direct suction connected to a vacuum suction unit [86].
To minimize infectious risk to medical staff during the perioperative period, detailed protective strategies have been proposed as briefly outlined below. Based on clinical information and expert recommendation, all elective cases are suggested to be canceled, with the focus to maintain only emergency operations and elective cancer surgeries [87,88]. A negative pressure isolation transfer cabin is recommended for staff wearing BSL-3 protective medical equipment to transport patients [89]. Ideally, it seems necessary to create specific transfer pathways, and patients be transferred directly to the operating room (OR), without stopping at the pre-operation or post-anesthesia care unit (PACU) areas. It is also suggested that BSL-3 protective medical equipment should be worn, including N95 masks, goggles, protective suits, face shields, caps, shoe covers, and gloves [45]. Furthermore, all staff should take a training course on PPE use [67]. A negative pressure (below - 4.7 Pa) OR must be established, preferably isolated from the main surgical theaters and with a separate ventilation system [85]. A checklist should be used for preparation and incubation, and enough time should be allocated for the preparation of airway equipment. It is recommended that one experienced anesthetist to deliver 100% O2 manually for 3–5 min and videolaryngoscopy be used to perform rapid sequence induction [90,91]. It is further recommended to use a high-quality HMEF (Heat and Moisture Exchange Filter) between the face mask and breathing circuit. Medical staff should use fast-drying hand antiseptics and change gloves immediately after contacting a patient, body fluids or contaminated materials [92]. Anesthetic equipment must be used by one person only and the anesthesia machine be strictly disinfected [93]. All protective gear should be disposed of properly. When using electrocautery devices during surgery, it is necessary to adjust to the lowest effective power in order to reduce the amount of surgical smoke [86,94]. Surgical smoke and pneumoperitoneum should be evacuated only using a direct suction connected to a vacuum suction unit [86]. Smoke evacuation electrosurgical devices should be used to minimize medical staff's exposure to surgical smoke. Postoperative patients should preferably recover in an isolation room with negative pressure when resources permitting in the PACU or intensive care unit (ICU). If negative pressure isolation rooms are unavailable, it is recommended to let the patients to recover in the OR prior to being transfer to a single patient room. Postoperatively, the anesthesia workstation needs to be disinfected for 2 h with an anesthesia circuit sterilizer (containing 12% hydrogen peroxide) [45], and the next operation must be performed beyond 2 h after the completion of the disinfection [89,95]. In particular, COVID-19 patients’ specimens should be clearly labeled and handled as infectious specimens for treatment by the pathology department [90].
Our meta-analysis has several limitations. First, our analysis was based on a small number of cases and the data availability for several parameters, such as medical staff infection. Second, it should be noted that some articles did not clearly provide information regarding the type of surgery and the kinds of post-operative complications, nor did they describe the detailed symptoms of COVID-19, and thus the number of patients in these studies could not be used for the calculation of the total number or percentage of patients included in each of the 4° of surgical difficulties, and also not suitable for the correlation analysis in relation to the severity of COVID symptoms. Lastly, among of 47 studies in this meta-analysis, 26 articles were mainly from China, and the other 21 articles were from the United States and Europe. This imbalance of sources increased the possibility of publication bias. Most of the included studies were case reports or case series, which may affect the representativeness of the results. Therefore, large sample and/or multicenter trials are needed to further explore the perioperative mortality rate of operative patients with COVID-19 and in particular the factors that have highest impact on the perioperative mortality or medical staff infection.
In summary, we found that operative patients with COVID-19 have high mortality rate, and that postoperative COVID-19 symptom and related respiratory complications were significantly associated with the death of operative patients. Medical staff who have closed contact with infected patients are at the highest potential risk of infection. Thus, it is urgently needed to apply standard measures to actively deal with postoperative complications of patients with COVID-19 in order to reduce the mortality rate, and to provide effective protection and safe environment to avoid the cross-infection during the perioperative period.
Declaration of Interests
We declare no competing interests associated with this work.
Acknowledgments
Contributors
Kun Wang and Zhengyuan Xia had the idea for the study. Kun Wang designed the study. Changshuai Wu and Jian Xu collected all data in the study. Zhenglian Gao, Xiaowang Zhang, Baohui Zhang and Jian Xu performed data analysis. Kun Wang drafted the manuscript. Zhengyuan Xia revised the final manuscript.
Funding
This work was supported by Heilongjiang Postdoctoral Scientific Research Developmental Fund (LBH-Q17127), and the National Natural Science Foundation of China (Nos. 81970247 and 81670770).
Acknowledgments
The authors thank Doctor Xiangdong Chen, Doctor Zhongyuan Xia and Doctor Shaoqing Lei for providing information in the preparation of this manuscript.
Data sharing
All data are available upon reasonable request to the corresponding author, and it will be shared according to the standards of ethical policies regulating data sharing of human subjects.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100612.
Appendix. Supplementary materials
References
- 1.Skoog H., Withrow K., Jeyarajan H. Tracheotomy in the SARS-CoV-2 pandemic. Head Neck. 2020;42(7):1392–1396. doi: 10.1002/hed.26214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. There is a current outbreak of coronavirus (COVID-19) disease. Available at: https://www.who.int/health-topics/coronavirus. Accessed October 5, 2020.
- 3.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian X., Ren R., Wang Y. Fighting against the common enemy of COVID-19: a practice of building a community with a shared future for mankind. Infect Dis Poverty. 2020;9(1):34. doi: 10.1186/s40249-020-00650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J. Pathogenicity and transmissibility of 2019-nCoV-a quick overview and comparison with other emerging viruses. Microbe Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J.T., Leung K., Leung G.M. Now casting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67(5):568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amodeo G., Bugada D., Franchi S. Immune function aftermajor surgical interventions: the effect of postoperative pain treatment. J Pain Res. 2018;11:1297–1305. doi: 10.2147/JPR.S158230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272(1):e27–e29. doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang L.Y., Wang J. Anesthesia and COVID-19: what we should know and what we should do. Semin Cardiothorac Vasc Anesth. 2020;24(2):127–137. doi: 10.1177/1089253220921590. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Liu Y., Gong Y. Perioperative management of patients infected with the novel coronavirus: recommendation from the joint task force of the Chinese society of anesthesiology and the Chinese association of anesthesiologists. Anesthesiology. 2020;132(6):1307–1316. doi: 10.1097/ALN.0000000000003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehl G., Laible M., Rauchenzauner M. Co-infection of SARS CoV-2 and influenza A in a pediatric patient in Germany. Klin Padiatr. 2020 doi: 10.1055/a-1163-7385. [DOI] [PubMed] [Google Scholar]
- 14.Hutton B., Salanti G., Caldwell D.M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 15.Rücker G., Schwarzer G., Carpenter J., Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28(5):721–738. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S., Ling K., Yan H. Anesthetic management of patients with COVID 19 infections during emergency procedures. J Cardiothorac Vasc Anesth. 2020;34(5):1125–1131. doi: 10.1053/j.jvca.2020.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aminian A., Kermansaravi M., Azizi S. Bariatric surgical practice during the initial phase of COVID-19 outbreak. Obes Surg. 2020:1–4. doi: 10.1007/s11695-020-04617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H., Zhao S., Han L. Anesthetic management of patients undergoing aortic dissection repair with suspected severe acute respiratory syndrome COVID-19 infection. J Cardiothorac Vasc Anesth. 2020;34(6):1402–1405. doi: 10.1053/j.jvca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.K., Peng S., Li L.Q. Clinical and transmission characteristics of Covid-19-a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40(2):295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272(1):e27–e29. doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai M., Wang G., Zhang L. Performing abdominal surgery during the COVID-19 epidemic in Wuhan, China: a single-centred, retrospective, observational study. Br J Surg. 2020;107(7):e183–e185. doi: 10.1002/bjs.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y., Xi H., Chen L. Emergency surgery in suspected COVID-19 patients with acute abdomen: case series and perspectives. Ann Surg. 2020;272(1):e38–e39. doi: 10.1097/SLA.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuhara S., Rosati C.M., El-Dalati S. Acute type A aortic dissection during COVID-19 outbreak. Ann Thorac Surg. 2020 doi: 10.1016/j.athoracsur.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W., Huang X., Zhao H., Jiang X. A COVID-19 patient who underwent endonasal endoscopic pituitary adenoma resection: a case report. Neurosurgery. 2020:nyaa147. doi: 10.1093/neuros/nyaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J.Y., Qiao K., Liu F. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J Engl. 2020;133(12):1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagana S.M., De Michele S., Lee M.J. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Wang Y., Zhao Y., Shi H., Zeng F., Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillen E., Pineiro G.J., Revuelta I. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation. Am J Transplant. 2020;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J., Lin H., Wu Y. COVID-19 in posttransplant patients-report of 2 cases. Am J Transplant. 2020;20(7):1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu J.J., Gaynor P., Kamath M. COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant. 2020;20(7):1911–1915. doi: 10.1111/ajt.15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bussalino E., De Maria A., Russo R., Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: a case report. Am J Transplant. 2020;20(7):1922–1924. doi: 10.1111/ajt.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prada V., Bellone E., Schenone A., Grandis M. The suspected SARS-Cov-2 infection in a Charcot-Marie-Tooth patient undergoing postsurgical rehabilitation: the value of telerehabilitation for evaluation and continuing treatment. Int J Rehabil Res. 2020 doi: 10.1097/MRR.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pernazza A., Mancini M., Rullo E. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020:1–6. doi: 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Y., Hao Z., Gao Y. Coronavirus disease 2019 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in Wuhan, People's Republic of China. J Thorac Oncol. 2020;15(6):1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pernazza A., Mancini M., Rullo E. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020:1–6. doi: 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benmoussa N., de Kerangal Q., Leymarie N. Failure of free flaps in head and neck oncology surgery in COVID-19 patients. Plast Reconstr Surg. 2020 doi: 10.1097/PRS.0000000000007120. [DOI] [PubMed] [Google Scholar]
- 40.Luong-Nguyen M., Hermand H., Abdalla S. Nosocomial infection with SARS-Cov-2 within departments of digestive surgery. J Visc Surg. 2020;157(3S1):S13–S18. doi: 10.1016/j.jviscsurg.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J., Wang A., Kang G., Li D., Hu W. Clinical course of patients infected with severe acute respiratory syndrome coronavirus 2 soon after thoracoscopic lung surgery. J Thorac Cardiovasc Surg. 2020 doi: 10.1016/j.jtcvs.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong Q., Liu Y.Y., Luo Q. Spinal anaesthesia for patients with coronavirus disease 2019 and possible transmission rates in anaesthetists: retrospective, single-centre, observational cohort study. Br J Anaesth. 2020;124(6):670–675. doi: 10.1016/j.bja.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Jiang Y., Wei M. Analysis of novel coronavirus pneumonia in Hubei during pregnancy. Chin J Obstet Gynecol. 2020;(03):166–171. [Google Scholar]
- 45.Chen R., Zhang Y., Huang L., Cheng B.H., Xia Z.Y., Meng Q.T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67(6):655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020:ciaa200. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Zhao R., Zheng S. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D., Li L., Wu X. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020;215(1):127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 50.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth. 2020;124(5):e216–e218. doi: 10.1016/j.bja.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu D., Sang L., Du S., Li T., Chang Y., Yang X.A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020 doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliva M., Hsu K., Alsamarai S., de Chavez V., Ferrara L. Clinical improvement of severe COVID-19 pneumonia in a pregnant patient after caesarean delivery. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makwe C.C., Okunade K.S., Rotimi M.K. Caesarean delivery of first prediagnosed COVID-19 pregnancy in Nigeria. Pan Afr Med J. 2020;36:1–4. doi: 10.11604/pamj.2020.36.100.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattone E., Sofia M., Schembari E. Acute acalculous cholecystitis on a COVID-19 patient: a case report. Ann Med Surg Lond. 2020;58:73–75. doi: 10.1016/j.amsu.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh S.L., Chia C.L.K., Chen Y.R. Laparoscopic surgery in a patient with atypical presentation of COVID-19: salient points to reduce the perils of surgery. Singap Med J. 2020;61(8):443–444. doi: 10.11622/smedj.2020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunkin J.A, Lindsey M.H, Stenquist D.S, Fuller B.C, Chen A.F, Shah V.M. Surgical treatment of acute periprosthetic knee infection with concurrent presumed COVID-19: a case report. JBJS Case Connect. 2020;10(3) doi: 10.2106/JBJS.CC.20.00226. [DOI] [PubMed] [Google Scholar]
- 57.Cao Y.-L., Han Y.-J., Chen P. Surgical treatment of thoracolumbar fracture with incomplete lower limb paralysis in a patient with COVID-19. Chin J Traumatol. 2020;23(4):211–215. doi: 10.1016/j.cjtee.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Um S.H., Kim D.-H., Youn M.-Y. Protection of surgical team from COVID-19 during bipolar hemiarthroplasty in an infected elderly patient. Clin Orthop Surg. 2020;12(3):286–290. doi: 10.4055/cios20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardiac surgery in patients with confirmed COVID-19 infection: early experience. J Card Surg. 2020;35(6):1351–1353. doi: 10.1111/jocs.14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori M., Geirsson A., Vallabhajosyula P., Assi R. Surgical management of thoracic aortic emergency with pre- and postoperative COVID-19 disease. J Card Surg. 2020:1–3. doi: 10.1111/jocs.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon S.A, Deep N.L, Jethanamest D. Exoscope and personal protective equipment use for otologic surgery in the era of COVID-1. Otolaryngol Head Neck Surg. 2020;163(1):179–181. doi: 10.1177/0194599820928975. [DOI] [PubMed] [Google Scholar]
- 62.Salna M., Polanco A., Bapat V., George I., Argenziano M., Takeda K. A case of coronavirus disease 2019 (COVID-19) presenting after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2020;160(4):e193–e195. doi: 10.1016/j.jtcvs.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daabiss M. American society of anaesthesiologists physical status classification. Indian J Anaesth. 2011;55(2):111–115. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amodeo G., Bugada D., Franchi S. Immune function after major surgical interventions: the effect of postoperative pain treatment. J Pain Res. 2018;11:1297–1305. doi: 10.2147/JPR.S158230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni Choileain N., Redmond H.P. Cell response to surgery. Arch Surg. 2006;141(11):1132–1140. doi: 10.1001/archsurg.141.11.1132. [DOI] [PubMed] [Google Scholar]
- 66.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Marzo F., Sartelli M., Cennamo R. Recommendations for general surgery activities in a pandemic scenario (SARS-CoV-2) Br J Surg. 2020 doi: 10.1002/bjs.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuech J.J., Gangloff A., Di Fiore F. Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg. 2020;157(3S1):S7–12. doi: 10.1016/j.jviscsurg.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kutikov A., Weinberg D.S, Edelman M.J. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambertini M., Toss A., Passaro A. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists' perspective. ESMO Open. 2020;5(2) doi: 10.1136/esmoopen-2020-000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turaga K.K., Girotra S. Are we harming cancer patients by delaying their cancer surgery during the covid-19 pandemic? Ann Surg. 2020:10. doi: 10.1097/SLA.0000000000003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Saverio S., Pata F., Gallo G., Carrano F. Coronavirus pandemic and colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020;22(6):625–634. doi: 10.1111/codi.15056. [DOI] [PubMed] [Google Scholar]
- 74.Gok A.F.K., Eryılmaz M., Ozmen M.M. Recommendations for trauma and emergency general surgery practice during COVID-19 pandemic. Ulus Travma Acil Cerrahi Derg. 2020;26(3):335–342. doi: 10.14744/tjtes.2020.79954. [DOI] [PubMed] [Google Scholar]
- 75.COVID Surg Collaborative Global guidance for surgical care during the COVID‐19 pandemic. Br J Surg. 2020;15(10):1002. doi: 10.1002/bjs.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Association for the Advancement of Medical Instrumentation. Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities; 2012 (ANSI/AAMI PB70:2012):6-7 (4.2.1-3).
- 77.Odor P.M., Neun M., Bampoe S. Anaesthesia and COVID-19: infection control. Br J Anaesth. 2020;125(1):16–24. doi: 10.1016/j.bja.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.European Centre for Disease Prevention and Control. Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19 2020.
- 79.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.NO Prevention of COVID-19 for Healthcare Providers https://clinicaltrials.gov/show/NCT04312243, 2020
- 81.Zheng M.H., Boni L., Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272(1):e5–e6. doi: 10.1097/SLA.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Risk of SARS-CoV-2 diffusion when performing minimally invasive surgery during the COVID-19 pandemic. Eur Urol. 2020;78(1):e12–e13. doi: 10.1016/j.eururo.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwak H.D., Kim S.-H., Seo Y.S. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73(12):857–863. doi: 10.1136/oemed-2016-103724. [DOI] [PubMed] [Google Scholar]
- 84.Choi S.H., Kwon T.G., Chung S.Kwang. Surgical smoke may be a biohazard to surgeons performing laparoscopic surgery. Surg Endosc. 2014;28(8):2374–2380. doi: 10.1007/s00464-014-3472-3. [DOI] [PubMed] [Google Scholar]
- 85.Novara G., Giannarini G., De Nunzio C., Porpiglia F., Ficarra V. Risk of SARS-CoV-2 diffusion when performing minimally invasive surgery during the COVID-19 pandemic. Eur Urol. 2020;78(1):e12–e13. doi: 10.1016/j.eururo.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng M.H., Boni L., Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272(1):e5–e6. doi: 10.1097/SLA.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Royal College of Surgeons. Guidance for surgeons working during the COVID-19 pandemic. Available at: https://www.rcseng.ac.uk/coronavirus/joint-guidance-forsurgeons-v1/.Accessed March 30, 2020.
- 88.American College of Surgeons. COVID-19: elective case triage guidelines for surgical care. Available at: https://www.facs.org/covid-19/clinical-guidance/elective-case. Accessed March 30, 2020.
- 89.Du Z., Wang T. Infection prevention and control in perioperative patients during the COVID-19 pandemic: protocol from a Tertiary General Hospital. J Minim Invasive Gynecol. 2020;27(5):1216–1217. doi: 10.1016/j.jmig.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Balas M., Al-Balas H.I., Al-Balas H. Surgery during the COVID-19 pandemic: a comprehensive overview and perioperative care. Am J Surg. 2020;219(6):903–906. doi: 10.1016/j.amjsurg.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Z. Liana, L. Nadav, K. Desire, A. Mike, R. S. Krishna. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updates/perioperative-considerations-forthe-2019-novel-coronavirus-covid-19/; 2020.
- 92.Family Health Committee of the People's Republic of China. Hand hygiene rules for medical staff [EB/OL].
- 93.Ti L.K., Ang L.S., Foong T.W., Ng B.S.W. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance. Can J Anaesth. 2020;67(6):756–758. doi: 10.1007/s12630-020-01617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao K.X., Zhang B.X., Zhang P., et al. Zhonghua Wai Ke Za Zhi 2020;58(0) E001. [DOI] [PubMed]
- 95.Park J., Yoo S.Y., Ko J.H. Infection prevention measures for surgical procedures during a middle east respiratory syndrome outbreak in a tertiary care hospital in South Korea. Sci Rep. 2020;10(1):325. doi: 10.1038/s41598-019-57216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.