Abstract
2019 saw advances in the generation of induced pluripotent stem cell (iPSC)-derived nephron progenitors and in our understanding of how nephrons form in a kidney organoid. Fundamental studies of regeneration in zebra fish continue to provide vital clues as to how we might use iPSC-derived cells to regenerate a human nephron in vivo.
The past few years have witnessed an explosion of interest in the idea of recreating human kidney tissue from induced pluripotent stem cells (iPSCs). Advances in this area draw heavily on studies in model organisms, particularly mouse, to identify the cell types and interactions that drive kidney organogenesis. Advances in single-cell transcriptomics have enabled precise characterization of equivalent cell types in the developing human kidney, while directed differentiation of iPSCs provides a potential source of cells with which to recapitulate the developmental process.
One cell type that is pivotal for kidney organogenesis is the nephron progenitor cell, which exists within an anatomically defined niche at the periphery of the developing kidney. This surprisingly motile mesenchymal population signals to the ureteric epithelium to stimulate branching, while simultaneously self-renewing to provide a source of cells that form nephrons. Nephron progenitors persist throughout fetal development, giving rise to >14,000 nephrons in the mouse kidney (approximately 1 million in human kidney). The progenitor population is lost shortly after birth in mouse (before birth in humans) and, consequentially, the postnatal kidney is unable to form new nephrons. For this reason, the nephron progenitor population has been the focus of intense interest in the field of development and regeneration to assess whether this nephron progenitor state can be recreated, whether nephron progenitors can be supported or expanded in vitro and whether these cells can be used for regenerative medicine. Notably, 2019 has seen a number of interesting advances in each of these areas.
Although lineage tracing studies performed in the mouse over a decade ago identified SIX2-expressing cap mesenchymal cells as the definitive population that gives rise to the nephron epithelium1, 2019 saw the first lineage tracing study within a kidney organoid, which showed that nephrons within human iPSC-derived kidney organoids are also derived from a SIX2-expressing population2. Although this finding was not surprising given the transcriptional and anatomical congruence between human and mouse, the study went on to carefully examine the timing of nephrogenesis within an organoid model. By genetically engineering CreERT2 into the SIX2 locus to enable time-restricted labelling of SIX2+ cells, we demonstrated that formation of nephrons within an organoid occurred within a short time frame. SIX2-expressing cells are still present in late organoids but they no longer contribute to nephron formation, presumably due to the absence of an appropriate stem cell niche.
Although recreating the signalling environment that defines a stem cell niche is a complex task, a number of groups have now identified methods to maintain nephron progenitors in vitro3–5. While each method uses FGF9 or FGF2, the addition of BMP ligands and inhibitors varies substantially between protocols. According to the protocol by Brown et al.3, BMP7 is required to support the nephron progenitor population, but inhibition of canonical SMAD signalling is needed to support BMP-mediated PI3K signalling, thereby preventing nephron formation. Indeed, the protocol by Li et al.4 inhibits both SMAD1/5 and SMAD2/3 signalling. In a 2016 study, Tanigawa et al.5 added BMP7 to their culture medium but also used LIF to drive STAT signalling and DAPT to block Notch signalling, ultimately inhibiting epithelium formation. Building on their previous work, in 2019 Tanigawa et al.6 reported a more effective method to maintain and propagate human iPSC-derived nephron progenitors in vitro. In this study, the researchers reassessed the role of TGFβ superfamily signalling, demonstrating a requirement for Activin A rather than BMP7 for efficient maintenance of progenitor identity. Rather than assuming that SIX2 expression alone was sufficient to identify nephron progenitors, they developed a more rigorous fluorescence-activated cell sorting (FACS) fractionation method using both a SIX2 reporter and selection for ITGA8+PDGFRA− cells. Under sub-optimal culture conditions, the researchers identified cells that expressed SIX2 but were not ITGA8+PDGFRA− progenitor cells — an interesting observation given the presence of a non-nephrogenic SIX2+ population within kidney organoids2. Importantly, this revised protocol is applicable to multiple iPSC lines, as antibody-based selection for ITGA8+PDGFRA− cells alone, in the absence of a genetically encoded SIX2 reporter, was sufficient to propagate human nephron progenitors.
Despite advances in our ability to generate human nephrons from iPSCs, connecting the resulting nephrons to a common exit path from the kidney presents a notable challenge. Even during development the mechanism by which new nephrons form a patent connection to the ureteric epithelium is poorly understood. Importantly, two studies from 2019 shed light on equivalent events that occur during neonephrogenesis in the zebrafish mesonephros7,8. In response to increased body weight or nephron loss resulting from injury, aggregates of lhx1a-expressing cells form and fuse to the adjacent distal tubule of the pronephros9. Although the existence of this fusion event has long been recognized, the new reports identify a requirement for Wnt–Frizzled signalling in the formation and invasion of these aggregates8, as well as a role for Fgf4 and Fgf10, produced by the distal nephron in luring the aggregates to the right place for fusion to occur7. Indeed, it is the upregulation of these Fgf ligands in response to injury, which is sensed by the Fgfr1 receptor on the aggregates, that stimulates neonephrogenesis in zebrafish7.
Although it is easy to dismiss such studies of mesonephric neonephrogenesis as irrelevant to the mammalian metanephros, mouse ureteric tips also express FGF ligands, and WNT signalling is known to be essential for nephrogenesis during mouse development. The iterative use of patterning cues and the conservation of key gene expression patterns between mesonephros and metanephros development10 indicate that the two processes are probably similar. Improved understanding of how the fusion of new nephrons is facilitated in the fish could prove paramount for driving the integration of transplanted iPSC-derived nephrons.
Advances in the field of kidney development and regenerative biology have in the past year provided insights into the similarities between mouse and human progenitor populations, and how nephron progenitors can be proficiently propagated and potentially plumbed into the filtration system (FIG. 1). However, the ability to generate a sufficient number of functional stem cell-derived nephrons in a form that is amenable to transplantation to replace organ function presents a major engineering challenge. A lack of ureteric tip signalling in kidney organoids may lead to progressive loss of nephron progenitors; however, it is also likely that the lack of an appropriate stroma or of the correct global tissue architecture are equally limiting. An alternative approach to transplanting kidney organoids might be to induce neonephrogenesis in the postnatal kidney by directly injecting human nephron progenitor cells. However, the ability to initiate neonephrogenesis in mammals is limited by our incomplete understanding of the niche signals required to maintain progenitors and to facilitate nephrogenesis and functional integration. What is clear is that much remains to be learned from model organisms that will steer progress in human regenerative medicine.
Fig. 1 |. Advances in our understanding of nephron development and regeneration.
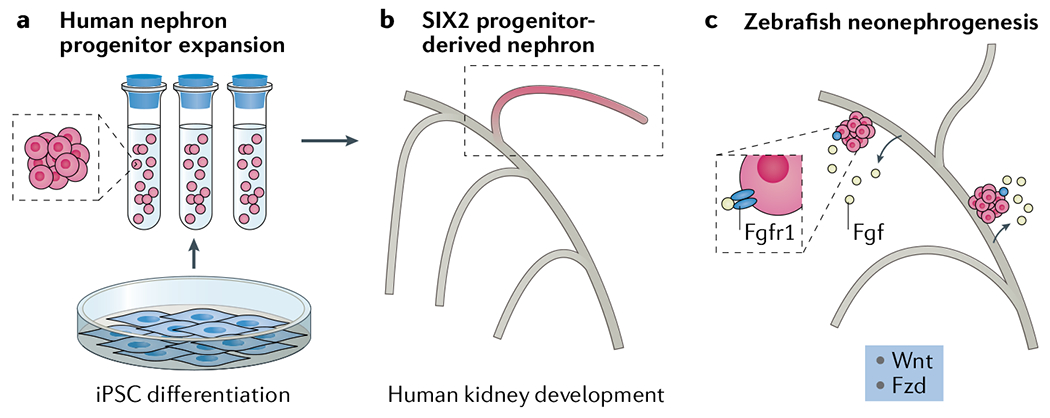
a | Optimization of culture methods have enabled the propagation of stem cell-derived nephron progenitor cells6, b | Lineage tracing in human induced pluripotent stem cell (iPSC)-derived organoids confirms that SIX2-expressing progenitors give rise to the entire nephron and provides tools to examine the dynamics of nephrogenesis during organoid growth2, c | Signalling mechanisms identified in zebrafish may provide insights into how stem cell-derived nephrons might be incorporated into the postnatal human kidney7,8. Fzd, Frizzled.
Key advances.
Lineage tracing in kidney organoids confirms nephron formation from a SIX2+ progenitor population but shows that nephron formation is temporally limited2.
Optimized culture conditions provide an efficient, broadly applicable method to maintain human nephron progenitors in culture6.
Studies in zebrafish have improved our understanding of the factors that facilitate the fusion of newly formed nephrons to adjacent epithelium7,8.
Acknowledgements
M.H.L. is a senior principal research fellow of the National Health and Medical Research Council of Australia (NHMRC; GNT1136085). Her research is supported by the National Institute of Health as part of ReBuilding a Kidney (DK107344) and the NHMRC (GNT1156440 and GNT1098654).
Footnotes
Competing interests
M.H.L. is an inventor on a patent related to kidney organoid generation and has consulted for and received research funding from Organovo Inc. K.T.L declares no competing interests.
References
- 1.Kobayashi A et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stern Cell 3, 169–181 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howden SE et al. Reporter-based fate mapping in human kidney organoids confirms nephron lineage relationships and reveals synchronous nephron formation. EMBO Rep. 20 e47483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AC, Muthukrishnan SD & Oxburgh L A synthetic niche for nephron progenitor cells. Dev. Cell 34 229–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19 516–529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanigawa S et al. Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell Rep. 15 801–813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanigawa S et al. Activin is superior to BMP7 for efficient maintenance of human iPSC-derived nephron progenitors. Stem Cell Reports 13 322–337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallegos TF et al. Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney. Dev. Biol. 454 44–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei CN et al. Wnt signaling mediates new nephron formation during zebrafish kidney regeneration. Development 146, dev 168294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diep CQ et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Wafure 470 95–100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgas K et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 332, 273–286 (2009). [DOI] [PubMed] [Google Scholar]


