Abstract
Isoorientin has anti-inflammatory effects; however, the mechanism remains unclear. We previously found isoorientin is an inhibitor of glycogen synthase kinase 3β (GSK3β) in vitro. Overactivation of GSK3β is associated with inflammatory responses. GSK3β is inactivated by phosphorylation at Ser9 (i.e., p-GSK3β). Lithium chloride (LiCl) inhibits GSK3β and also increases p-GSK3β (Ser9). The present study investigated the anti-inflammatory effect and mechanism of isoorientin via GSK3β regulation in lipopolysaccharide- (LPS-) induced RAW264.7 murine macrophage-like cells and endotoxemia mice. LiCl was used as a control. While AKT phosphorylates GSK3β, MK-2206, a selective AKT inhibitor, was used to activate GSK3β via AKT inhibition (i.e., not phosphorylate GSK3β at Ser9). The proinflammatory cytokines TNF-α, IL-6, and IL-1β were detected by ELISA or quantitative real-time PCR, while COX-2 by Western blotting. The p-GSK3β and GSK3β downstream signal molecules, including NF-κB, ERK, Nrf2, and HO-1, as well as the tight junction proteins ZO-1 and occludin were measured by Western blotting. The results showed that isoorientin decreased the production of TNF-α, IL-6, and IL-1β and increased the expression of p-GSK3β in vitro and in vivo, similar to LiCl. Coadministration of isoorientin and LiCl showed antagonistic effects. Isoorientin decreased the expression of COX-2, inhibited the activation of ERK and NF-κB, and increased the activation of Nrf2/HO-1 in LPS-induced RAW264.7 cells. Isoorientin increased the expressions of occludin and ZO-1 in the brain of endotoxemia mice. In summary, isoorientin can inhibit GSK3β by increasing p-GSK3β and regulate the downstream signal molecules to inhibit inflammation and protect the integrity of the blood-brain barrier and the homeostasis in the brain.
1. Introduction
Isoorientin is a 6-C-glycosylflavone with a molecular formula of C21H20O11. It is present in many plant species, such as corn (Zea mays) silks and pollens, kudzu (Pueraria tuberosa), Patrinia villosa, [1–4]. Isoorientin exhibits antioxidant, antiviral, analgesic, antitumor, and anti-inflammatory activities [5–8]. Isoorientin reduces the development of inflammation in carrageenan-induced paw edema mice [6]. It enhances the activity of antioxidant enzymes, inhibits the release of inflammatory factors (IL-1β, IL-6, and TNF-α), and reduces liver oxidative damage and hepatitis in high-fructose-treated mice [9]. Isoorientin inhibits the activation of MAPKs and NF-κB nuclear translocation in LPS-stimulated BV-2 microglia cells and consequently blocks the expression of inflammatory cytokines [10]. Isoorientin is a potential drug for the treatment of the inflammation-related diseases. The anti-inflammatory mechanism of isoorientin, however, has been unclear.
Our recent study showed that isoorientin is a substrate competitive inhibitor of GSK3β [11]. Overactivated GSK3β plays an important role in inflammatory response and the phosphorylation of the Tau protein, which is involved in the neurodegenerative pathological process [11]. Isoorientin reduces the hyperphosphorylation of the Tau protein and plays neuroprotective effects in SH-SY5Y cells [11]. Notably, GSK3 disorders are involved in a numbers of diseases, such as diabetes, reperfusion injury, mental stability, cancer, and neurodegenerative diseases [12–15], which are all related with inflammation. Sepsis leads to systemic inflammation and the destruction of homeostasis in the brain [16, 17]. Cognitive and memory impairments occur in rats or mice with endotoxemia in the open-field and Morris water maze experiments [18]. GSK3β plays an important role in inflammatory responses in endotoxemia mice [19]. Phosphorylation of GSK3β at the Ser9 site inactivates GSK3β [20–22]. GSK3β regulates its downstream signal molecules such as NF-κB, ERK, Nrf2, and HO-1 in several or animal models [23–25].
The macrophage is the crucial part of the innate immunity system to trigger acute inflammatory responses. Although the anti-inflammatory properties of isoorientin have been revealed, the underlying mechanism remains indistinct. The present study was to explore the anti-inflammatory mechanism of isoorientin targeting GSK3β in comparison with LiCl that is a GSK3β inhibitor and also increases p-GSK3β [26, 27]. This study investigated the effects of isoorientin on the inactive form GSK3β (phosphorylation at Ser9) and its downstream signal molecules in macrophages, as well as the protective effect on the brain in endotoxemia mice.
2. Methods
2.1. Reagents
Isoorientin (HPLC purity ≥ 98%), LiCl, and LPS (from Escherichia coli 0111:B4) were purchased from Sigma (HPLC purity ≥ 98%, St. Louis, MO, USA). MK-2206 was purchased from Selleck Chemicals (Houston, Texas, USA). Monoclonal antibodies against p-GSK3β, GSK3β, p-ERK1/2, ERK1/2, COX-2, NF-κBp65, IκB-α, HO-1, Nrf2, ZO-1, and occludin were purchased form Cell Signaling Technology (Danvers, MA, USA). The antibody against GAPDH was obtained from TransGen Biotech (Beijing, China). The horseradish peroxidase- (HRP-) conjugated anti-mouse and anti-rabbit IgG were purchased from MultiSciences (Hangzhou, China). Mouse TNF-α, IL-1β, and IL-6 ELISA detection kits were obtained from eBioscience (San Diego, CA, USA). ReverTra Ace qPCR RT Master Mix with gDNA Remover and SYBR® Green Realtime PCR Master Mix were purchased from Toyobo Co., Ltd. (Japan).
2.2. Cell Culture and Treatment
RAW264.7 murine macrophage-like cells purchased from the China Center for Type Culture Collection (Wuhan, China) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (HyClonemao) containing 10% fetal bovine serum (FBS, Gibco) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) in an atmosphere of 5% CO2 at 37 °C. Given the results of the growth curve, the cells were seeded at the density of 2 × 105/mL. The cells were pretreated with isoorientin at different concentrations for 1 h, followed by stimulation with LPS (50 ng/mL) for an appropriate time.
2.3. Evaluation of the Effect of Coadministration of Isoorientin and LiCl
The anti-inflammatory doses were preliminarily defined. One hour after exposure to single or mixed LiCl and isoorientin, RAW264.7 cells were stimulated with LPS at 50 ng/mL for 8 h. The proinflammatory cytokine TNF-α in the supernatant was detected by ELISA. The interactions between isoorientin and LiCl were analyzed according to the published method of Jin's Q formula [28]. Q = Ea+b/(Ea + Eb − Ea × Eb), where Ea, Eb, and Ea+b represent the inhibition ratio of isoorientin, LiCl, and mixture of isoorientin and LiCl to TNF-α, respectively. Q < 0.85 suggests an antagonistic effect, 0.85 ≤ Q < 1.15 suggests an additive effect, and Q ≥ 1.15 suggests a synergistic effect.
2.4. Animals and Ethics Statement
The male BALB/c mice (6-8 weeks, 22 ± 2 g) were purchased from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China). The mice were adapted to the environment for 5 days prior to the experiment and were given food and drink randomly. The temperature of the room was 22 ± 2°C with a 12 h light/dark cycle. Animal care and treatment were performed in accordance with the Laboratory Animal Research Committee Guidelines of Guangzhou University of Chinese Medicine, Guangzhou, China.
2.5. Experimental Design and Animal Procedures
Mice were randomly divided into 5 groups (6 mice per group): control (saline), LPS (5 mg/kg), LPS (5 mg/kg) + isoorientin (25 mg/kg and 50 mg/kg), and LPS (5 mg/kg) + LiCl (100 mg/kg); isoorientin and LiCl were given by intragastric administration (ig) once a day for 5 days. Thirty minutes after the last administration of isoorientin or LiCl, LPS was injected intraperitoneally (ip) at a dose of 5 mg/kg. After 6 h of LPS injection, blood and tissues were collected. The blood was left at room temperature for 1 h and then centrifuged to obtain the sera.
2.6. ELISA for Cytokines
The levels of proinflammatory cytokines in sera or cell supernatants were measured by ELISA kits according to the instructions.
2.7. Western Blotting Analysis
The protein samples from RAW264.7 cells and mouse cortical tissue were lysed with RIPA lysis buffer (Kangwei Century Biotechnology, Beijing, China) containing protease and phosphatase inhibitors. Nuclear and cytoplasmic proteins of cells were obtained using a nuclear and cytoplasmic protein extraction kit according to the instructions (Kaiji Biotechnology, Jiangsu, China). Protein concentrations were measured by the BCA protein kit (TransGen Biotech, Beijing, China). Equal amounts of total proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to PVDF membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBST), washed with TBST, incubated with TBST containing primary antibodies(1 : 1000) and 5% bovine serum albumin (BSA) overnight at 4 °C, and subsequently incubated with TBST containing secondary antibodies (1 : 5000) and 5% nonfat dry milk at room temperature for 2 h. The enhanced chemiluminescence of protein blots was measured on a Multifunctional Imaging Analysis System (Bio-Rad, Hercules, CA, USA).
2.8. Quantitative RT-PCR (qPCR)
Total mRNA was isolated from tissues and was quantified. The purity and concentration of extracted total RNA were measured on a NanoPhotometer NP80 (Implen, Germany). The A260/A280 absorption ratio was between 1.8 and 2.2. Reverse transcription reactions were conducted according to the manufacturer's instruction of the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd.), followed by real-time PCR using the SYBR® Green Realtime PCR Master Mix (Toyobo Co., Ltd.). The primers and the product sizes were IL-1β (sense 5-TCCAGGATGAGGACATGAGCAC-3, antisense 5-GAACGTCACACACCAGCAGGTTA-3, product size 105 bp), TNF-α (sense 5-CAGGCGGTGCCTATGTCTCA-3, antisense 5-GGCTACAGGCTTGTCACTCGAA-3, product size 199 bp), IL-6 (sense 5-AGGATACCACTCCCAACAGACC-3, antisense 5-GCACAACTCTTTTCTCATTTCCAC-3, product size 101 bp), and GAPDH (sense 5-TGTGTCCGTCGTGGATCTGA-3, antisense 5-TTGCTGTTGAAGTCGCAGGAG-3, product size 150 bp). qPCR was performed on a 7500 Real-Time PCR System (Applied Biosystems, USA) as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 62°C for 30 s. The comparative Ct method (2-ΔΔCt) was used to analyze the relative expression of those genes by taking GAPDH as an endogenous control.
2.9. Statistical Analysis
SPSS 17.0 was used for statistical analysis. The data were expressed as mean ± SEM. The differences between experimental groups were analyzed with one-way ANOVA, while multiple comparisons were performed with the least significant difference (LSD) method. P < 0.05 was considered statistically significant.
3. Results
3.1. Isoorientin Inhibited Inflammatory Responses in LPS-Stimulated RAW264.7 Cells
The secretion of inflammatory cytokines TNF-α and IL-6 and the expression of COX-2 were detected to illustrate the anti-inflammatory effect of isoorientin. Compared with the control group, LPS (50 ng/mL) significantly increased the secretion of TNF-α and IL-6 (Figures 1(a) and 1(b)) and the expression of COX-2 (Figure 1(c)), which were significantly decreased by isoorientin (6.25 and 25 μM) and LiCl (100 μM).
Figure 1.
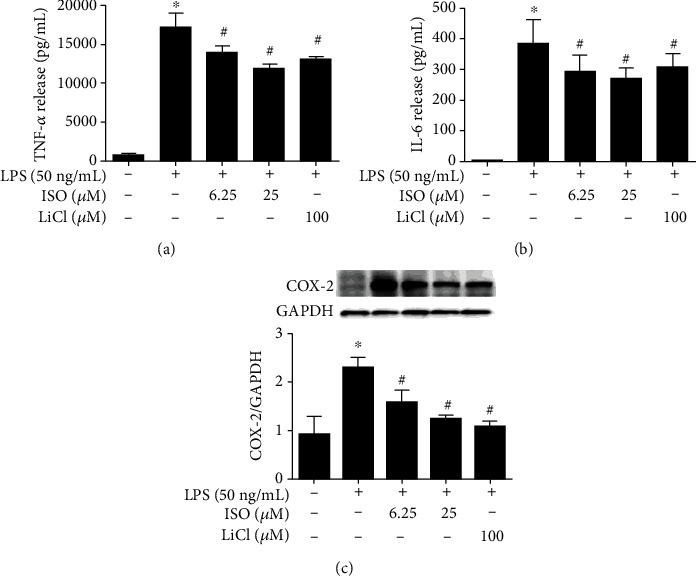
Effects of isoorientin on inflammatory cytokines and COX-2. RAW264.7 cells were treated with isoorientin (ISO) (6.25 and 25 μM) or LiCl (100 μM) for 30 min and then stimulated with LPS (50 ng/mL). After 8 h of stimulation, the cell supernatants were collected, and the levels of TNF-α (a) and IL-6 (b) were detected by ELISA (n = 4, 4 replicates). The cells were collected to measure the expression of COX-2 by Western blotting (n = 3, 3 independent experiments) (c). The data were expressed as mean ± SEM. ∗P < 0.05 versus the control group, #P < 0.05 versus the LPS group.
3.2. The Coadministration of Isoorientin and LiCl Showed an Antagonistic Effect
Our preliminary experiments defined that doses of isoorientin at 6.25 and 25 μM and LiCl at 25 and 100 μM exerted anti-inflammatory effects. Here, we investigated the effect of the coadministration of isoorientin and LiCl by analyzing the secretion of TNF-α. According to Jin's Q formula, Q < 0.85 showed an antagonistic effect, not an additive effect, in the coadministration group (Table 1). The results hinted that isoorientin might act on the same target with LiCl.
Table 1.
Effects of coadministered isoorientin (ISO) and LiC1 in RAW264.7 cells (mean ± SEM, n = 3).
| Compounds (μM) | Inhibition (%) Ea or Eb ± SEM |
Compounds (μM) | Inhibition (%) Ea+b ± SEM |
Q values |
|---|---|---|---|---|
| ISO 6.25 | E a 16.6 ± 6.5a | ISO 6.25+LiC1 25 | 10.1 ± 3.3 | 0.31 |
| ISO 25 | E a 29.8 ± 3.6a | ISO 6.25+LiC1 100 | 27.6 ± 7.9 | 0.79 |
| LiC 25 | E b 18.8 ± 3.3a | ISO 25+LiC1 25 | 28.8 ± 9.9 | 0.67 |
| LiC1 100 | E b 21.7 ± 6.1a | ISO 25+LiC1 100 | 35.0 ± 9.3 | 0.78 |
RAW264.7 cells were treated with isoorientin (6.25 and 25 μM) without or with LiCl (0, 25, and 100 μM) for 30 min and then stimulated with LPS (50 ng/mL). After 8 h stimulation, cell supernatants were collected, and the levels of TNF-α were detected by ELISA. The data were expressed as mean ± SEM (n = 3, 3 replicates). aP < 0.05 in comparison with the LPS group.
3.3. Isoorientin Increased the Expression of p-GSK3β in LPS-Stimulated RAW264.7 Cells
The activity of GSK3β is inhibited upon its phosphorylation at Ser9 [20–22]. LiCl can increase the phosphorylation of Ser9. In Figure 2(a), LPS stimulation resulted in a significant decrease in the phosphorylation of GSK3β (Ser9) in RAW264.7 cells, whereas isoorientin (6.25 and 25 μM) reversed the effects of LPS on p-GSK3β, similar to LiCl. The expressions of GSK3β were not affected significantly. The results suggested that isoorientin increased p-GSK3β to inhibit the activity of GSK3β.
Figure 2.
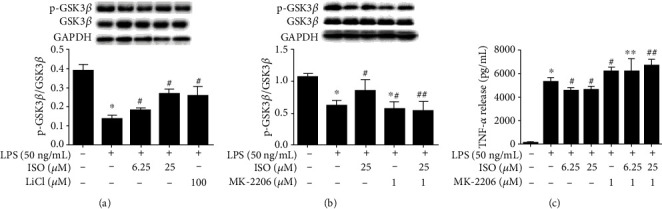
Effects of isoorientin on the phosphorylation of GSK3β (Ser9) and TNF-α in LPS-induced RAW264.7 cells. (a) Effects of isoorientin and LiCl on p-GSK3β and (b) effects of MK-2206 on p-GSK3β. RAW264.7 cells were treated with isoorientin (6.25 and 25 μM) or LiCl (100 μM) for 1 h without (a) or with (b) prior treatment of MK-2206 (1 μM) for 30 min and then stimulated with LPS (50 ng/mL). After 30 min of LPS stimulation, the cells were collected for immunoblotting of p-GSK3β, GSK3β, and GAPDH (n = 3, 3 independent experiments). After 8 h of LPS stimulation, the cell supernatants were collected, and the levels of TNF-α were detected by ELISA (n = 4, 4 replicates) (c). The data were expressed as mean ± SEM. Statistical analysis was done using ANOVA. ∗P < 0.05 versus the control group, #P < 0.05 versus the LPS group. ∗∗P < 0.05 versus the isoorientin 6.25 μM group, ##P < 0.05 versus the isoorientin 25 μM group.
3.4. MK-2206 Attenuated the Effects of Isoorientin on p-GSK3β and TNF-α
MK-2206 is a highly selective inhibitor of AKT. As previously reported, AKT is an important protein kinase to phosphorylate GSK3β [29]. In the present experiment, MK-2206 was used to inhibit the phosphorylation of GSK3β in RAW264.7 cells. MK-2206 pretreatment reversed the GSK3β phosphorylation induced by isoorientin (Figure 2(b)) but had no significant effects on the expressions of GSK3β. Isoorientin (6.25 and 25 μM) decreased the production of TNF-α induced by LPS, while MK-2206 (1 μM) attenuated the effect of isoorientin (Figure 2(c)). These results suggested that isoorientin inhibited the production of TNF-α by increasing the phosphorylation of GSK3β.
3.5. Isoorientin Regulated ERK, NF-κB, and Nrf2/HO-1 Signaling Molecules
It is well known that MAPK/ERK, NF-κB, and Nrf2 participate in inflammatory responses, which are downstream signaling molecules of GSK3β. We wondered whether isoorientin regulated these downstream signaling molecules. Isoorientin (6.25 and 25 μM) and LiCl (100 μM) dramatically attenuated the phosphorylation of ERK1/2 (p-ERK1/2) in LPS-stimulated RAW264.7 cells, while the total protein of ERK1/2 remained unchanged in each group (Figure 3(a)). Isoorientin and LiCl also increased the expression of IκB-α in the cytoplasmic fraction and suppressed the expression of NF-κBp65 in the nuclear fraction induced by LPS (Figure 3(b)), which suggested that isoorientin and LiCl inhibited the activation of the NF-κB signaling pathway. The expression of Nrf2 in the nucleus and HO-1 in the cytoplasm increased upon LPS stimulation, while further increasing significantly upon isoorientin treatment (Figure 3(c)).
Figure 3.
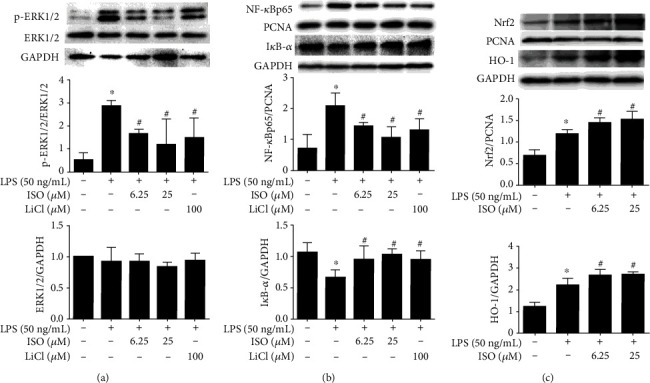
Effects of isoorientin on the activation of ERK (a), NF-κB (b), and Nrf2/HO-1 (c) in LPS-activated RAW264.7 cells. RAW264.7 cells were treated with isoorientin (6.25 and 25 μM) or LiCl (100 μM) for 1 h with or without stimulation of LPS (0 and 50 ng/mL). After the indicated time of LPS stimulation, the cells were collected for immunoblotting of p-ERK1/2 and ERK1/2 (15 min), Nucl-NF-kBp65 (1 h), cyto-IκB-α (1 h), Nucl-Nrf2 (18 h), and cyto-HO-1 (18 h). The data were expressed as mean ± SEM (n = 3, 3 independent experiments). Statistical analysis was done using ANOVA. ∗P < 0.05 versus the control group, #P < 0.05 versus the LPS group.
3.6. Isoorientin Inhibited Proinflammatory Cytokines in the Sera and Cortices of Endotoxemia Mice
The levels of IL-1β, IL-6, and TNF-α in the sera detected by ELISA and the mRNA levels in the cortices detected by qPCR increased dramatically in the endotoxemia mice. Isoorientin (50 mg/kg) and LiCl (100 mg/kg) inhibited significantly the production of IL-1β, IL-6, and TNF-α in the sera and in the cortices (Figure 4).
Figure 4.
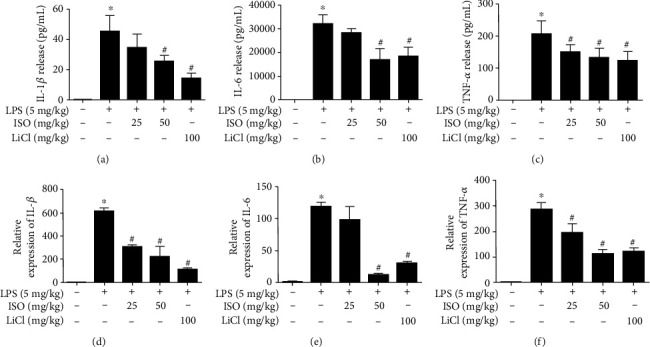
Isoorientin inhibited proinflammatory cytokines in the sera and cortices of endotoxemia mice. Isoorientin (25 and 50 mg/kg/d, ig) and LiCl (100 mg/kg/d, ig) were given once a day for 5 days, and after 30 min of the last administration, LPS (5 mg/kg, ip) was injected. The blood and tissues were collected after 6 h of LPS injection. IL-1β (a), IL-6 (b), and TNF-α (c) in the sera were detected by ELISA (n = 6, 6 biological replicates). IL-1β (d), IL-6 (e), and TNF-α (f) in the cortices were detected by qPCR (n = 3, 3 biological replicates). The data were expressed as mean ± SEM. Statistical analysis was done using ANOVA. ∗P < 0.05 versus the control group. #P < 0.05 versus the LPS group.
3.7. Isoorientin Increased Occludin and ZO-1 of Blood-Brain Barrier (BBB) Components in Endotoxemia Mice
Tight junction proteins, such as occludin and ZO-1, are the important components of BBB to perform normal functions. The occludin and ZO-1 decreased in the cortices of endotoxemia mice compared to the control, while isoorientin (25 and 50 mg/kg) and LiCl (100 mg/kg) increased the expressions of occludin and ZO-1 in endotoxemia mice (Figure 5(a)), which meant preventive effects of isoorientin on the disruption of BBB.
Figure 5.
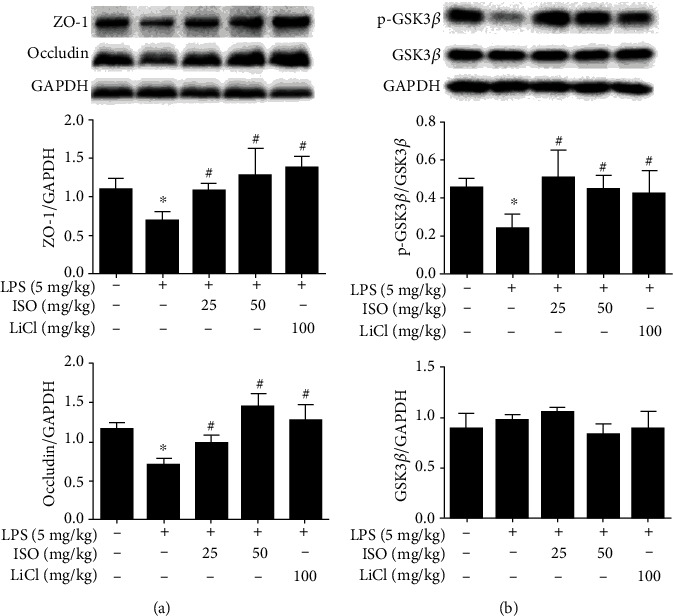
Effects of isoorientin on ZO-1 (a), occludin (a), and p-GSK3β (b) in the brain of endotoxemia mice. Isoorientin (25 and 50 mg/kg, ig) and LiCl (100 mg/kg, ig) were given once a day for 5 days, and after 30 min of the last administration, LPS (5 mg/kg, ip) was given. The brain tissues were collected after 6 h of LPS injection. Western blotting was used to measure the expression of ZO-1, occludin, p-GSK3β, and GSK3β. The data were expressed as mean ± SEM (n = 3, 3 biological replicates). Statistical analysis was done using ANOVA. ∗P < 0.05 versus the control group, #P < 0.05 versus the LPS group.
3.8. Isoorientin Increased the Expression of p-GSK3β in the Brain of Endotoxemia Mice
Isoorientin (25 and 50 mg/kg) and LiCl (100 mg/kg) increased the phosphorylation of GSK3β (Ser9) reduced by LPS (Figure 5(b)). The total protein of GSK3β did not change markedly among the groups. The results suggested that isoorientin increased p-GSK3β to inhibit the activity of GSK3β in the brain.
4. Discussion
GSK3β is a serine/threonine protein kinase. GSK3β plays an important role in regulating cellular inflammatory response, nerve, glucose metabolism, heart, and reproductive function [1, 12, 15]. Inhibition to the activity of GSK3β reduced prostaglandin E2, serotonin, histamine, and other inflammatory mediators in collagen II-induced rheumatoid arthritis in rats [30] and protected the nervous system from HIV-associated neurocognitive disorders [31]. GSK3β is a potential target for the treatment of immune diseases [32]. Phosphorylation of GSK3β at Ser9 (p-GSK3β) has a greater effect on GSK3β activity than phosphorylation at Tyr216 [20]. GSK3β is inactivated when phosphorylation occurs at Ser9 [20–22].
The PI3K/AKT signaling pathway plays an important role in regulating GSK3β activity via upregulating the phosphorylation of GSK3β (Ser9) in Drosophila and dorsoventral patterning in Xenopus embryos [33]. Modulating the PI3K/Akt/GSK3β signaling pathway affects the duration and intensity of the Toll-like receptor- (TLR-) mediated inflammation in septicemic shock [34]. GSK3β acts as an upstream molecule to regulate Nrf2 phosphorylation and Nrf2 detachment from the antioxidant response element (ARE). Parkinson's disease (PD) was alleviated by regulating the AKT/GSK3β/Nrf2 signaling pathway in a rat model of PD [23]. NF-κB, a transcription factor with multiple transcriptional regulatory effects, is an important downstream pathway in the LPS-mediated inflammatory response signal transduction pathway in macrophages [35]. NF-κB plays a key role in proinflammatory effects of GSK3β in human monocytes. The GSK3β inhibitor SB216763 inhibits the transcriptional activity of NF-κB and reduces the production of inflammatory factors induced by TLR in human monocytes [25]. The MAPK signaling pathway regulates oxidative stress and injury response in cells. GSK3β knockdown blocks the IFN-α-induced phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 (Thr202/Tyr204) in human Jurkat T cells [24].
Isoorientin is anti-inflammatory in carrageenan-induced paw edema mice and high-fructose-treated mice [6, 9]. It significantly blocks the inflammatory response in BV-2 microglia cells stimulated by LPS [10]. However, the anti-inflammatory mechanism of isoorientin has not been elucidated. In our previous study, isoorientin showed the ability of binding with GSK3β in vitro and reducing its activity in molecular docking and enzyme kinetics studies [11]. Lithium ion (Li+), an inhibitor of GSK3β, acts on GSK3β directly by competition with magnesium ion in the ATP binding pocket and has already been used for the treatment of bipolar disorders. Li+ also increases the phosphorylation at serine 9 of GSK3β to indirectly modulate GSK3β activity by activating Akt [26, 27, 36]. In the present study, we investigated whether isoorientin modulated inflammatory response by regulating p-GSK3β and exerted protective effects on brain injury.
In our study, isoorientin (≤100 μM) and LiCl (100 μM) had no inhibitory effect on the growth of RAW264.7 (data not shown). LPS (50 ng/kg) remarkably increased the release of TNF-α. Isoorientin at 6.25 μM and 25 μM decreased the expressions of proinflammatory cytokines and COX-2. In order to investigate the anti-inflammatory mechanism of isoorientin targeting GSK3β, TNF-α was detected to evaluate the effect of the coadministration of isoorientin and LiCl. The results suggested the coadministration exerted an antagonistic effect, but not an additive effect, indicating the same biochemical target of isoorientin and LiCl, namely, GSK3β (Table 1).
Furthermore, isoorientin upregulated p-GSK3β, similar to LiCl. Upregulation of p-GSK3β is well known to contribute to the inactivity of GSK3β. To verify the biochemical target of isoorientin, an AKT inhibitor, MK-2206 was used to inhibit the phosphorylation of GSK3β. Upon exposure to MK-2206 at 1 μM (IC50 12 μM) alone, the viability of RAW264.7 cells did not alter dramatically (data not shown). MK-2206 (1 μM) reversed the effects of isoorientin on TNF-α and p-GSK3β (Figure 2), suggesting inhibition of inflammation by isoorientin via upregulating p-GSK3β. The downstream signaling molecules of GSK3β were investigated in LPS-induced RAW264.7 cells. Isoorientin inhibited the activation of transcription factor NF-κB and the ERK signaling pathway and activated the Nrf2/HO-1 signaling pathway (Figure 3).
Clinical studies have shown that sepsis patients have impairments in cognitive and memory functions [37, 38]. Endotoxemia mice show structural disorders in hippocampal neurons and cell necrosis in histopathological observations [39] and display symptoms such as disturbance of consciousness, abnormal behavior, and impaired sensory functions [40–42]. The BBB maintains immune privilege in the brain. Inflammation can increase the permeability of the BBB and disturb the homeostasis of the brain. IL-1β, IL-6, and TNF-α increased the permeability of BBB by downregulating tight junction proteins in endothelial cells [43]. Long-lasting inflammation damages the brain, which is common in neurodegenerative diseases [44, 45]. To clarify the protective effect of isoorientin on the brain, we detected the inflammatory cytokines in peripheral and central nervous systems, the BBB integrity, and the p-GSK3β (Ser9) in the brain of endotoxemia mice.
Anti-inflammatory doses of isoorientin and LiCl in vivo were determined according to previous studies [46, 47]. In the present study, LPS (5 mg/kg) significantly increased the levels of peripheral and central nervous system inflammatory cytokines, which were reduced by isoorientin (50 mg/kg) and LiCl (100 mg/kg) (Figure 4).
Occludin and ZO-1 are two key tight junction proteins in BBB, which determine the paracellular permeability to different ions or large molecules [48]. The decreased expression of occludin can be used as a marker of BBB damage [49]. Isoorientin (25 and 50 mg/kg) and LiCl (100 mg/kg) upregulated the expression of occludin, ZO-1 and p-GSK3β in the brain of endotoxemia mice (Figure 5), showing a good potential of reversing the destruction of BBB and treatment for GSK3β-related brain diseases. This study provided good evidence for clarifying the role of isoorientin in endotoxemia by protecting the BBB and regulating p-GSK3β in the brain.
Isoorientin binds with GSK3β in vitro and inhibits its activity [11]. Given that LiCl increases p-GSK3β to inactivate its activity, we found that isoorientin also increased p-GSK3β. These results suggested that isoorientin also regulated the upstream molecules of GSK3β to inhibit GSK3β. However, the merits of isoorientin superior to LiCl need further studies.
In conclusion, isoorientin increased the phosphorylation of GSK3β (Ser9) to inactivate its activity and regulated NF-κB, ERK, and Nrf2/HO-1 signaling pathways to inhibit the production of proinflammatory cytokines and COX-2 in macrophages induced by LPS (Figure 6(a)). Isoorientin inhibited inflammatory responses in endotoxemia mice, increased p-GSK3β (Ser9), and protected the integrity of BBB by increasing the tight junction protein occludin and ZO-1 in the brain (Figure 6(b)). This was the first study to elucidate the anti-inflammatory mechanism of isoorientin from the perspective of GSK3β and to analyze the protective effect on inflammation-related brain injury.
Figure 6.
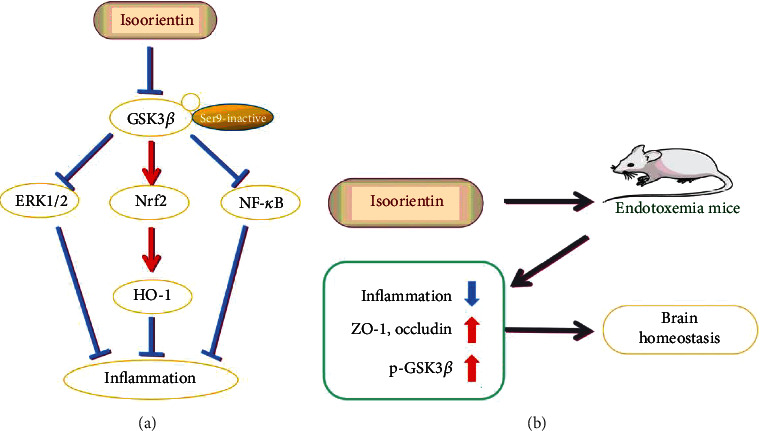
Proposed mechanism of isoorientin inhibiting inflammation in the monocyte macrophage RAW264.7 cells (a) and endotoxemia mice (b) via GSK3β regulation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81973545), the USDA (Hatch HAW5032-R), and the Hawaii Community Foundation (18ADV-90801).
Abbreviations
- BBB:
Blood-brain barrier
- COX-2:
Cyclooxygenase-2
- ERK1/2:
Extracellular regulated protein kinases
- GAPDH:
Glyceraldehyde-3-phosphate dehydrogenase
- GSK3β:
Glycogen synthase kinase 3β
- HO-1:
Heme oxygenase 1
- ISO:
Isoorientin
- IκB-α:
Nuclear factor kappa B inhibitor alpha
- LiCl:
Lithium chloride
- IL:
Interleukin
- LPS:
Lipopolysaccharide
- NF-κB:
Nuclear factor kappa B
- Nrf2:
Nuclear factor-E2-related factor 2
- TNF:
Tumor necrosis factor
- ZO-1:
Zonula occludens-1.
Contributor Information
Qing X. Li, Email: qingl@hawaii.edu.
Yan Dong, Email: dondy001@gzucm.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article, containing Table 1 and Figures 1–5. Other data that might be useful for the findings of this study will be supplied as supplementary information by the corresponding author (Yan Dong) upon request.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Y.D. and Q.X.L. conceived the study, designed the experiments, supervised all the research, analyzed the data, and revised the manuscript. Y.G.L and Y.J.Z. completed the experiments and analyzed the data, and Y.G.L wrote the manuscript. X.Q.T., J.Y.L., Y.K.Z., L.Y., and S.S.B. carried out the experiments. Q.D. designed part of the experiments. Yingui Li and Yijing Zhao are co-first authors.
References
- 1.Anilkumar K., Reddy G. V., Azad R., et al. Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of Pueraria tuberosa. Oxidative Medicine and Cellular Longevity. 2017;2017:10. doi: 10.1155/2017/5498054.5498054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng J., Fan G., Hong Z., Chai Y., Wu Y. Preparative separation of isovitexin and isoorientin from Patrinia villosa Juss by high-speed counter-current chromatography. Journal of Chromatography A. 2005;1074(1-2):111–115. doi: 10.1016/j.chroma.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 3.Yuan L., Wang J., Wu W., Liu Q., Liu X. Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomedicine & Pharmacotherapy. 2016;81:356–362. doi: 10.1016/j.biopha.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Widstrom N. W., Snook M. E. A gene controlling biosynthesis of isoorientin, a compound in corn silks antibiotic to the corn earworm. Entomologia Experimentalis et Applicata. 1998;89(2):119–124. doi: 10.1046/j.1570-7458.1998.00390.x. [DOI] [Google Scholar]
- 5.Tunalier Z., Koşar M., Küpeli E., Çaliş İ., Başer K. H. C. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. Journal of Ethnopharmacology. 2007;110(3):539–547. doi: 10.1016/j.jep.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Kupeli E., Aslan M., Gurbuz I., Yesilada E. Evaluation of in vivo biological activity profile of isoorientin. Zeitschrift für Naturforschung C. 2004;59(11-12):787–790. doi: 10.1515/znc-2004-11-1204. [DOI] [PubMed] [Google Scholar]
- 7.Yuan L., Wang J., Xiao H., Xiao C., Wang Y., Liu X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicology and Applied Pharmacology. 2012;265(1):83–92. doi: 10.1016/j.taap.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X.-Z., Shen W.-W., Gong C.-Y., et al. Antiviral activity of Isoorientin against respiratory syncytial virus in vitro and in vivo. Journal of Sun Yat-sen University(Medical Sciences) 2015;36:352–359. [Google Scholar]
- 9.Yuan L., Han X., Li W., Ren D., Yang X. Isoorientin prevents hyperlipidemia and liver injury by regulating lipid metabolism, antioxidant capability, and inflammatory cytokine release in high-fructose-fed mice. Journal of Agricultural and Food Chemistry. 2016;64(13):2682–2689. doi: 10.1021/acs.jafc.6b00290. [DOI] [PubMed] [Google Scholar]
- 10.Yuan L., Wu Y., Ren X., Liu Q., Wang J., Liu X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia. Molecular and Cellular Biochemistry. 2014;386(1-2):153–165. doi: 10.1007/s11010-013-1854-9. [DOI] [PubMed] [Google Scholar]
- 11.Liang Z., Zhang B., Su W. W., Williams P. G., Li Q. X. C-Glycosylflavones alleviate tau phosphorylation and amyloid neurotoxicity through GSK3β inhibition. ACS Chemical Neuroscience. 2016;7(7):912–923. doi: 10.1021/acschemneuro.6b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitasi C. L., Liu J., Gausserès B., et al. Implication of glycogen synthase kinase 3 in diabetes-associated islet inflammation. The Journal of endocrinology. 2020;244(1):133–148. doi: 10.1530/JOE-19-0239. [DOI] [PubMed] [Google Scholar]
- 13.Slim C., Zaouali M. A., Nassrallah H., et al. Protective potential effects of fucoidan in hepatic cold ischemia-rerfusion injury in rats. International Journal of Biological Macromolecules. 2020;155:498–507. doi: 10.1016/j.ijbiomac.2020.03.245. [DOI] [PubMed] [Google Scholar]
- 14.Glibo M., Serman A., Karin-Kujundzic V., et al. The role of glycogen synthase kinase 3 (GSK3) in cancer with emphasis on ovarian cancer development and progression: A comprehensive review. Bosnian Journal of Basic Medical Sciences. 2020 doi: 10.17305/bjbms.2020.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onishi T., Iwashita H., Uno Y., et al. A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S)-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer's disease. Journal of Neurochemistry. 2011;119(6):1330–1340. doi: 10.1111/j.1471-4159.2011.07532.x. [DOI] [PubMed] [Google Scholar]
- 16.Ge L., Hu Q., Chen J., Shi M., Yang H., Zhu G. Inhibition of TNF-α sepsis of lipopolysaccharide induction using nano cerium oxide system. Materials Science and Engineering: C. 2017;77:405–410. doi: 10.1016/j.msec.2017.03.207. [DOI] [PubMed] [Google Scholar]
- 17.Han Q., Lin Q., Huang P., et al. Microglia-derived IL-1β contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. Journal of Neuroinflammation. 2017;14(1):p. 52. doi: 10.1186/s12974-017-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Xie K., Chen H., et al. Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Research. 2014;1589:78–92. doi: 10.1016/j.brainres.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Noh K. T., Son K. H., Jung I. D., et al. Protein Kinase C δ (PKCδ)-Extracellular Signal-regulated Kinase 1/2 (ERK1/2) Signaling Cascade Regulates Glycogen Synthase Kinase-3 (GSK-3) Inhibition-mediated Interleukin-10 (IL-10) Expression in Lipopolysaccharide (LPS)-induced Endotoxemia. ournal of Biological Chemistry. 2012;287(17):14226–14233. doi: 10.1074/jbc.m111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borghi R., Piccini A., Delacourte A., Strocchi P., Zaccheo D., Tabaton M. Protein levels of glycogen synthase 3 kinase are normal in progressive supranuclear palsy. Neuroscience Letters. 2004;366(1):67–70. doi: 10.1016/j.neulet.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Frame S., Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359(1):1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q. M., Fiol C. J., DePaoli-Roach A. A., Roach P. J. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. Journal of Biological Chemistry. 1994;269(20):14566–14574. [PubMed] [Google Scholar]
- 23.Huang B., Liu J., Meng T., et al. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson's disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Frontiers in Immunology. 2018;9:p. 2527. doi: 10.3389/fimmu.2018.02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao C. W., Lin C. F., Wu H. T., et al. Glycogen synthase kinase-3β is critical for Interferon-α-induced serotonin uptake in human Jurkat T cells. Journal of Cellular Physiology. 2012;227(6):2556–2566. doi: 10.1002/jcp.22994. [DOI] [PubMed] [Google Scholar]
- 25.Martin M., Rehani K., Jope R. S., Michalek S. M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nature Immunology. 2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Jiang R., Wang L., Chen X., Wang Y. Ginsenoside Rg1 improves differentiation by inhibiting senescence of human bone marrow mesenchymal stem cell via GSK-3β and β-catenin. Molecular Immunology. 2020;2020:1–16. doi: 10.1155/2020/2365814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquali L., Busceti C. L., Fulceri F., Paparelli A., Fornai F. Intracellular pathways underlying the effects of lithium. Behavioural Pharmacology. 2010;21(5-6):473–492. doi: 10.1097/FBP.0b013e32833da5da. [DOI] [PubMed] [Google Scholar]
- 28.Jin Z. J. Addition in drug combination (author's transl) Acta pharmacologica Sinica. 1980;1(2):70–76. [PubMed] [Google Scholar]
- 29.Zhang H. H., Lipovsky A. I., Dibble C. C., Sahin M., Manning B. D. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Molecular Cell. 2006;24(2):185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H., Liu J., Zeng J., Hu B., Fang X., Li L. Inhibition of GSK-3β alleviates collagen II-Induced rheumatoid arthritis in rats. Medical Science Monitor. 2016;22:1047–1052. doi: 10.12659/MSM.897739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ances B. M., Letendre S. L., Alexander T., Ellis R. J. Role of psychiatric medications as adjunct therapy in the treatment of HIV associated neurocognitive disorders. International Review of Psychiatry. 2009;20(1):89–93. doi: 10.1080/09540260701877670. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P., Katz J., Michalek S. M. Glycogen synthase kinase-3β (GSK3β) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Molecular Immunology. 2009;46(4):677–687. doi: 10.1016/j.molimm.2008.08.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross D. A. E., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Kumar A., Lamont R. J., Scott D. A. GSK3β and the control of infectious bacterial diseases. Trends in Microbiology. 2014;22(4):208–217. doi: 10.1016/j.tim.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharif O., Bolshakov V. N., Raines S., Newham P., Perkins N. D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunology. 2007;8(1):p. 1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin L., Latypova X., Wilson C. M., et al. Tau protein kinases: involvement in Alzheimer's disease. Ageing Research Reviews. 2013;12(1):289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen D. N., Huyghens L., Zhang H., Schiettecatte J., Smitz J., Vincent J.-L. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/712742.712742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonneville R., Verdonk F., Rauturier C., et al. Understanding brain dysfunction in sepsis. Annals of Intensive Care. 2013;3(1):p. 15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandes M. S., D’Avila J. C., Trevelin S. C., et al. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. Journal of Neuroinflammation. 2014;11(1):p. 36. doi: 10.1186/1742-2094-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R., Chen W., Lu Y., et al. Dioscin relieves endotoxemia induced acute neuro-inflammation and protect neurogenesis via improving 5-HT metabolism. Scientific Reports. 2017;7(1, article 40035) doi: 10.1038/srep40035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widmann C. N., Heneka M. T. Long-term cerebral consequences of sepsis. The Lancet Neurology. 2014;13(6):630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 42.Du Y., Meng Y., Lv X., et al. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins ameliorating brain endotoxemia in mice. International Journal of Clinical and Experimental Pathology. 2014;7:8443–8452. [PMC free article] [PubMed] [Google Scholar]
- 43.Yi X., Xu C., Huang P., et al. 1-Trifluoromethoxyphenyl-3-(1-Propionylpiperidin-4-yl) Urea Protects the Blood-Brain Barrier Against Ischemic Injury by Upregulating Tight Junction Protein Expression, Mitigating Apoptosis and Inflammation In Vivo and In Vitro Model. Frontiers in Pharmacology. 2020;11 doi: 10.3389/fphar.2020.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seabrook T. J., Thomas K., Jiang L., et al. Dendrimeric Aβ1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiology of Aging. 2007;28(6):813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Bohrmann B., Baumann K., Benz J., et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. Journal of Alzheimer's Disease. 2012;28(1):49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 46.Song Y., Kim H. D., Lee M. K., et al. Maysin and its flavonoid derivative from centipedegrass attenuates amyloid plaques by inducting humoral immune response with Th2 skewed cytokine response in the Tg (APPswe, PS1dE9) Alzheimer's mouse model. PLoS One. 2017;12(1, article e169509) doi: 10.1371/journal.pone.0169509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang J. Lithium chloride improves the learning and memory ability of APP/PS1 double-transgenic mice by reduce the levels of oxidative stress. Guizhou Medical University; 2019. [Google Scholar]
- 48.Hu Y. J., Wang Y. D., Tan F. Q., Yang W. X. Regulation of paracellular permeability: factors and mechanisms. Molecular Biology Reports. 2013;40(11):6123–6142. doi: 10.1007/s11033-013-2724-y. [DOI] [PubMed] [Google Scholar]
- 49.Sun W., Yang K. T., Sheng L., Su Z. Q. A study on the ultrastructure of blood brain barrier and the expression of occludin in focal brain ischemia-reperfusion injury rats. Apoplexy and Nervous Diseases. 2007;4:425–427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article, containing Table 1 and Figures 1–5. Other data that might be useful for the findings of this study will be supplied as supplementary information by the corresponding author (Yan Dong) upon request.


